Identification of Cryptotermes brevis (Walker, 1853) and Kalotermes flavicollis (Fabricius, 1793) Termite Species by Detritus Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
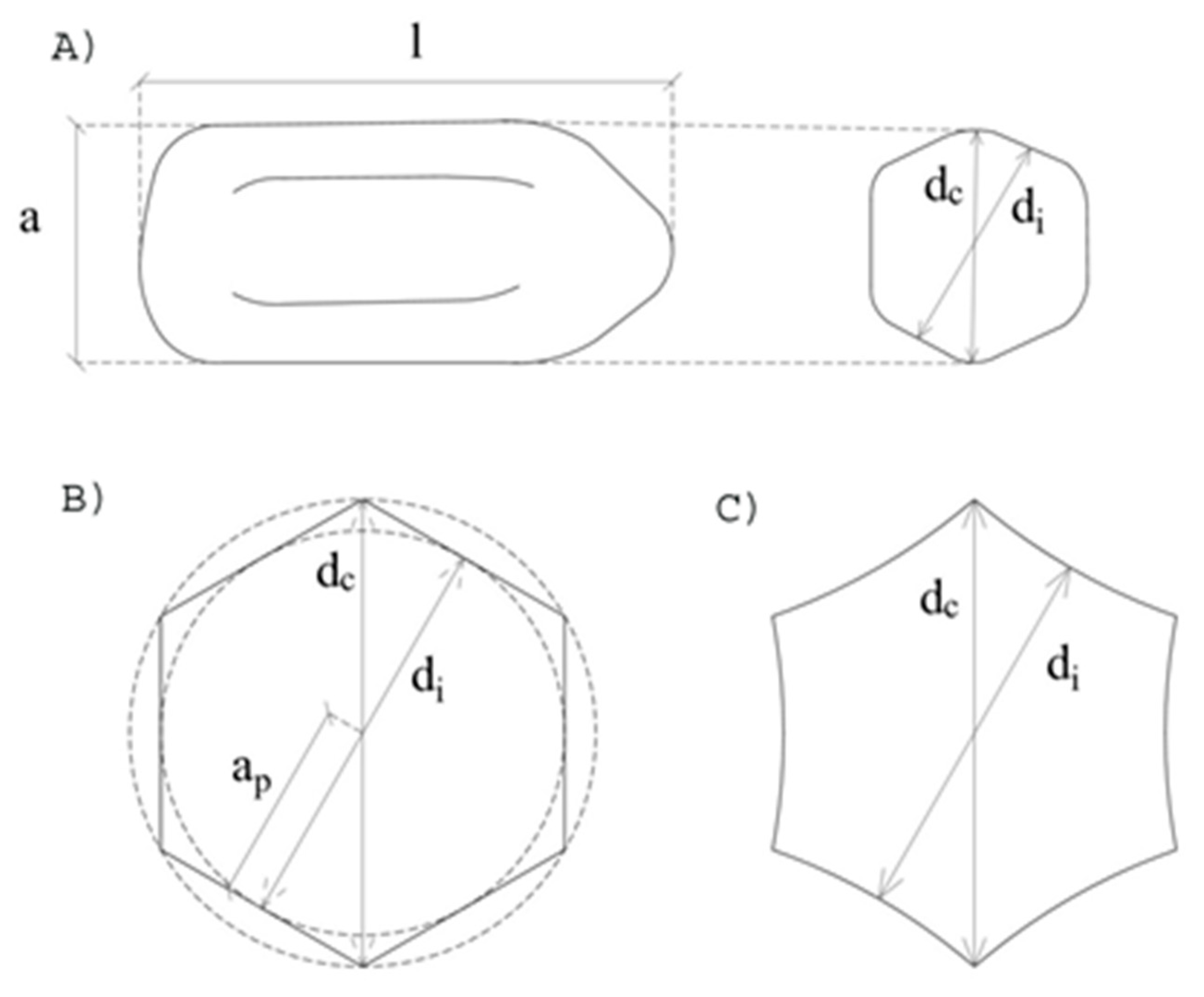
2.2. Morphological Characterization
2.3. Prediction Models
3. Results and Discussion
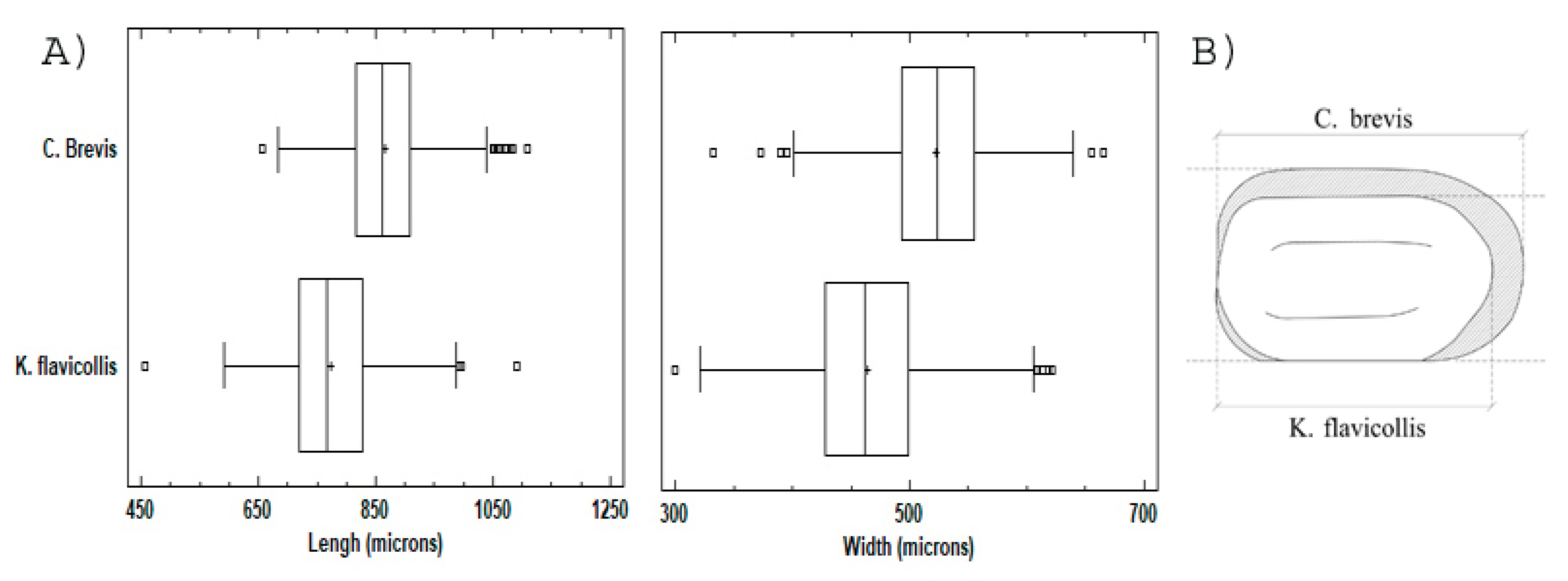
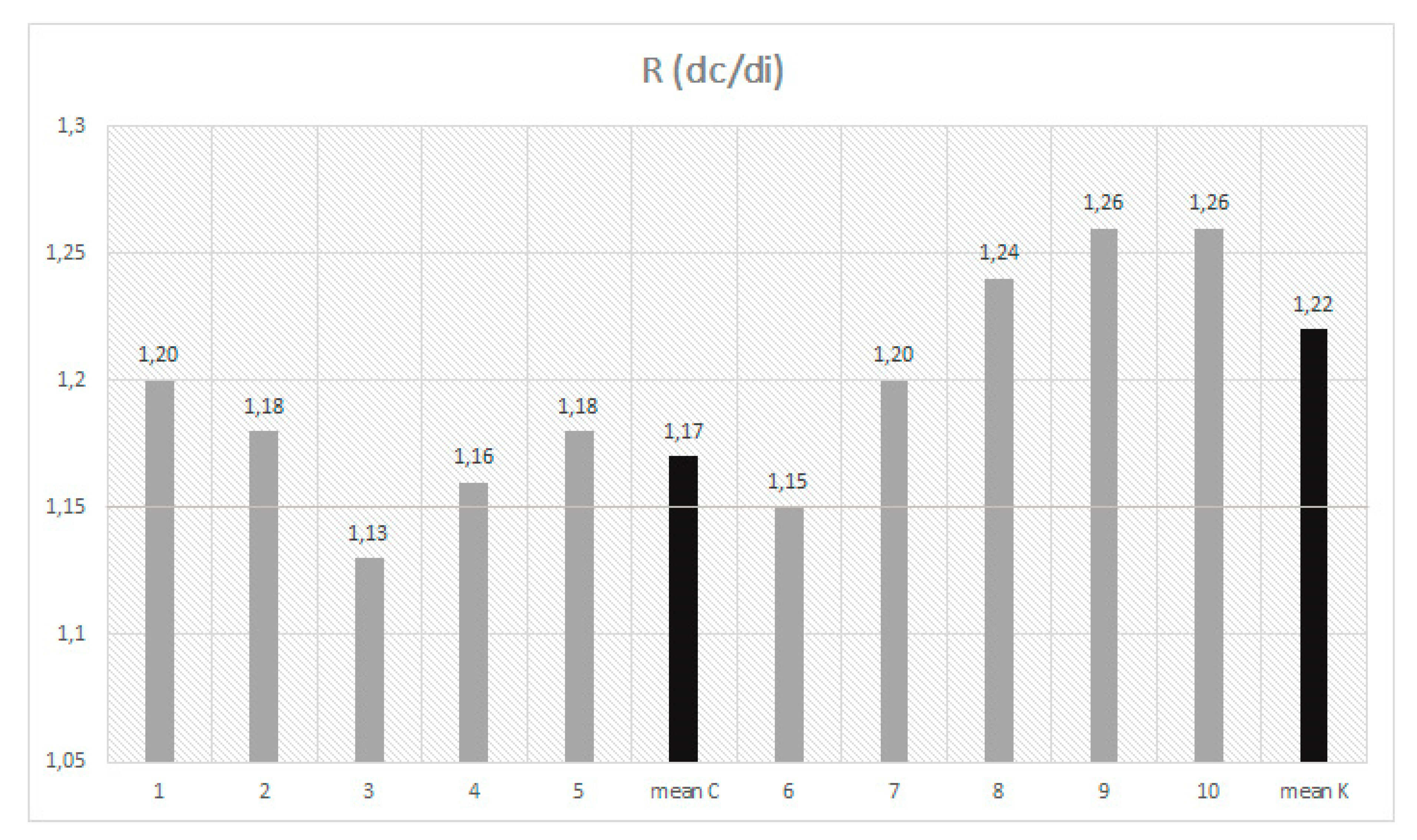
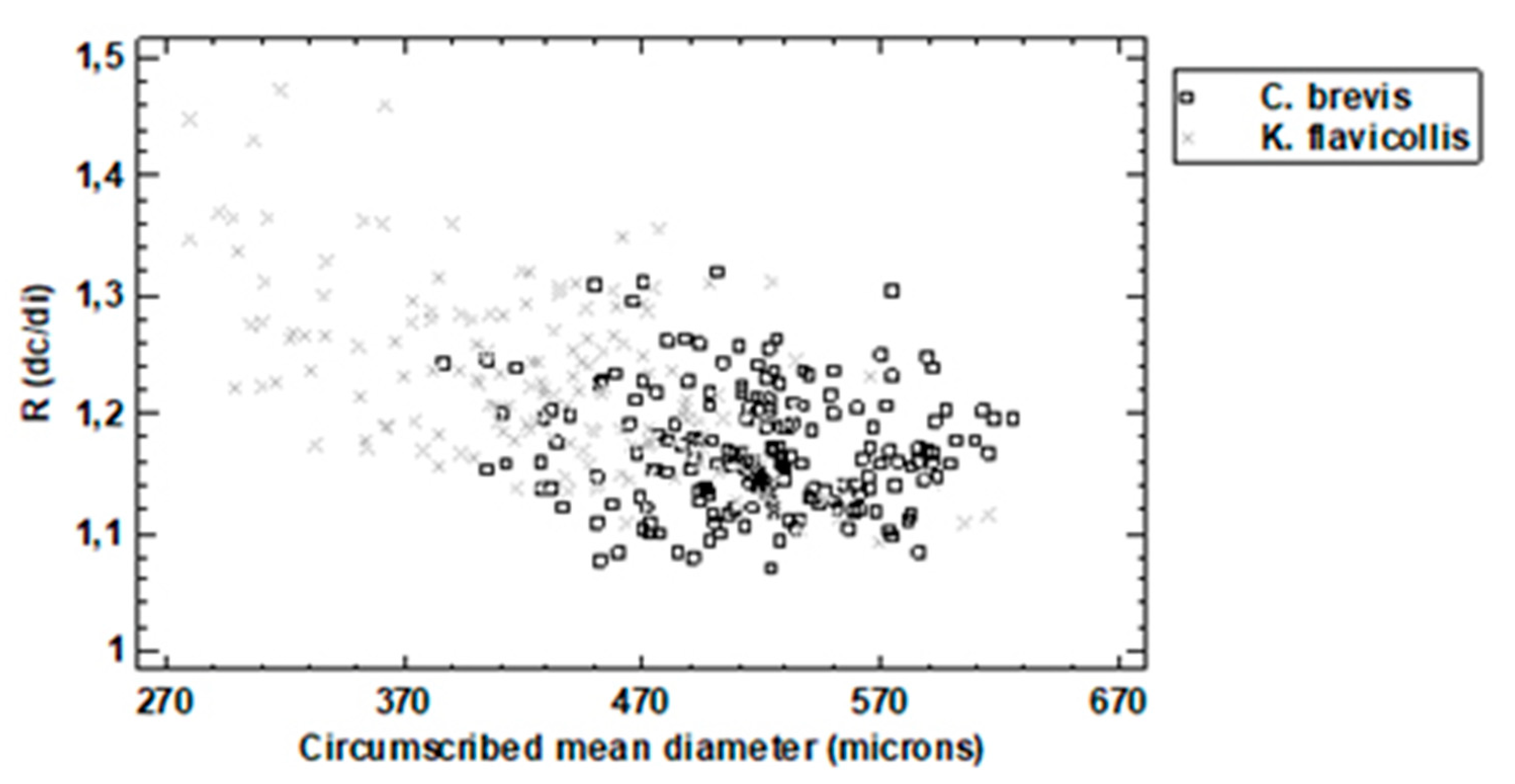
3.1. Dimensional Analysis
3.2. Shape and Color Analysis (Aspect)
3.3. Prediction Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benito, M.J. El termes de madera seca (Cryptotermes brevis) en las islas Canarias. Serv. Plagas For. Rev. Montes 1957, 75, 147–161. [Google Scholar]
- Benito, M.J. Los termitos en España. Biología, daños y métodos para combatir la especie subterránea Reticulitermes lucífugus. In Servicio de Plagas Forestales. Serie B, nº 6; Dirección General de Montes, Caza y Pesca fluvial: Ministerio de Agricultura, Spain, 1958; p. 23. [Google Scholar]
- Becker, G.; Kny, U. Survival and development of the drywood termite Cryptotermes brevis (Walker) in Berlin. Anz. Schadl. Pflanz. 1977, 50, 177–179. [Google Scholar]
- Rainieri, V.; Rey, A.; Marini, M.; Zafaggnini, V. A new discovery of Cryptotermes brevis in Genoa, Italy (Isoptera). Boll. Soc. Entomol. Italiana. 2001, 133, 99–102. [Google Scholar]
