Carbon and Macronutrient Budgets in an Alder Plantation Grown on a Reclaimed Combustion Waste Landfill
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Study
2.3. Alder Biomass Study
2.4. Nutrient Stock and Carbon Accumulation
2.5. Statistical Analyses
3. Results
3.1. Basic Soil Parameters
3.2. Carbon and Macronutrient Stocks in Technosols
3.3. Alder Biomass
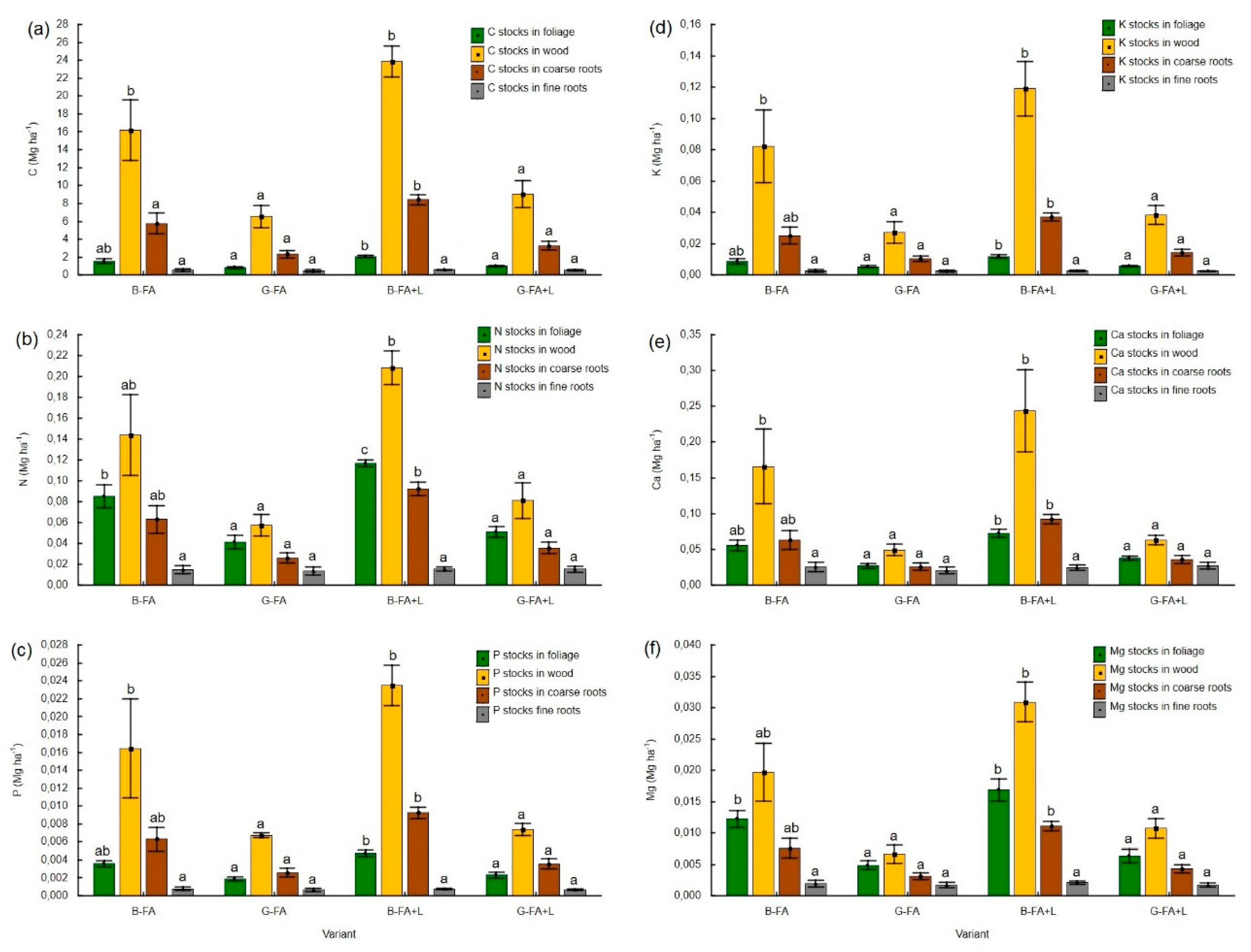
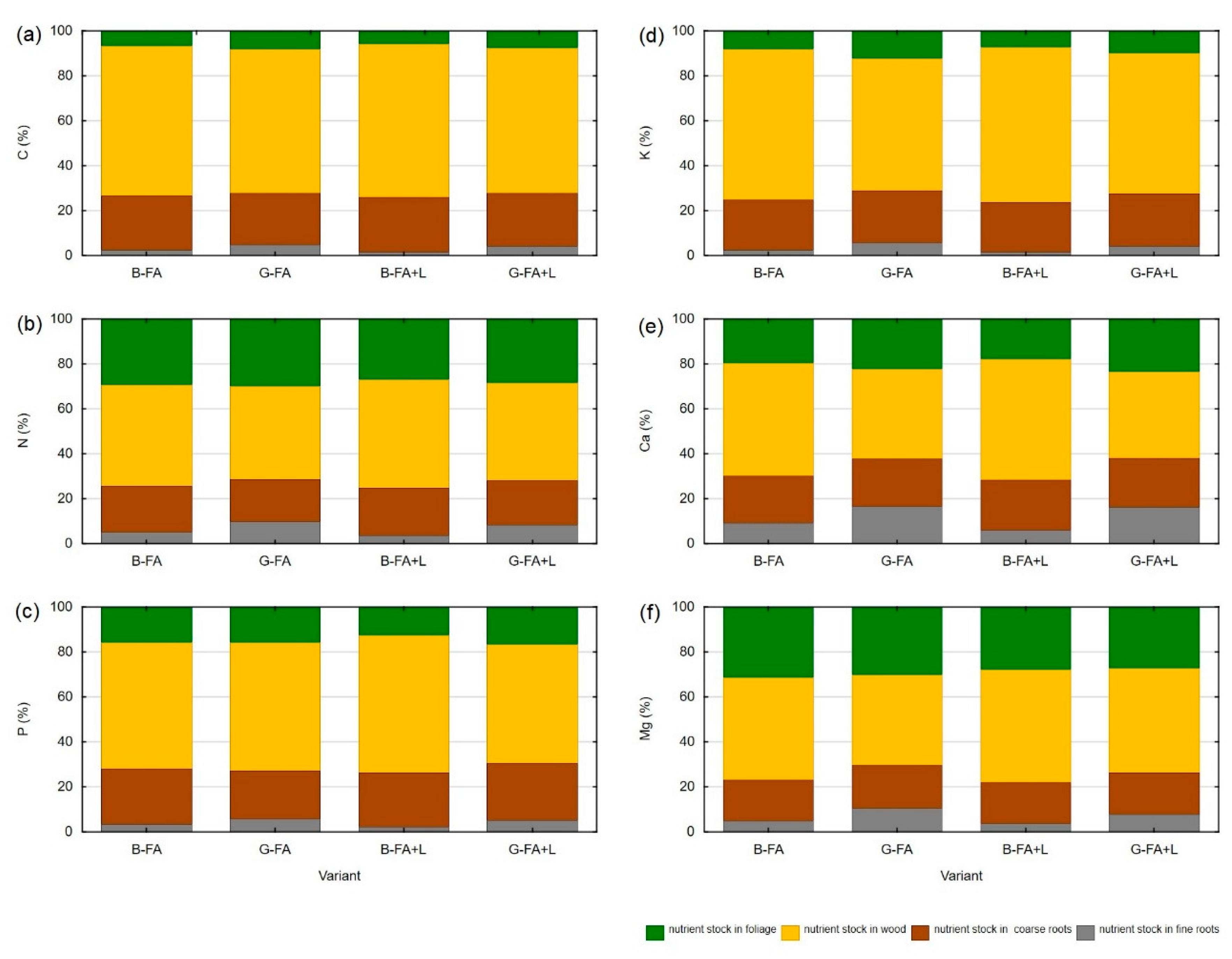
3.4. Carbon and Macronutrient Stocks in Biomass
3.5. Whole Ecosystem Carbon Accumulation
3.6. Relationship between the Carbon and Nutrient Stocks in the Biomass and Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McCarthy, M.; Dhir, R.K. Development of high volume fly ash cements for use in concrete construction. Fuel 2005, 84, 1423–1432. [Google Scholar] [CrossRef]
- Sua-iam, G.; Makul, N. Utilization of high volumes of unprocessed lignite-coal fly ash and rice husk ash in self-consolidating concrete. J. Clean. Prod. 2014, 78, 184–194. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth-Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Iyer, R.S.; Scott, J.A. Power station fly ash—A review of value-added utilization outside of the construction industry. Resour. Conserv. Recycl. 2001, 31, 217–228. [Google Scholar] [CrossRef]
- Żołnierz, L.; Weber, J.; Gilewska, M.; Strączyńska, S.; Pruchniewicz, D. The spontaneous development of understory vegetation on reclaimed and afforested post-mine excavation filled with fly ash. Catena 2016, 136, 84–90. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Woś, B.; Pająk, M.; Wanic, T.; Krzaklewski, W.; Chodak, M. Reclamation of a lignite combustion waste disposal site with alders (Alnus sp.): Assessment of tree growth and nutrient status within 10 years of the experiment. Environ. Sci. Pollut. Res. 2018, 25, 17091–17099. [Google Scholar] [CrossRef]
- Krzaklewski, W.; Pietrzykowski, M.; Woś, B. Survival and growth of alders (Alnus glutinosa (L.) Gaertn. and Alnus incana (L.) Moench) on fly ash technosols at different substrate improvement. Ecol. Eng. 2012, 49, 35–40. [Google Scholar] [CrossRef]
- Weber, J.; Strączyńska, S.; Kocowicz, A.; Gilewska, M.; Bogacz, A.; Gwiżdż, M.; Debicka, M. Properties of soil materials derived from fly ash 11 years after revegetation of post-mining excavation. Catena 2015, 133, 250–254. [Google Scholar] [CrossRef]
