Dendrochronological Analyses and Whole-Tree Dissections Reveal Caliciopsis Canker (Caliciopsis pinea) Damage Associated with the Declining Growth and Climatic Stressors of Eastern White Pine (Pinus strobus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Site and Stand Selection
2.3. Field Sampling
2.4. Cross-Section Preparation and Tree Ring Measurements
2.5. Analyses
- X = year for which Dscore is being calculated,
- BAI = basal area increment for year in subscript,
- VARx = the pooled variance of the means (Equation (2))
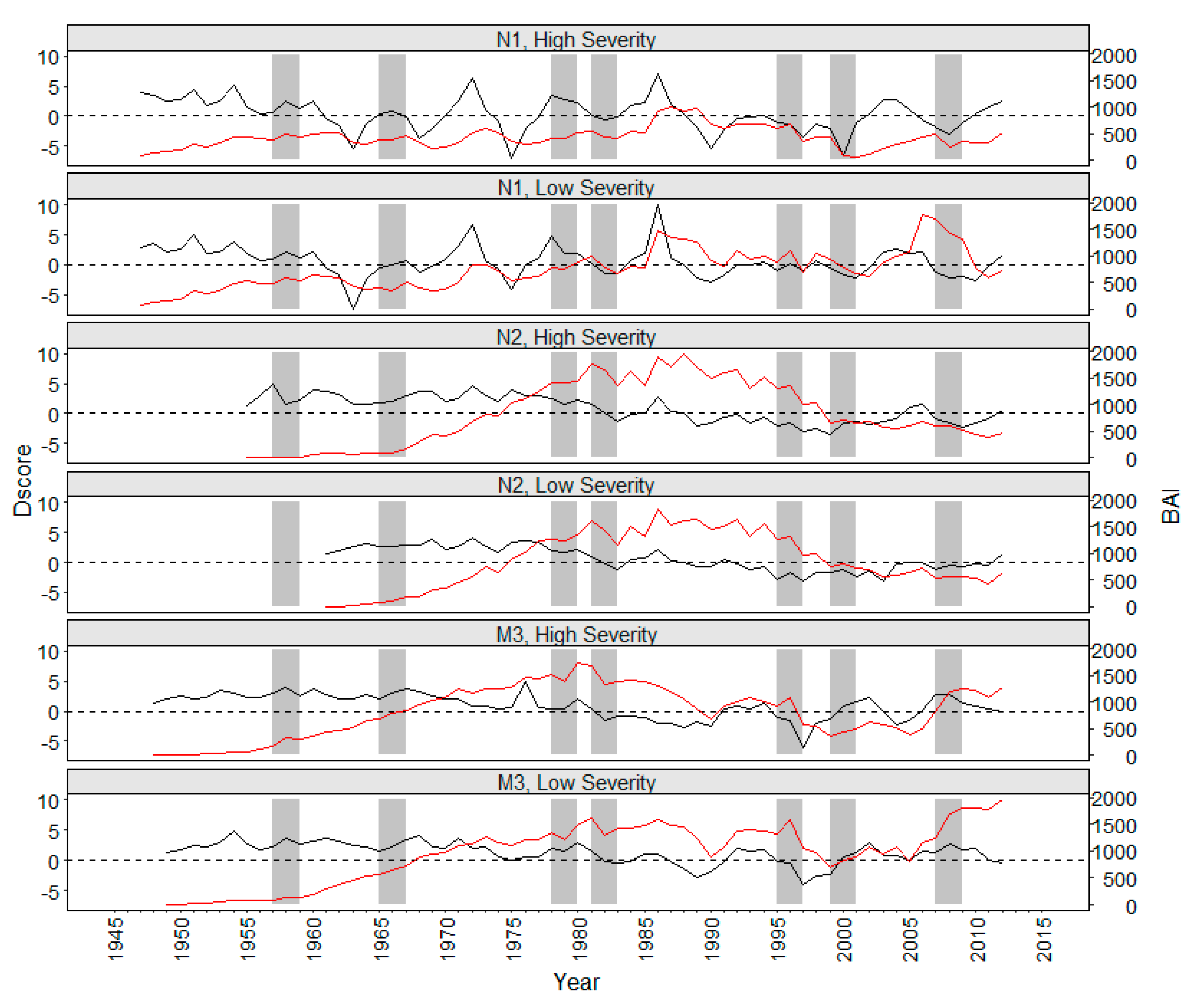
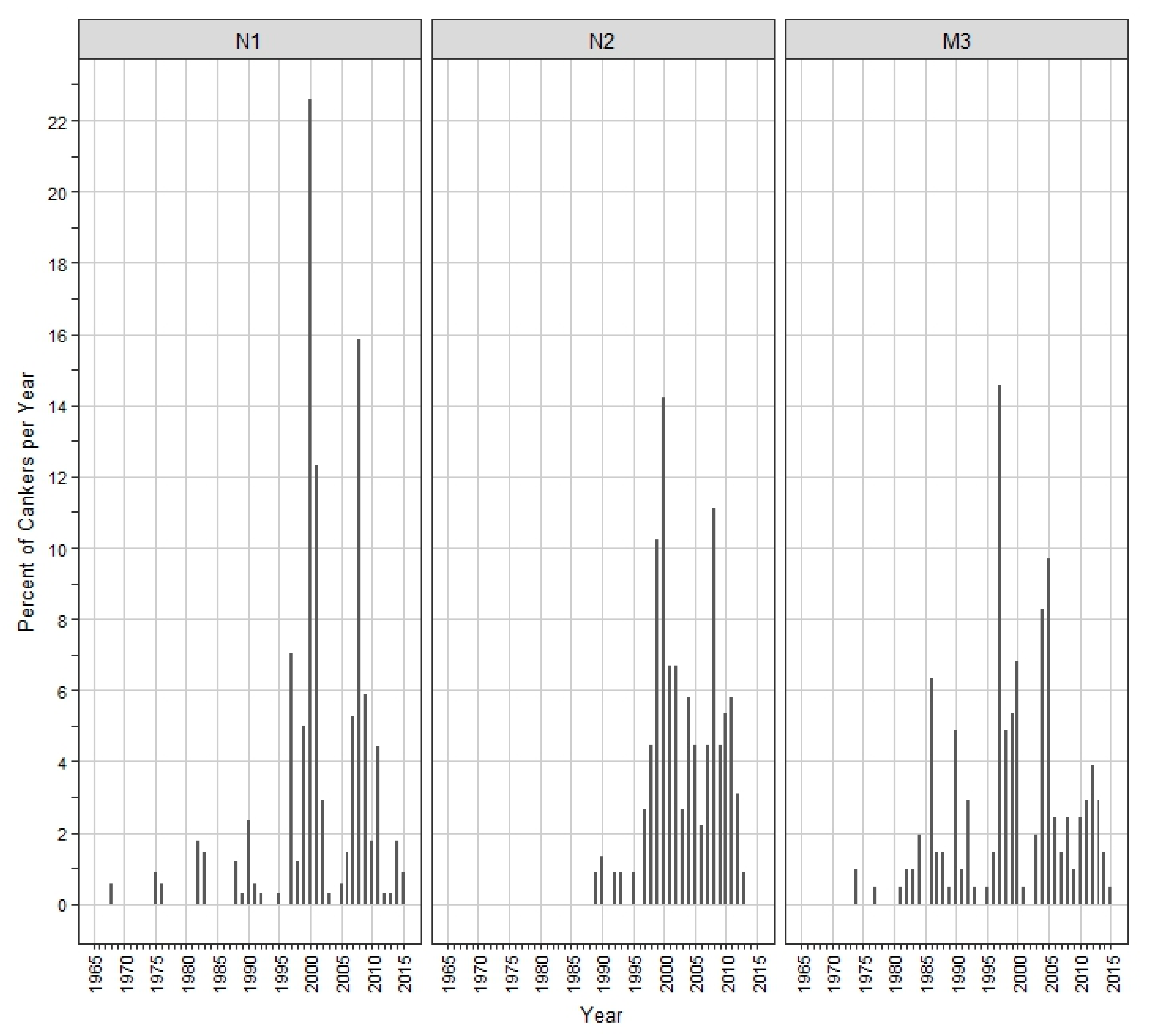
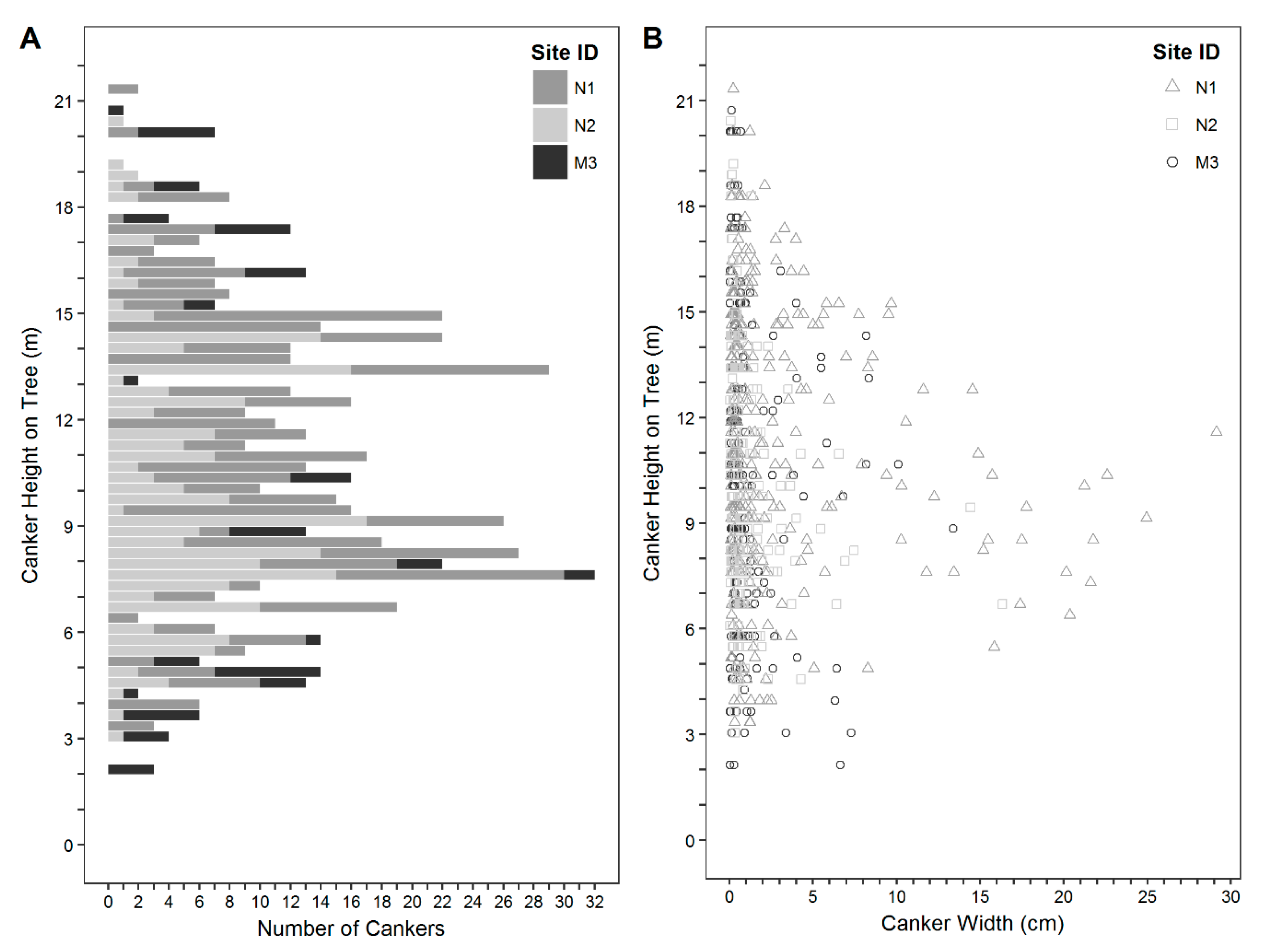
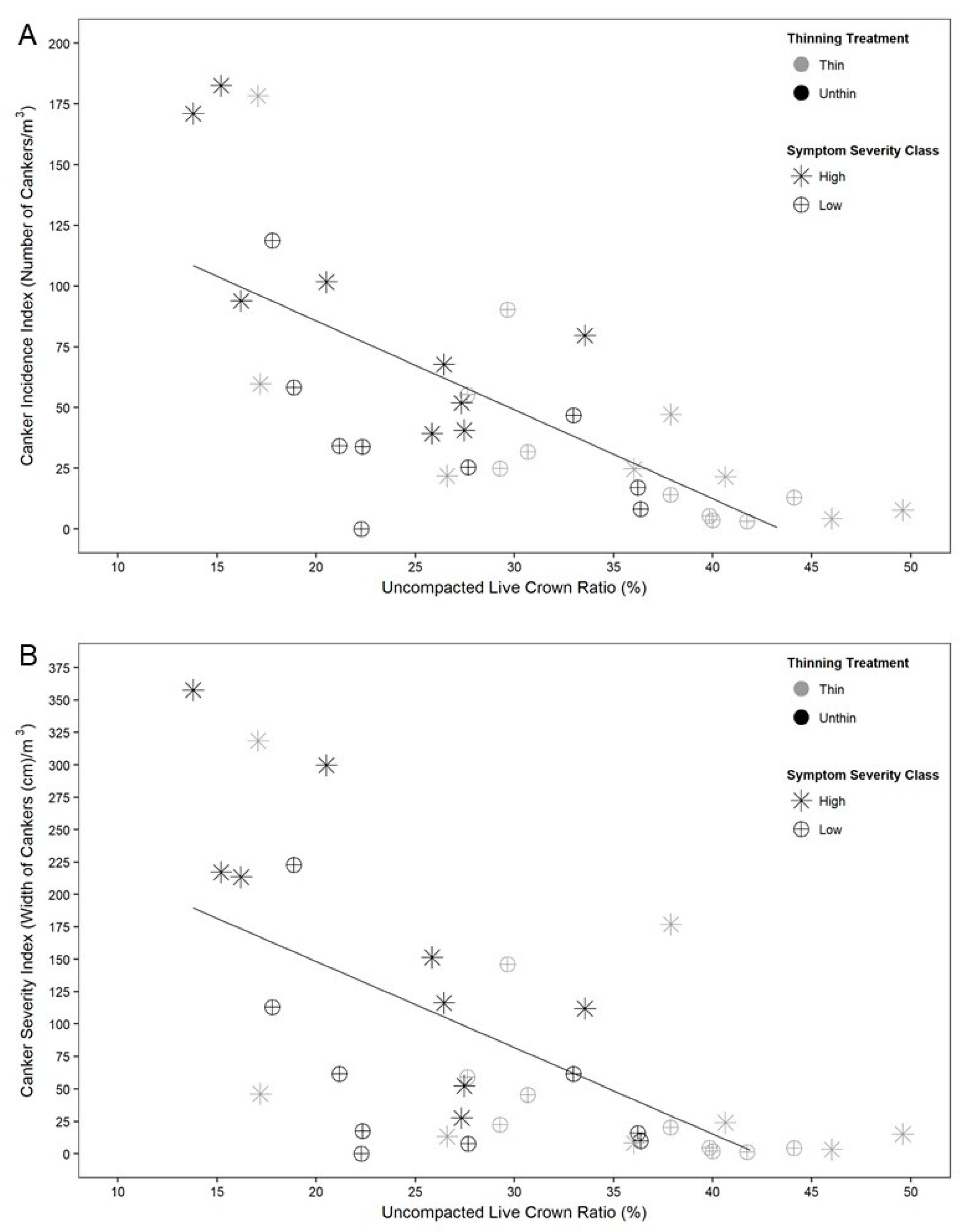
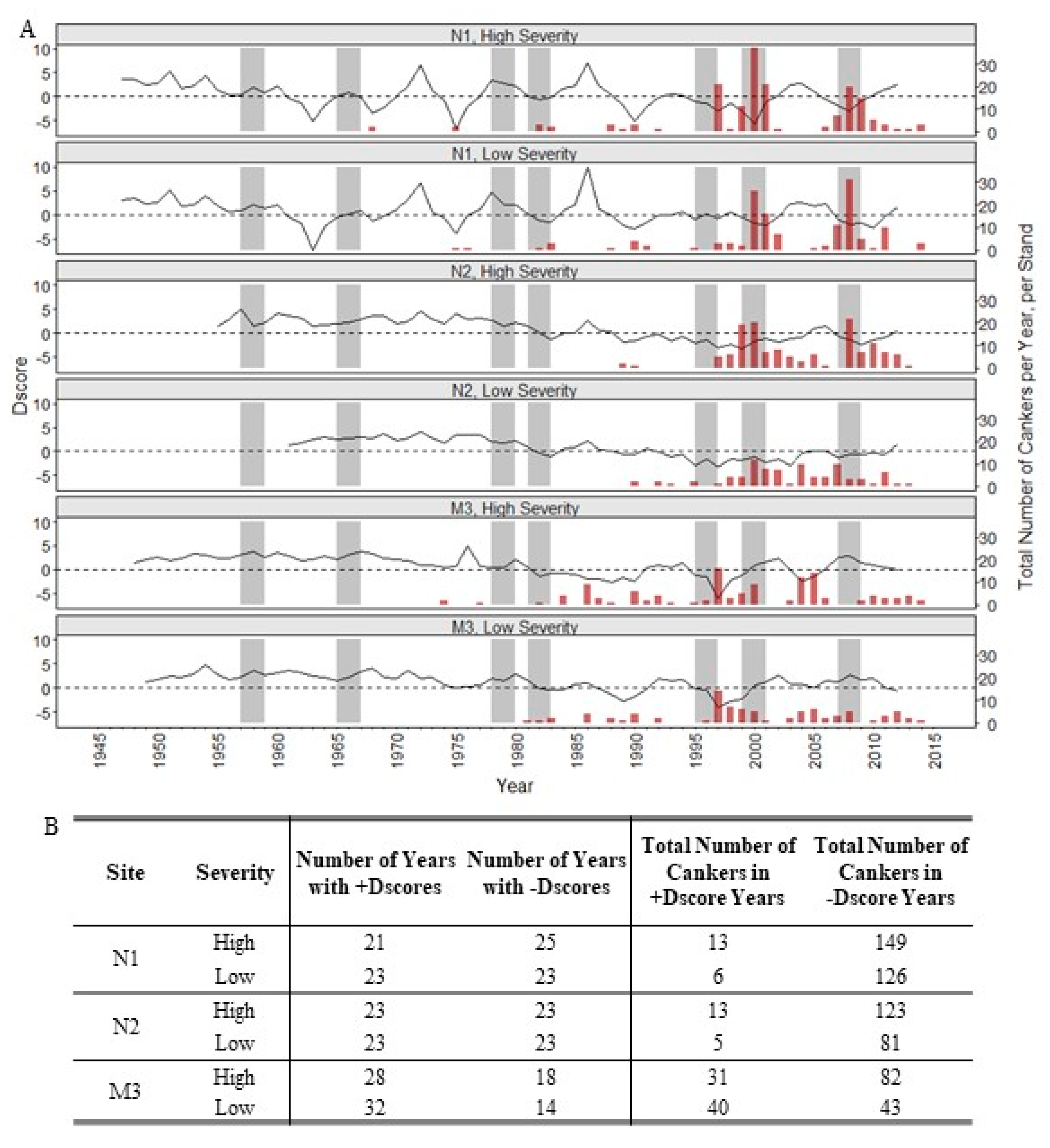
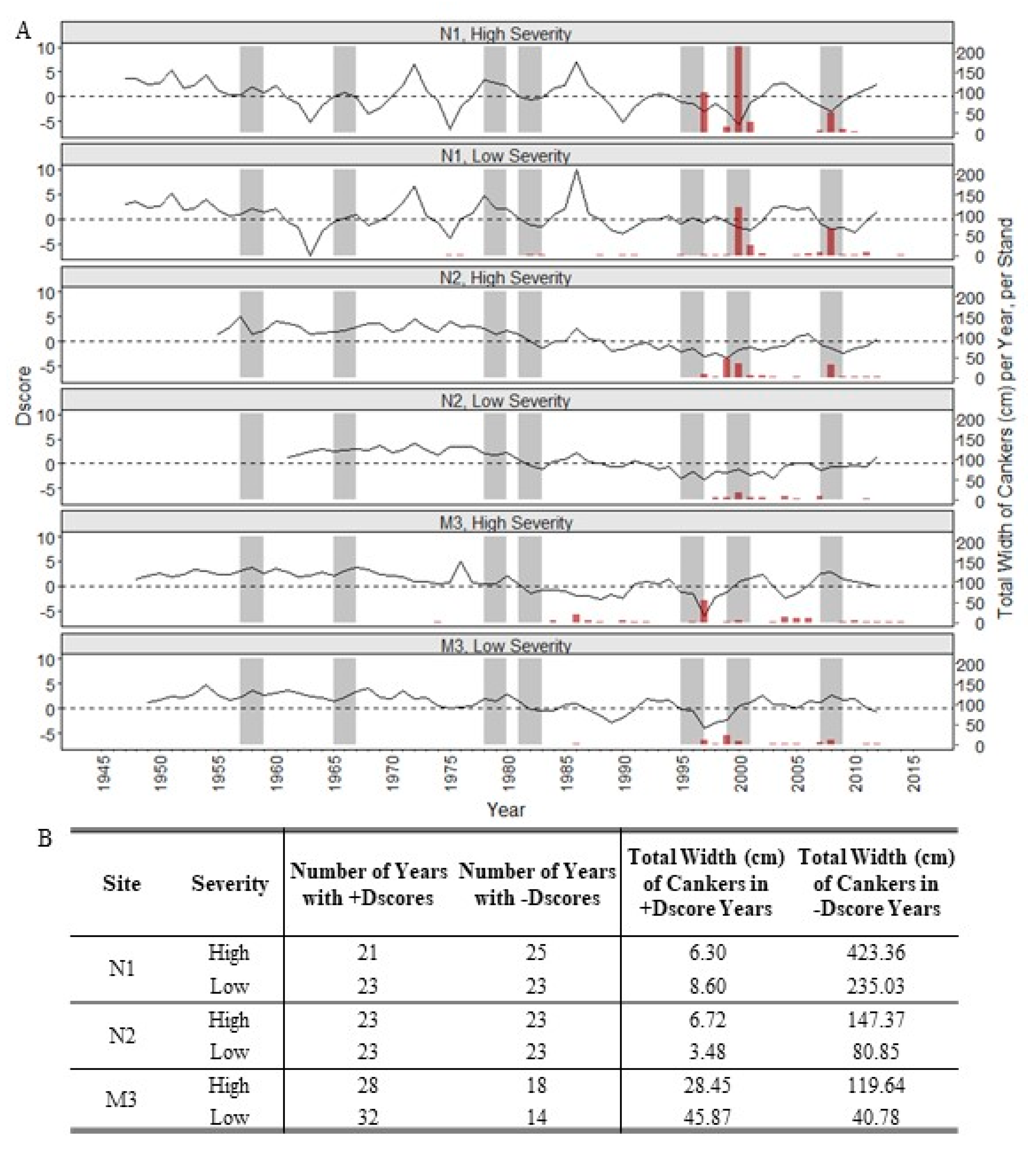
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Climate Measure * | N1 | N2 | M3 |
|---|---|---|---|
| Mean annual temperature (°C) | 7.10 a | 7.56 b | 6.91 a |
| Minimum annual temperature (°C) | 0.79 a | 1.22 b | 0.80 a |
| Maximum annual temperature (°C) | 13.40 a | 13.90 b | 13.03 c |
| Total annual precipitation (cm) | 114.22 a | 111.20 a | 117.88 a |
| Site | N1 | N2 | M3 | Two-Way ANOVA (Model: Metric ~ Treat × Severity + Error(Site/(Treat × Severity)) | |||
|---|---|---|---|---|---|---|---|
| Thinning Treatment | Thin | Unthin | Thin | Unthin | Thin | Unthin | |
| Diameter at breast height (cm) | 25.7 | 23.1 | 31.0 | 23.6 | 37.0 | 23.5 | p < 0.01, df = 1 (treat) |
| p = 0.09, df = 1 (severity) | |||||||
| p = 0.21, df = 1 (site) | |||||||
| p = 0.75, df = 1 (treat × severity) | |||||||
| Tree height (m) | 23.1 | 21.6 | 23.7 | 20.6 | 25.1 | 22.3 | p < 0.01, df = 1 (treat) |