- Nunes, L.; Gaju, M.; Krecek, J.; Molero, R.; Ferreira, M.; De Roca, C.B. First records of urban invasive Cryptotermes brevis (Isoptera: Kalotermitidae) in continental Spain and Portugal. J. Appl. Entomol. 2010, 134, 637–640. [Google Scholar] [CrossRef]
- Guerreiro, O.; Cardoso, P.M.; Ferreira, J.M.F.; Ferreira, M.T.; Borges, P.A.V. Potential distribution and cost estimation of the damage caused by Cryptotermes brevis (isóptera: Kalotermitidae) in the Azores. J. Econ. Entomol. 2014, 107, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.A.; Forschler, B.T.; Kenneth Grace, J. Biology of invasive termites: A worldwide review. Annu. Rev. Entomol. 2012, 58, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Barco Villalba, N. Análisis de las Características Morfológicas y Anatómicas del Detrito, Galerías y Larvas de Hylotrupes Bajulus L. y su Evolución en Condiciones Controladas de Laboratorio; TFC EUITF For.-UPM. Director: Madrid, Spain, 2010; p. 58. [Google Scholar]
- Bobadilla, I.; Arriaga, F.; Luengo, E.; Martínez, R. Dimensional and morphological analysis of the detritus from six European wood boring insects. Maderas Cienc. Tecnol. 2015, 17, 893–904. [Google Scholar] [CrossRef]