- Haynes, R.J. Reclamation and revegetation of fly ash disposal sites-challenges and research needs. J. Environ. Manag. 2009, 90, 43–53. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Zagórski, Z.; Mendak, E.; Bartmiński, P.; Szara, E.; Kondras, M.; Oktaba, L.; Turek, A.; Rogoziński, R. Technogenic soils (Technosols) developed from fly ash and bottom ash from thermal power stations combusting bituminous coal and lignite. Part I. Properties, classification, and indicators of early pedogenesis. Catena 2017, 157, 75–89. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Krzaklewski, W.; Woś, B. Preliminary assessment of growth and survival of green alder (Alnus viridis), a potential biological stabilizer on fly ash disposal sites. J. For. Res. 2015, 26, 131–136. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Plant regeneration potential in fly ash ecosystem. Urban For. Urban Green. 2016, 15, 40–44. [Google Scholar] [CrossRef]
- Pietrzykowski, M. Tree response to soil reconstruction on reclaimed post-mining sites—A key issue in forest ecosystem restoration. In Problems of Forestry in the Mountains and Industrial Region; Orzeł, S., Ed.; Agricultural University of Krakow Publishing: Krakow, Poland, 2015; Chapter 1; pp. 15–39, (In Polish, English Summary). [Google Scholar]
- Pietrzykowski, M.; Krzaklewski, W. Soil organic matter, C and N accumulation during natural succession and reclamation in an opencast sand quarry (southern Poland). Arch. Agron. Soil Sci. 2007, 53, 473–483. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Daniels, W.L. Estimation of carbon sequestration by pine (Pinus sylvestris L.) ecosystems developed on reforested post-mining sites in Poland on differing mine soil substrates. Ecol. Eng. 2014, 73, 209–218. [Google Scholar] [CrossRef]
- Čížková, B.; Woś, B.; Pietrzykowski, M.; Frouz, J. Development of soil chemical and microbial properties in reclaimed and unreclaimed grasslands in heaps after opencast lignite mining. Ecol. Eng. 2018, 123, 103–111. [Google Scholar] [CrossRef]
- Balboa-Murias, M.A.; Rojo, A.; Álvarez, J.G.; Merino, A. Carbon and nutrient stocks in mature Quercus robur L. stands in NW Spain. Ann. For. Sci. 2005, 63, 557–565. [Google Scholar] [CrossRef]
- Bungart, R.; Hüttl, R.F. Production of biomass for energy in post-mining landscapes and nutrient dynamics. Biomass Bioenergy 2001, 20, 181–187. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Woś, B.; Haus, N. Scots pine needles macronutrient (N, P, K, CA, MG, and S) supply at different reclaimed mine soil substrates–as an indicator of the stability of developed forest ecosystems. Environ. Monit. Assess. 2013, 185, 7445–7457. [Google Scholar] [CrossRef]