| p = 0.05, df = 1 (severity) | |||||||
| p = 0.57, df = 1 (site) | |||||||
| p = 0.91, df = 1 (treat × severity) | |||||||
| Tree volume (m3) | 0.59 | 0.43 | 0.80 | 0.44 | 1.23 | 0.44 | p < 0.01, df = 1 (treat) |
| p = 0.16, df = 1 (severity) | |||||||
| p = 0.23, df = 1 (site) | |||||||
| p = 1.00, df = 1 (treat × severity) | |||||||
| Uncompacted live crown ratio (%) | 29.9 | 21.1 | 37.6 | 24.9 | 35.4 | 27.7 | p < 0.01, df = 1 (treat) |
| p = 0.28, df = 1 (severity) | |||||||
| p = 0.43, df = 1 (site) | |||||||
| p = 0.92, df = 1 (treat × severity) | |||||||
| Site | N1 | N2 | M3 | Two-Way ANOVA (Model: Metric ~ Treat × Severity + Error(Site/(Treat × Severity)) | |||
|---|---|---|---|---|---|---|---|
| Symptom Severity | High | Low | High | Low | High | Low | |
| Diameter at breast height (cm) | 20.8 | 28.1 | 27.5 | 27 | 31.2 | 32.7 | p < 0.01, df = 1 (treat) |
| p = 0.09, df = 1 (severity) | |||||||
| p = 0.21, df = 1 (site) | |||||||
| p = 0.75, df = 1 (treat × severity) | |||||||
| Tree height (m) | 20.9 | 23.9 | 22.4 | 21.8 | 23.1 | 24.7 | p < 0.01, df = 1 (treat) |
| p = 0.05, df = 1 (severity) | |||||||
| p = 0.57, df = 1 (site) | |||||||
| p = 0.91, df = 1 (treat × severity) | |||||||
| Tree volume (m3) | 0.34 | 0.68 | 0.65 | 0.59 | 0.91 | 0.97 | p < 0.01, df = 1 (treat) |
| p = 0.16, df = 1 (severity) | |||||||
| p = 0.23, df = 1 (site) | |||||||
| p = 1.00, df = 1 (treat × severity) | |||||||
| Uncompacted live crown ratio (%) | 21.8 | 29.2 | 31.4 | 31.0 | 31.9 | 33.7 | p < 0.01, df = 1 (treat) |
| p = 0.28, df = 1 (severity) | |||||||
| p = 0.43, df = 1 (site) | |||||||
| p = 0.92, df = 1 (treat × severity) | |||||||
References