- Recarte Gallego, J. Diferenciación de las Especies de Termitas Cryptotermes brevis (Walker, 1853) y Kalotermes flavicollis (Fabricius, 1793) Mediante el Análisis Morfológico y Dimensional de sus Detritos; TFC ETSI de Montes, Forestal y del Medio Natural-UPM. Dir: Madrid, Spain, 2017; p. 47. [Google Scholar]
- Rivera Rubio, M. Análisis Morfológico y Dimensional del Detritus de las Especies de Insectos Xilófagos más Frecuentes en la Madera Estructural; TFC ETSI de Montes, Forestal y del Medio Natural-UPM. Dir: Madrid, Spain, 2017; p. 90. [Google Scholar]
- Rodriguez Barreal, J.A. Patología de la Madera; ETSI de Montes, Ed.; FUCOVASA-Mundiprensa: Madrid, Spain, 1998; p. 349. [Google Scholar]
- Arriaga, F.; Peraza, F.; Esteban, M.; Bobadilla, I.; García, F. Intervención en Estructuras de Madera; Aitim: Madrid, Spain, 2002; p. 476. [Google Scholar]
- Hay, C.J. Frass of some wood boring insects in living oak. Ann. Entomol. Soc. Am. 1968, 61, 225–258. [Google Scholar] [CrossRef]
- Oevering, P.; Pitman, A.J. Characteristics of attack of coastal timbres by Pselactus sapadix Herbst and an investigation of its life history. Holzforschung 2002, 56, 335–359. [Google Scholar] [CrossRef]
- Haverty, M.; Woodrow, J.; Nelson, l.; Grace, K. Identification of termite species by the hydrocarbons in their faeces. J. Chem. Ecol. 2005, 31. [Google Scholar] [CrossRef]