- Pandey, V.C. Suitability of Ricinus communis L. cultivation for phytoremediation of fly ash disposal sites. Ecol. Eng. 2013, 57, 336–341. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Biol. 2008, 7, 47–59. [Google Scholar] [CrossRef]
- Rees, F.; Dagois, R.; Derrien, D.; Fiorelli, J.-L.; Watteau, F.; Morel, J.L.; Schwartz, C.; Simonnot, M.O.; Séré, G. Storage of carbon in constructed technosols: In situ monitoring over a decade. Geoderma 2019, 337, 641–648. [Google Scholar] [CrossRef]
- Resh, S.C.; Binkley, D.; Parrotta, J.A. Greater soil carbon sequestration under Nitrogen-fixing trees compared with Eucalyptus species. Ecosystems 2002, 5, 217–231. [Google Scholar] [CrossRef]
- Binkley, D. How nitrogen-fixing trees change soil carbon. In Tree Species Effects on Soils: Implications for Global Change; Binkley, D., Menyailo, O., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2005; pp. 155–164. [Google Scholar]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Ostrowska, S.; Gawlinski, Z.; Szczubialka, Z. Procedures for Soil and Plants Analysis; Institute of Environmental Protection: Warsaw, Poland, 1991. (In Polish) [Google Scholar]
- van Reeuwijk, L.P. Procedures for Soil Analysis; ISRIC: Wageningen, The Netherlands, 2002. [Google Scholar]
- De Vos, B.; Van Meirvenne, M.; Quataert, P.; Deckers, J.; Muys, B. Predictive quality of pedotransfer functions for estimating bulk density of forest soils. Soil Sci. Am. J. 2005, 69, 500–510. [Google Scholar] [CrossRef]
- Ochał, W. The above-ground biomass of trees in young black alder (Alnus glutinosa (L.) Gaertn.) stands. Acta Agrar. Silvestria Ser. Silvestris 2013, 51, 75–89, (In Polish, English Summary). [Google Scholar]
- Frouz, J.; Dvorščík, P.; Vávrová, A.; Doušová, O.; Kadochová, Š.; Matějíček, L. Development of canopy cover and woody vegetation biomass on reclaimed and unreclaimed post-mining sites. Ecol. Eng. 2015, 84, 233–239. [Google Scholar] [CrossRef]
- Külaots, I.; Hurt, R.H.; Suuberg, E.M. Size distribution of unburned carbon in coal fly ash and its implications. Fuel 2004, 83, 223–230. [Google Scholar] [CrossRef]
- StatSoft Inc. (1984–2018) STATISTICA (Data Analysis Software System); Version 13.1. Available online: https://www.statsoft.pl/statistica_13/ (accessed on 8 March 2020).