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Ramsfield, T.D.; Bentz, B.J.; Faccoli, M.; Jactel, H.; Brockerhoff, E.G. Forest health in a changing world: Effects of globalization and climate change on forest insect and pathogen impacts. For. Int. J. For. Res. 2016, 89, 245–252. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- Logan, J.A.; Régnière, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Dukes, J.S.; Pontius, J.; Orwig, D.; Garnas, J.R.; Rodgers, V.L.; Brazee, N.; Cooke, B.; Theoharides, K.A.; Stange, E.E.; Harrington, R.; et al. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 2009, 39, 231–248. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Wyka, S.A.; Munck, I.A.; Brazee, N.J.; Broders, K.D. Response of eastern white pine and associated foliar, blister rust, canker, and root rot pathogens to climate change. For. Ecol. Manag. 2018, 423, 18–26. [Google Scholar] [CrossRef]
- Costanza, K.K.L.; Whitney, T.D.; McIntire, C.D.; Livingston, W.H.; Gandhi, K.J.K. A synthesis of emerging health issues of eastern white pine (Pinus strobus) in eastern North America. For. Ecol. Manag. 2018, 423, 3–17. [Google Scholar] [CrossRef]
- Linder, S.; Benson, M.L.; Myers, B.J.; Raison, R.J. Canopy dynamics and growth of Pinus radiata: I. Effects of irrigation and fertilization during a drought. Can. J. For. Res. 1987, 17, 1157–1165. [Google Scholar] [CrossRef]
- Maier, C.A.; Teskey, R.O. Internal and external control of net photosynthesis and stomatal conductance of mature eastern white pine (Pinus strobus). Can. J. For. Res. 1992, 22, 1387–1394. [Google Scholar] [CrossRef]
- Vose, J.M.; Swank, W.T. Effects of long-term drought on the hydrology and growth of a white pine plantation in the southern Appalachians. For. Ecol. Manag. 1994, 64, 25–39. [Google Scholar] [CrossRef]