- Hickin Norman, E.; Edwards, R. The Insect Factor in Wood Decay: An Account of Wood Boring Snsects with Particular Reference to Timber Indoors; Hutchinson and Co. Publishers Ltd.: London, UK, 1975; p. 383. [Google Scholar]



| Sample | Species | Origin | Source | No. of Measurements | ||
|---|---|---|---|---|---|---|
| Length | Width | Diameter | ||||
| 1 | Cryptotermes brevis (Walker, 1853) | Barcelona | 1 | 200 | 200 | 41 |
| 2 | La Gomera | 2 | 200 | 200 | 30 | |
| 3 | Gran Canaria | 3 | 200 | 200 | 39 | |
| 4 | Barcelona | 2 | 200 | 200 | 39 | |
| 5 | Tenerife | 2 | 200 | 200 | 40 | |
| 6 | Kalotermes flavicollis (Fabricius, 1793) | Barcelona | 2 | 200 | 200 | 30 |
| 7 | Madrid. Ciudad Universitaria | 4 | 200 | 200 | 44 | |
| 8 | Madrid. Vicálvaro | 4 | 200 | 200 | 32 | |
| 9 | Madrid. Parque del Oeste | 4 | 200 | 200 | 32 | |
| 10 | Madrid. | 4 | 200 | 200 | 41 | |
| Overall mean | 2000 | 2000 | 368 | |||
| Sample | Sp. | l (µm) | CV (%) | SE (%) | a (µm) | CV (%) | SE (%) | dc (µm) | CV (%) | SE (%) | di (µm) | CV (%) | SE (%) | R (dc/di) | CV (%) | SE (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cb | 867 z | 6.7 | 4.1 | 524 z | 6.7 | 2.5 | 523 z | 5.8 | 4.8 | 438 z | 7.9 | 5.4 | 1.20 z | 4.1 | 0.008 |
| 2 | 933 y | 7.1 | 4.7 | 555 y | 6.8 | 2.7 | 550 y | 9.5 | 9.5 | 466 y | 10.2 | 8.7 | 1.18 zy | 4.2 | 0.009 | |