- Johansson, T. Biomass equations for determining fractions of common and grey alders growing on abandoned farmland and some practical implications. Biomass Bioenergy 2000, 18, 147–159. [Google Scholar] [CrossRef]
- Aosaar, J.; Varik, M.; Uri, U. Biomass production potential of grey alder (Alnus incana (L.) Moench.) in Scandinavia and Eastern Europe: A review. Biomass Bioenergy 2012, 45, 11–26. [Google Scholar] [CrossRef]
- Vares, A. Biomass, nitrogen and phosphorus allocation in above-ground parts of black alder (Alnus glutinosa (L.) Gaertn.) plantations. Balt. For. 2000, 6, 47–52. [Google Scholar]
- Burke, M.K.; Raynal, D.J. Fine root growth phenology, production and turnover in a northern hardwood forest ecosystem. Plant Soil 1994, 162, 135–146. [Google Scholar] [CrossRef]
- Józefowska, A.; Woś, B.; Pietrzykowski, M. Tree species and soil substrate effects on soil biota during early soil forming stages at afforested mine sites. Appl. Soil Ecol. 2016, 102, 70–79. [Google Scholar] [CrossRef]
- Pietrzykowski, M. Scots pine (Pinus sylvestris L.) ecosystem macronutrients budget on reclaimed mine sites–stand trees supply and stability. Nat. Sci. 2010, 2, 590–599. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The Mineral Nutrition of Wild Plants Revisited: A Re-evaluation of Processes and Patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar]
- Marchner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elservier Ltd.: Oxford, UK, 2012; pp. 315–330. [Google Scholar]
- Pietrzykowski, M.; Woś, B.; Pająk, M.; Wanic, T.; Krzaklewski, W.; Chodak, M. The impact of alders (Alnus spp.) on the physico-chemical properties of technosols on a lignite combustion waste disposal site. Ecol. Eng. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Orczewska, A.; Piotrowska, A.; Lemanowicz, J. Soil acid phosphomonoesterase activity and phosphorus forms in ancient and post-agricultural black alder [Alnus glutinosa (L.) Gaertn.] woodlands. Acta Soc. Bot. Pol. 2012, 81, 81–86. [Google Scholar] [CrossRef]
- Compton, J.E.; Cole, D.W. Phosphorus cycling and soil P fractions in Douglas-fir and red alder stands. For. Ecol. Manag. 1998, 110, 101–112. [Google Scholar] [CrossRef]
- Giardina, C.P.; Huffman, S.; Binkley, D.; Caldwell, B.A. Alders increase soil phosphorus availability in a Douglas-fir plantation. Can. J. For. Res. 1995, 25, 1652–1657. [Google Scholar] [CrossRef]