- Boyer, J.S. Biochemical and biophysical aspects of water deficits and the predisposition to disease. Annu. Rev. Phytopathol. 1995, 33, 251–274. [Google Scholar] [CrossRef] [PubMed]
- Desprez-Loustau, M.-L.; Marçais, B.; Nageleisen, L.-M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Livingston, W.H.; Kenefic, L.S. Low densities in white pine stands reduce risk of drought-incited decline. For. Ecol. Manag. 2018, 423, 84–93. [Google Scholar] [CrossRef]
- Hepting, G.H. Climate and forest diseases. Annu. Rev. Phytopathol. 1963, 1, 31–50. [Google Scholar] [CrossRef]
- Schoeneweiss, D.F. Predisposition, stress, and plant disease. Annu. Rev. Phytopathol. 1975, 13, 193–211. [Google Scholar] [CrossRef]
- Schoeneweiss, D.F. The role of environmental stress in diseases of woody plants. Plant. Dis. 1981, 65, 308–314. [Google Scholar] [CrossRef]
- Ayres, P.G. Growth responses induced by pathogens and other stresses. In Response of Plants to Multiple Stresses; Mooney, H.A., Winner, W.E., Pell, E.J., Chu, E., Eds.; Academic Press: Cambridge, MA, USA, 1991; pp. 227–248. [Google Scholar]
- Van Arsdel, E.P. Environment in relation to white pine blister rust infection. In Biology of Rust Resistance in Forest Trees: Proceedings of a NATO-IUFRO Advanced Study Institute; USDA Forest Service Miscellaneous Publication No. 1221; University of Idaho: Moscow, ID, USA, 1972. [Google Scholar]
- Peck, C.H. Report of the Botanist. Annu. Rep. N. Y. State Mus. Nat. Hist. 1880, 33, 32. [Google Scholar]
- Fitzpatrick, H.M. Revisionary studies in the Coryneliaceae. II. The genus Caliciopsis. Mycologia 1942, 34, 489–514. [Google Scholar] [CrossRef]
