Patterns of Density and Production in the Community Forests of the Sierra Madre Occidental, Mexico
Abstract
:1. Introduction
2. Materials and Methods
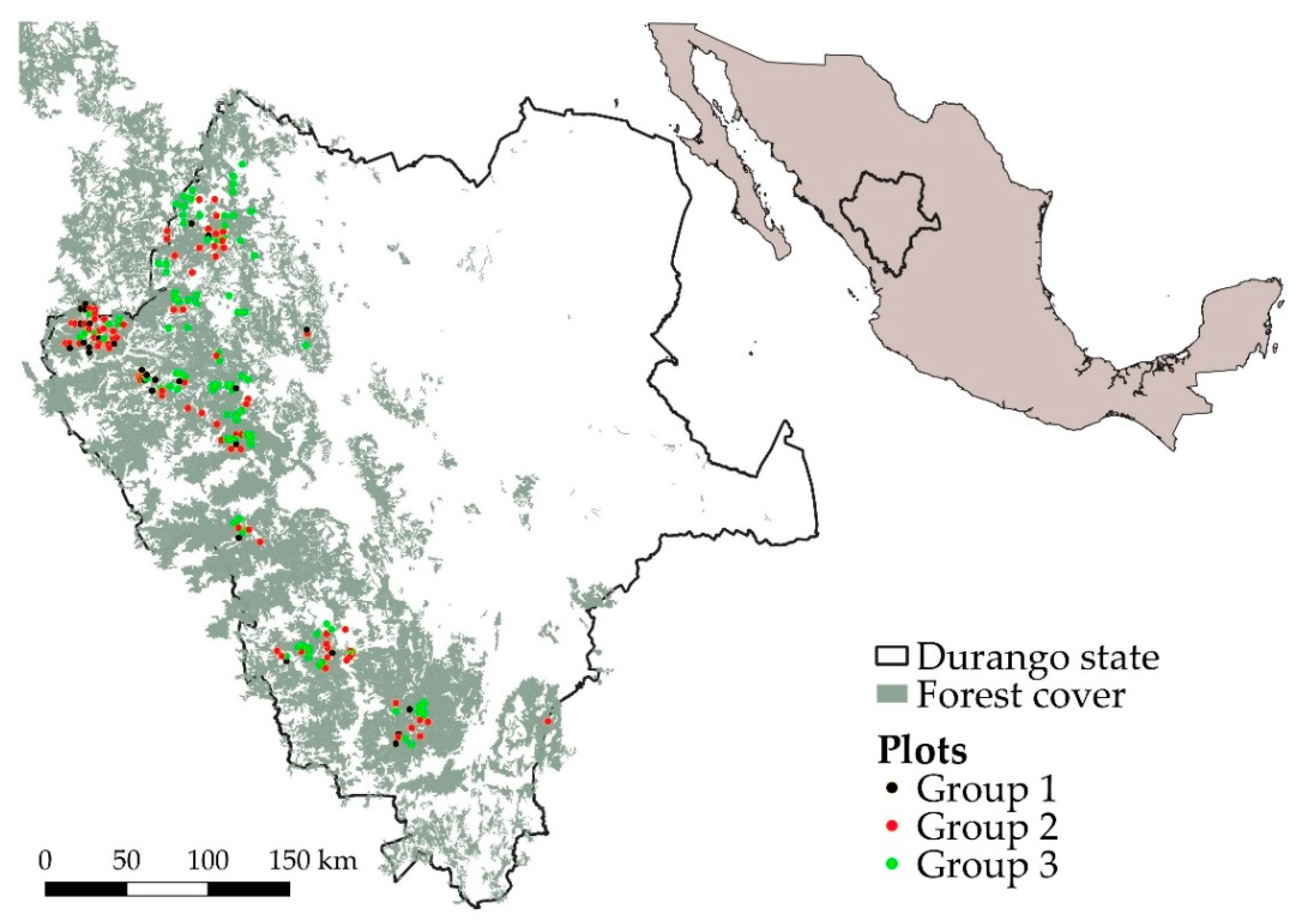