- Vindušková, O.; Frouz, J. Soil carbon accumulation after open-cast coal and oil shale mining in Northern Hemisphere: A quantitative review. Environ. Earth Sci. 2013, 69, 1685–1698. [Google Scholar] [CrossRef]
- Frouz, J.; Pižl, V.; Cienciala, E.; Kalčík, J. Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 2009, 94, 111–121. [Google Scholar] [CrossRef]
- Bartuska, M.; Frouz, J. Carbon accumulation and changes in soil chemistry in reclaimed open-cast coal mining heaps near Sokolov using repeated measurement of chronosequence sites. Eur. J. Soil Sci. 2015, 66, 104–111. [Google Scholar] [CrossRef]
- Claessens, H.; Oosterbaan, A.; Savill, P.; Rondeux, J. A review of the characteristics of black alder (Alnus glutinosa (L.) Gaertn.) and their implications for silvicultural practices. Forestry 2010, 83, 163–175. [Google Scholar] [CrossRef]


| Variant | Silt | Clay | BD 1 | pH in H2O | SOC 6 | Ntot | Ptot | Ktot | Catot | Mgtot | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | ||||
| (%) | (g cm−3) | (%) | |||||||||||||||
| Freshly deposited fly ash | 1 ± 0a 3 | 1 ± 0a | - | - | 8.52 ± 0.08b | - | 1.56 ± 0.01a | - | 0.025 ± 0.001a | - | 0.02 ± 0.00a | - | 0.05 ± 0.00a | - | 5.55 ± 0.08b | - | 0.37 ± 0.00b |
| B-FA 2 | 19 ± 13b | 1 ± 1a | 0.43 ± 0.11a | 6.39 ± 0.09a | 7.86 ± 0.03a | 38.42 ± 2.38a | 3.33 ± 0.90a | 1.94 ± 0.14a | 0.04 ± 0.01a | 0.07 ± 0.00a | 0.01 ± 0.00a | 0.11±0.01ab | 0.04 ± 0.01a | 3.37 ± 0.47a | 6.30 ± 0.55a | 0.33 ± 0.03a | 0.32 ± 0.03a |
| G-FA | 15 ± 3b | 1 ± 1a | 0.56 ± 0.10a | 6.56 ± 0.19a | 7.83 ± 0.03a | 36.17 ± 5.47a | 1.48 ± 0.68a | 1.84 ± 0.32a | 0.03 ± 0.00a | 0.10 ± 0.01b | 0.01 ± 0.00a | 0.12±0.02ab | 0.04 ± 0.01a | 3.53 ± 0.63a | 4.58 ± 1.38a | 0.30 ± 0.07a | 0.25 ± 0.05a |
| B-FA+L | 10 ± 3b | 1 ± 0a | 0.49 ± 0.06a | 6.45 ± 0.33a | 7.79 ± 0.07a | 35.66 ± 8.41a | 1.83 ± 0.65a | 1.86 ± 0.25a | 0.03 ± 0.01a | 0.07 ± 0.01a | 0.01 ± 0.00a | 0.10±0.03a | 0.04 ± 0.01a | 3.66 ± 1.14a | 5.18 ± 0.15a | 0.34 ± 0.08a | 0.28 ± 0.01a |
| G-FA+L | 23 ± 15b | 2 ± 1a | 0.41 ± 0.05a | 6.35 ± 0.04a | 7.86 ± 0.03a | 42.07 ± 2.36a | 3.35 ± 0.71a | 2.14 ± 0.15a | 0.04 ± 0.01a | 0.10 ± 0.01b | 0.01 ± 0.00a | 0.15±0.01b | 0.04 ± 0.00a | 2.67 ± 0.29a | 6.14 ± 1.32a | 0.25 ± 0.01a | 0.32 ± 0.05a |
| Sp 4 | n.s. 5 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Tr | n.s. | n.s. | n.s | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 50.05; 0.000 | n.s. | 8.47; 0.013 | n.s. | n.s. | n.s. | n.s. | n.s. |