- Funk, A. Studies in the genus Caliciopsis. Can. J. Bot. 1963, 41, 503–543. [Google Scholar] [CrossRef]
- Munck, I.A.; Livingston, W.H.; Lombard, K.; Luther, T.; Ostrofsky, W.D.; Weimer, J.; Wyka, S.; Broders, K. Extent and severity of Caliciopsis canker in New England, USA: An emerging disease of eastern white pine (Pinus strobus L.). Forests 2015, 6, 4360–4373. [Google Scholar] [CrossRef]
- Ray, W.W. Pathogenicity and cultural experiments with Caliciopsis pinea. Mycologia 1936, 28, 201–208. [Google Scholar] [CrossRef]
- Delatour, C. Contribution à l’étude du Caliciopsis pinea Peck: Résultats complémentaires d’inoculations artificielles. Ann. Sci. For. 1969, 26, 285–295. [Google Scholar] [CrossRef]
- Capretti, P. Caliciopsis pinea Peck parassita di Pinus pinaster e Pinus insignis. Phytopathol. Mediterr. 1978, 17, 101–104. [Google Scholar]
- Lombard, K. Caliciopsis Canker (Pine Canker): Caliciopsis Pinea; UNH Cooperative Extension Publication: Durham, NH, USA, 2003; p. 1. [Google Scholar]
- Lanier, L.; Delatour, C. Étude du pouvoir pathogène du Caliciopsis pinea Peck sur le Pin maritime des Landes. Rev. For. Fr. 1967, 5, 332–337. [Google Scholar]
- Mech, A.M.; Asaro, C.; Cram, M.M.; Coyle, D.R.; Gullan, P.J.; Cook, L.G.; Gandhi, K.J.K. Matsucoccus macrocicatrices (Hemiptera: Matsucoccidae): First report, distribution, and association with symptomatic eastern white pine in the southeastern United States. For. Entomol. 2013, 106, 2391–2398. [Google Scholar] [CrossRef]
- Fitzpatrick, H.M. Monograph of the Coryneliaceae. Mycologia 1920, 12, 206–237. [Google Scholar] [CrossRef]