| 3 | 867 z | 5.4 | 3.3 | 536 x | 6.7 | 2.5 | 537 zy | 6.9 | 5.9 | 475 y | 6.6 | 5.0 | 1.13 x | 3.5 | 0.006 | |
| 4 | 816 x | 6.6 | 3.8 | 501 w | 8.9 | 3.2 | 492 x | 10.5 | 8.2 | 424 z | 10.7 | 7.3 | 1.16 y | 3.6 | 0.007 | |
| 5 | 843 w | 7.6 | 4.6 | 495 w | 8.8 | 3.1 | 498 x | 9.1 | 7.1 | 423 z | 11 | 7.3 | 1.18 z | 3.8 | 0.007 | |
| Mean | 865 Z | 8.1 | 2.2 | 522 Z | 8.7 | 1.4 | 518 Z | 9.3 | 3.5 | 444 Z | 10.4 | 3.4 | 1.17 Z | 4.3 | 0.004 | |
| 6 | Kf | 813 xv | 9.4 | 5.4 | 524 z | 8.4 | 3.1 | 526 z | 9.6 | 9.2 | 459 y | 11.1 | 9.3 | 1.15 yx | 3.3 | 0.007 |
| 7 | 801 v | 9.1 | 5.2 | 481 v | 8.1 | 2.8 | 467 w | 8.8 | 6.2 | 389 x | 10.3 | 6.0 | 1.2 z | 3.7 | 0.007 | |
| 8 | 731 u | 8.6 | 4.5 | 435 u | 7.8 | 2.4 | 433 v | 6.9 | 5.2 | 351 w | 7.1 | 4.4 | 1.24 w | 4.8 | 0.010 | |
| 9 | 773 t | 12.1 | 6.6 | 437 ut | 12.9 | 4.0 | 402 u | 16.4 | 11.7 | 323 v | 20.4 | 11.6 | 1.26 w | 6.2 | 0.014 | |
| 10 | 754 s | 7.8 | 4.1 | 445 t | 9.3 | 2.9 | 375 t | 15.8 | 9.3 | 300 u | 19.4 | 9.1 | 1.26 w | 6.3 | 0.012 | |
| Mean | 774 Y | 10.3 | 2.5 | 464 Y | 11.9 | 1.7 | 438 Y | 16.4 | 5.4 | 362 Y | 20.3 | 5.5 | 1.22 Y | 6 | 0.005 |
| Dimensional Variable | C. brevis | K. flavicolis | |
|---|---|---|---|
| Model 1 (75.19%) | Length (L) | 0.111221 | 0.100192 |
| Width (W) | 0.110188 | 0.096958 | |
| Constant (C1) | −77.5688 | −62.0013 | |