| Sp × Tr | n.s. | n.s. | 5.89; 0.032 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Variant | SOC | Total Forms | Available and Exchangeable Forms | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ntot | Ptot | Ktot | Catot | Mgtot | Pav | K+ | Ca2+ | Mg2+ | ||||||||
| Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | Oi + Oe | 0–30 cm | 0–30 cm | ||||
| (Mg ha−1) | ||||||||||||||||
| B-FA 1 | 1.23 ± 0.29a 2 | 45.45 ± 12.7a | 0.062 ± 0.015a | 0.516 ± 0.192a | 0.002 ± 0.001a | 0.19 ± 0.04a | 0.004 ± 0.001a | 0.54 ± 0.19a | 0.108 ± 0.028a | 84.1 ± 16.34a | 0.010 ± 0.002a | 4.18 ± 0.75a | 0.02 ± 0.01a | 0.05 ± 0.01a | 12.71 ± 2.23a | 0.16 ± 0.03a |
| G-FA | 1.02 ± 0.52a | 22.11 ± 9.28a | 0.053 ± 0.030a | 0.504 ± 0.050a | 0.003 ± 0.001a | 0.20 ± 0.02a | 0.004 ± 0.002a | 0.77 ± 0.22a | 0.095 ± 0.028a | 72.34 ± 15.90a | 0.009 ± 0.004a | 3.96 ± 0.54a | 0.03 ± 0.01a | 0.05 ± 0.00a | 9.98 ± 1.13a | 0.14 ± 0.02a |
| B-FA+L | 1.39 ± 0.10a | 26.7 ± 10.28a | 0.074 ± 0.010a | 0.441 ± 0.164a | 0.003 ± 0.001a | 0.18 ± 0.01a | 0.004 ± 0.001a | 0.56 ± 0.18a | 0.157 ± 0.084a | 75.10 ± 2.84a | 0.014 ± 0.007a | 4.01 ± 0.08a | 0.02 ± 0.01a | 0.05 ± 0.00a | 10.09 ± 0.76a | 0.13 ± 0.01a |
| G-FA+L | 1.27 ± 0.50a | 41.96 ± 9.37a | 0.066 ± 0.028a | 0.492 ± 0.056a | 0.003 ± 0.001a | 0.17 ± 0.03a | 0.005 ± 0.002a | 0.54 ± 0.07a | 0.082 ± 0.034a | 77.39 ± 17.76a | 0.008 ± 0.003a | 4.01 ± 0.66a | 0.02 ± 0.00a | 0.04 ± 0.01a | 10.92 ± 2.47a | 0.14 ± 0.03a |
| Sp 3 | n.s. 4 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Tr | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Sp × Tr | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Characteristic | Species (Sp) | Soil Treatment (Tr) | Interaction Sp × Tr | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Btot 1 | 32.12 | 0.0001 | 5.59 | 0.0358 | n.s.1 | n.s. |
| C stocks | 32.13 | 0.0001 | 5.66 | 0.0348 | n.s. | n.s. |
| N stocks | 28.79 | 0.0002 | 4.80 | 0.0490 | n.s. | n.s. |
| P stocks | 24.73 | 0.0003 | n.s. | n.s. | n.s. | n.s. |
| K stocks | 23.46 | 0.0004 | n.s. | n.s. | n.s. | n.s. |
| Ca stocks | 18.74 | 0.0010 | n.s. | n.s. | n.s. | n.s. |
| Mg stocks | 37.43 | 0.0001 | 6.46 | 0.0259 | n.s. | n.s. |
| Variant | C Accumulation | ||
|---|---|---|---|
| (Mg ha−1 yr−1) | |||
| Soil | Biomass | Ecosystem | |
| B-FA 1 | 4.67 ± 1.26a 2 | 4.28 ± 0.81bc | 8.94 ± 0.88b |