- Costanza, K.K.L.; Crandall, M.S.; Rice, R.W.; Livingston, W.H.; Munck, I.A. Economic implications of a native tree disease, Caliciopsis canker, on the white pine (Pinus strobus) lumber industry in the northeastern United States. Can. J. For. Res. 2019, 49, 521–530. [Google Scholar] [CrossRef]
- Asaro, C. What’s killing white pine in the highlands of western Virginia? In Forest Health Review; Virginia Department of Forestry: Charlottesville, VA, USA, 2011; p. 12. [Google Scholar]
- USDA Forest Service. 2000 Forest Health Highlights: New Hampshire; USDA Forest Service, Northeastern Area State and Private Forestry: Durham, NH, USA, 2000; p. 2.
- O’Brien, J. White pine branch mortality. In 2007 Michigan Forest Health Highlights; Michigan Department of Natural Resources, Forest, Mineral, and Fire Management Division: Lansing, MI, USA, 2007; pp. 19–20. [Google Scholar]
- Rose, J.A. Caliciopsis canker of white pine (Caliciopsis pinea). Market. Bull. 2011, 2. [Google Scholar]
- Bordeleau, C.; Gagnon, G.; Innes, L.; Lachance, C.; Marchand, L.; Morneau, L.; Paré, D.; Prémont, M.; Simard, S. Insectes, Maladies, et Feux Dans les Forêts Québécoises en 2003; Gouvernement du Québec, Ministère des Ressources Naturelles, de la Faune et des Parcs: Québec, QC, Canada, 2004; p. 60.
- Swetnam, T.W.; Thompson, M.A.; Sutherland, E.K. Spruce Budworms Handbook: Using Dendrochronology to Measure Radial Growth of Defoliated Trees; Agricultural Handbook No. 639; USDA Forest Service: Washington, DC, USA, 1985; p. 38.
- Boulanger, Y.; Arsenault, D. Spruce budworm outbreaks in eastern Quebec over the last 450 years. Can. J. For. Res. 2004, 34, 1035–1043. [Google Scholar] [CrossRef]