| Model 2 (76.90%) | Circumscribed Diameter (dc) | 0.334091 | 0.314628 |
| R factor (dc/di) | 433.907 | 438.855 | |
| Constant (C2) | −341.057 | −337.666 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobadilla, I.; Martínez, R.D.; Martínez-Ramírez, M.; Arriaga, F. Identification of Cryptotermes brevis (Walker, 1853) and Kalotermes flavicollis (Fabricius, 1793) Termite Species by Detritus Analysis. Forests 2020, 11, 408. https://doi.org/10.3390/f11040408
Bobadilla I, Martínez RD, Martínez-Ramírez M, Arriaga F. Identification of Cryptotermes brevis (Walker, 1853) and Kalotermes flavicollis (Fabricius, 1793) Termite Species by Detritus Analysis. Forests. 2020; 11(4):408. https://doi.org/10.3390/f11040408
Chicago/Turabian StyleBobadilla, Ignacio, Roberto D. Martínez, Manuel Martínez-Ramírez, and Francisco Arriaga. 2020. "Identification of Cryptotermes brevis (Walker, 1853) and Kalotermes flavicollis (Fabricius, 1793) Termite Species by Detritus Analysis" Forests 11, no. 4: 408. https://doi.org/10.3390/f11040408
APA StyleBobadilla, I., Martínez, R. D., Martínez-Ramírez, M., & Arriaga, F. (2020). Identification of Cryptotermes brevis (Walker, 1853) and Kalotermes flavicollis (Fabricius, 1793) Termite Species by Detritus Analysis. Forests, 11(4), 408. https://doi.org/10.3390/f11040408