| G-FA | 2.31 ± 0.95a | 2.16 ± 0.34a | 4.47 ± 1.16a |
| B-FA+L | 2.81 ± 1.03a | 5.89 ± 0.38c | 8.70 ± 0.72b |
| G-FA+L | 4.32 ± 0.96a | 2.80 ± 0.29ab | 7.12 ± 1.17ab |
| Sp 3 | n.s. 4 | 27.23; 0.000 | 9.10; 0.011 |
| Tr | n.s. | 5.10; 0.043 | n.s. |
| Sp × Tr | n.s. | n.s. | n.s. |
| Variants | Btot:SOC | Btot:Ntot | Btot:Ptot | Btot:Ktot | Btot:Catot | Btot:Mgtot | Btot:Pav | Btot:K+ | Btot:Ca2+ | Btot:Mg2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| B-FA 1 | 1.37 ± 1.02a 2 | 0.64 ± 0.22a | 0.21 ± 0.11a | 0.38 ± 0.16a | 0.005 ± 0.002ab | 0.012 ± 0.005a | 1.32 ± 0.68a | 3.03 ± 1.09b | 0.033 ± 0.016ab | 0.35 ± 0.14a |
| G-FA | 1.21 ± 0.71a | 0.28 ± 0.07a | 0.06 ± 0.01a | 0.08 ± 0.03a | 0.002 ± 0.000a | 0.004 ± 0.000a | 0.48 ± 0.10a | 0.95 ± 0.13a | 0.013 ± 0.003a | 0.12 ± 0.01a |
| B-FA+L | 1.91 ± 0.56a | 0.98 ± 0.64a | 0.21 ± 0.02b | 0.65 ± 0.37a | 0.006 ± 0.001b | 0.015 ± 0.001b | 7.28 ± 3.77b | 3.63 ± 0.54b | 0.045 ± 0.010b | 0.48 ± 0.06b |
| G-FA+L | 0.40 ± 0.12a | 0.53 ± 0.12a | 0.09 ± 0.02a | 0.12 ± 0.01a | 0.003 ± 0.001ab | 0.006 ± 0.001a | 0.72 ± 0.08a | 1.83 ± 0.34ab | 0.019 ± 0.005ab | 0.19 ± 0.05a |
| Sp 3 | n.s. 4 | n.s. | 5.22; 0.04 | n.s. | 6.00; 0.03 | 12.01; 0.004 | 8.46; 0.007 | 9.45; 0.009 | 5.64; 0.035 | 9.99; 0.008 |
| Tr | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Sp × Tr | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woś, B.; Józefowska, A.; Pająk, M.; Chodak, M.; Frouz, J.; Pietrzykowski, M. Carbon and Macronutrient Budgets in an Alder Plantation Grown on a Reclaimed Combustion Waste Landfill. Forests 2020, 11, 430. https://doi.org/10.3390/f11040430
Woś B, Józefowska A, Pająk M, Chodak M, Frouz J, Pietrzykowski M. Carbon and Macronutrient Budgets in an Alder Plantation Grown on a Reclaimed Combustion Waste Landfill. Forests. 2020; 11(4):430. https://doi.org/10.3390/f11040430
Chicago/Turabian StyleWoś, Bartłomiej, Agnieszka Józefowska, Marek Pająk, Marcin Chodak, Jan Frouz, and Marcin Pietrzykowski. 2020. "Carbon and Macronutrient Budgets in an Alder Plantation Grown on a Reclaimed Combustion Waste Landfill" Forests 11, no. 4: 430. https://doi.org/10.3390/f11040430
APA StyleWoś, B., Józefowska, A., Pająk, M., Chodak, M., Frouz, J., & Pietrzykowski, M. (2020). Carbon and Macronutrient Budgets in an Alder Plantation Grown on a Reclaimed Combustion Waste Landfill. Forests, 11(4), 430. https://doi.org/10.3390/f11040430