- Speer, J.H.; Holmes, R.L. Effects of Pandora moth outbreaks on ponderosa pine wood volume. Tree-Ring Res. 2004, 60, 69–76. [Google Scholar] [CrossRef]
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Welsh, C.; Lewis, K.; Woods, A. The outbreak history of Dothiostroma needle blight: An emerging forest disease in northwestern British Columbia, Canada. Can. J. For. Res. 2009, 39, 2505–2519. [Google Scholar] [CrossRef]
- Griffith, G.E.; Omernik, J.M.; Bryce, S.A.; Royte, J.; Hoar, W.D.; Homer, J.W.; Keirstead, D.; Metzler, K.J.; Hellyer, G. Ecoregions of New England (Color Poster with Map, Descriptive Text, Summary Tables, and Photographs): Reston, VA, U.S. Geological Survey (Map Scale 1:1,325,000). 2009. Available online: https://www.epa.gov/eco-research/ecoregions-publications (accessed on 3 March 2020).
- PRISM Climate Group, Oregon State University. PRISM Climate Data. 2017. Available online: http://prism.oregonstate.edu (accessed on 9 July 2017).
- Fraver, S.; Bradford, J.B.; Palik, B.J. Improving tree age estimates derived from increment cores: A case study of red pine. For. Sci. 2011, 57, 164–170. [Google Scholar]
- Costanza, K.K.L. Fungal DNA Used to Assess Communities Associated with Caliciopsis Canker Damage in New England’s Pinus Strobus Forests. Ph.D. Thesis, University of Maine, Orono, ME, USA, 2017. [Google Scholar]
- USDA Forest Service. Forest Inventory and Analysis National Core Field Guide. Volume 1: Field Data Collection Procedures for Phase 2 Plots. Version 6.1; USDA Forest Service: Washington, DC, USA, 2014; p. 433.
- Rasband, W.S. 1997–2016. ImageJ. US National Institutes of Health: Bethesda, MD, USA. Available online: https://imagej.nih.gov/ij/ (accessed on 21 February 2017).
- Regent Instruments Canada Inc. WinDENDRO for Tree-Ring Analysis, Version 2012b; Regent Instruments: Québec, QC, Canada, 2012. [Google Scholar]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Schulz, A.N.; Mech, A.M.; Asaro, C.; Coyle, D.R.; Cram, M.M.; Lucardi, R.D.; Gandhi, K.J.K. Assessment of abiotic and biotic factors associated with eastern white pine (Pinus strobus L.) dieback in the Southern Appalachian Mountains. For. Ecol. Manag. 2018, 423, 59–69. [Google Scholar] [CrossRef]
- Honer, T.G. Standard Volume Tables and Merchantable Conversion Factors for the Commercial Tree Species of Central and Eastern Canada; Information Report FMR-X-5; Canadian Department of Forestry and Rural Development, Forest Management Research and Services Institute: Ottawa, ON, Canada, 1967; p. 21.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 3 March 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Livingston, W.H.; Pontius, J.; Costanza, K.K.L.; Trosper, S. Using changes in basal area increments to map relative risk of HWA impacts on hemlock growth across the Northeastern U.S. Biol. Invasions 2017, 19, 1577–1595. [Google Scholar] [CrossRef]
- New Hampshire Department of Environmental Services (NHDES). New Hampshire Drought Management Plan. NHDES-WRB-90-1; 1990; p. 48. Available online: https://drought.unl.edu/archive/plans/drought/state/NH_1990.pdf (accessed on 16 September 2015).
- Lombard, P.J. Drought Conditions in Maine, 1999–2002: A Historical Perspective. Water-Resources Investigations Report 03-4310; U.S. Geological Survey: Augusta, ME, USA, 2004; p. 47.
- National Oceanic and Atmospheric Administration (NOAA). Climatological Rankings. 2017. Available online: https://www.ncdc.noaa.gov/temp-and-precip/climatological-rankings (accessed on 25 October 2017).
- Munck, I.A.; Luther, T.; Wyka, S.; Keirstead, D.; McCracken, K.; Ostrofsky, W.; Searles, W.; Lombard, K.; Weimer, J.; Allen, B. Soil and stocking effects on Caliciopsis canker of Pinus strobus L. Forests 2016, 7, 269. [Google Scholar] [CrossRef]
- Johnson, A.H.; McLaughlin, S.B.; Adams, M.B.; Cook, E.R.; DeHayes, D.H.; Eagar, C.; Fernandez, I.J.; Johnson, D.W.; Kohut, R.J.; Mohnen, V.A.; et al. Synthesis and conclusions from epidemiological and mechanistic studies of red spruce decline. In Ecology and Decline of Red Spruce in the Eastern United States; Eager, C., Adams, M.B., Eds.; Springer: New York, NY, USA, 1992; pp. 385–412. [Google Scholar]
- Livingston, W.H.; Munck, I.A.; Lombard, K.; Weimer, J.; Bergdahl, A.; Kenefic, L.S.; Schultz, B.; Seymour, R.S. Field manual for managing eastern white pine health in New England. Maine Forest and Agricultural Experiment Station. Misc. Publ. 2019, 764, 20. [Google Scholar]
- D’Amato, A.W.; Troumbly, S.J.; Saunders, M.R.; Puettmann, K.J.; Albers, M.A. Growth and survival of Picea glauca following thinning of plantations affected by eastern spruce budworm. North. J. Appl. For. 2011, 28, 72–78. [Google Scholar] [CrossRef]
- Wyka, S.A.; Smith, C.; Munck, I.A.; Rock, B.N.; Ziniti, B.L.; Broders, K. Emergence of white pine needle damage in the northeastern United States is associated with changes in pathogen pressure in response to climate change. Glob. Chang. Biol. 2017, 23, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Manion, P.D. Tree Disease Concepts; Prentice Hall: Englewood Cliffs, NJ, USA, 1991; p. 402. [Google Scholar]
- D’Amato, A.W.; Bradford, J.B.; Fraver, S.; Palik, B.J. Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol. Appl. 2013, 23, 1735–1742. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanza, K.K.L.; Livingston, W.H.; Fraver, S.; Munck, I.A. Dendrochronological Analyses and Whole-Tree Dissections Reveal Caliciopsis Canker (Caliciopsis pinea) Damage Associated with the Declining Growth and Climatic Stressors of Eastern White Pine (Pinus strobus). Forests 2020, 11, 347. https://doi.org/10.3390/f11030347
Costanza KKL, Livingston WH, Fraver S, Munck IA. Dendrochronological Analyses and Whole-Tree Dissections Reveal Caliciopsis Canker (Caliciopsis pinea) Damage Associated with the Declining Growth and Climatic Stressors of Eastern White Pine (Pinus strobus). Forests. 2020; 11(3):347. https://doi.org/10.3390/f11030347
Chicago/Turabian StyleCostanza, Kara K.L., William H. Livingston, Shawn Fraver, and Isabel A. Munck. 2020. "Dendrochronological Analyses and Whole-Tree Dissections Reveal Caliciopsis Canker (Caliciopsis pinea) Damage Associated with the Declining Growth and Climatic Stressors of Eastern White Pine (Pinus strobus)" Forests 11, no. 3: 347. https://doi.org/10.3390/f11030347
APA StyleCostanza, K. K. L., Livingston, W. H., Fraver, S., & Munck, I. A. (2020). Dendrochronological Analyses and Whole-Tree Dissections Reveal Caliciopsis Canker (Caliciopsis pinea) Damage Associated with the Declining Growth and Climatic Stressors of Eastern White Pine (Pinus strobus). Forests, 11(3), 347. https://doi.org/10.3390/f11030347