2.1. Permanent Field Plots
2.2. Stratification of Field Plots
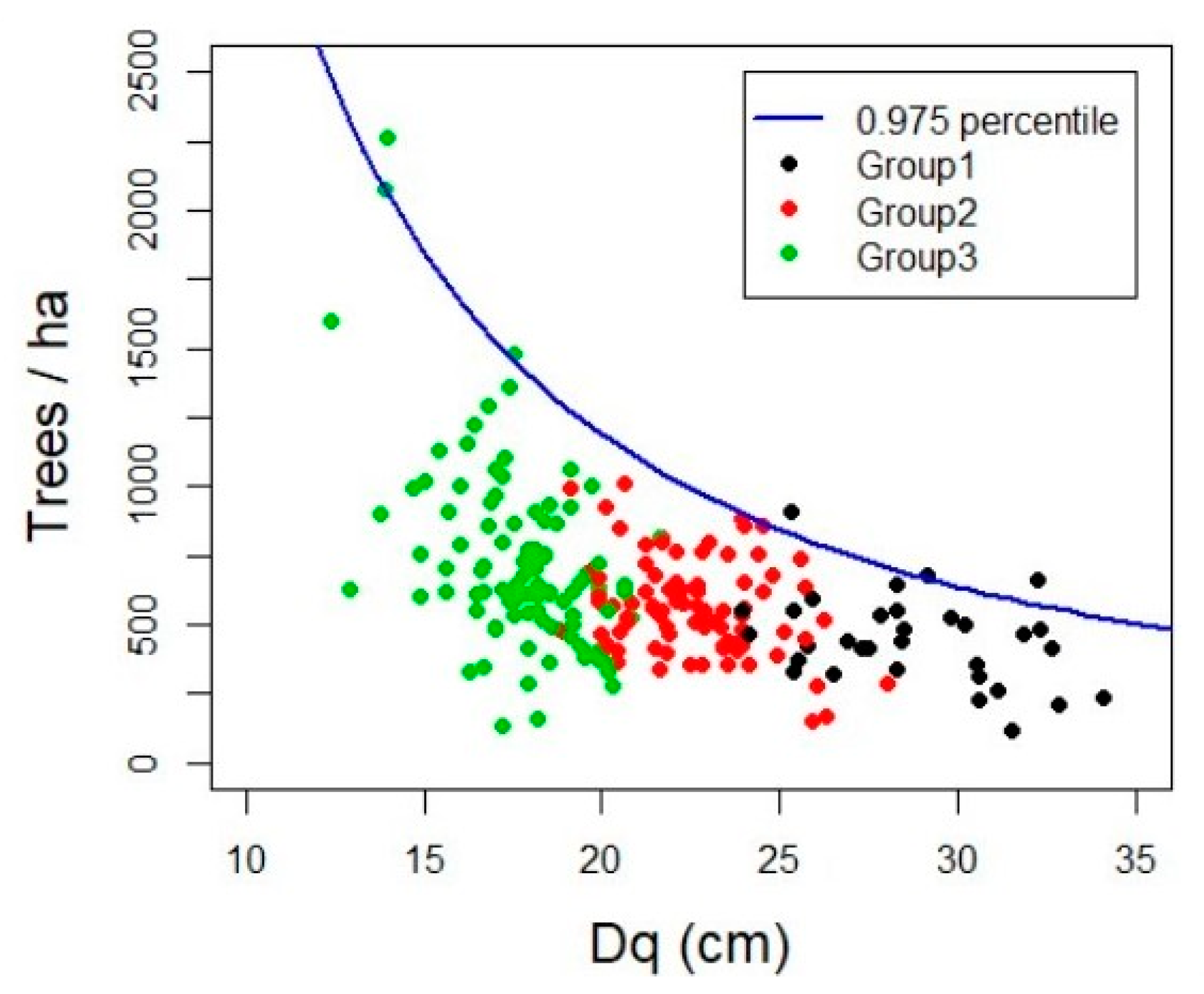
2.3. Maximum Forest Density
2.4. Forest Diversity
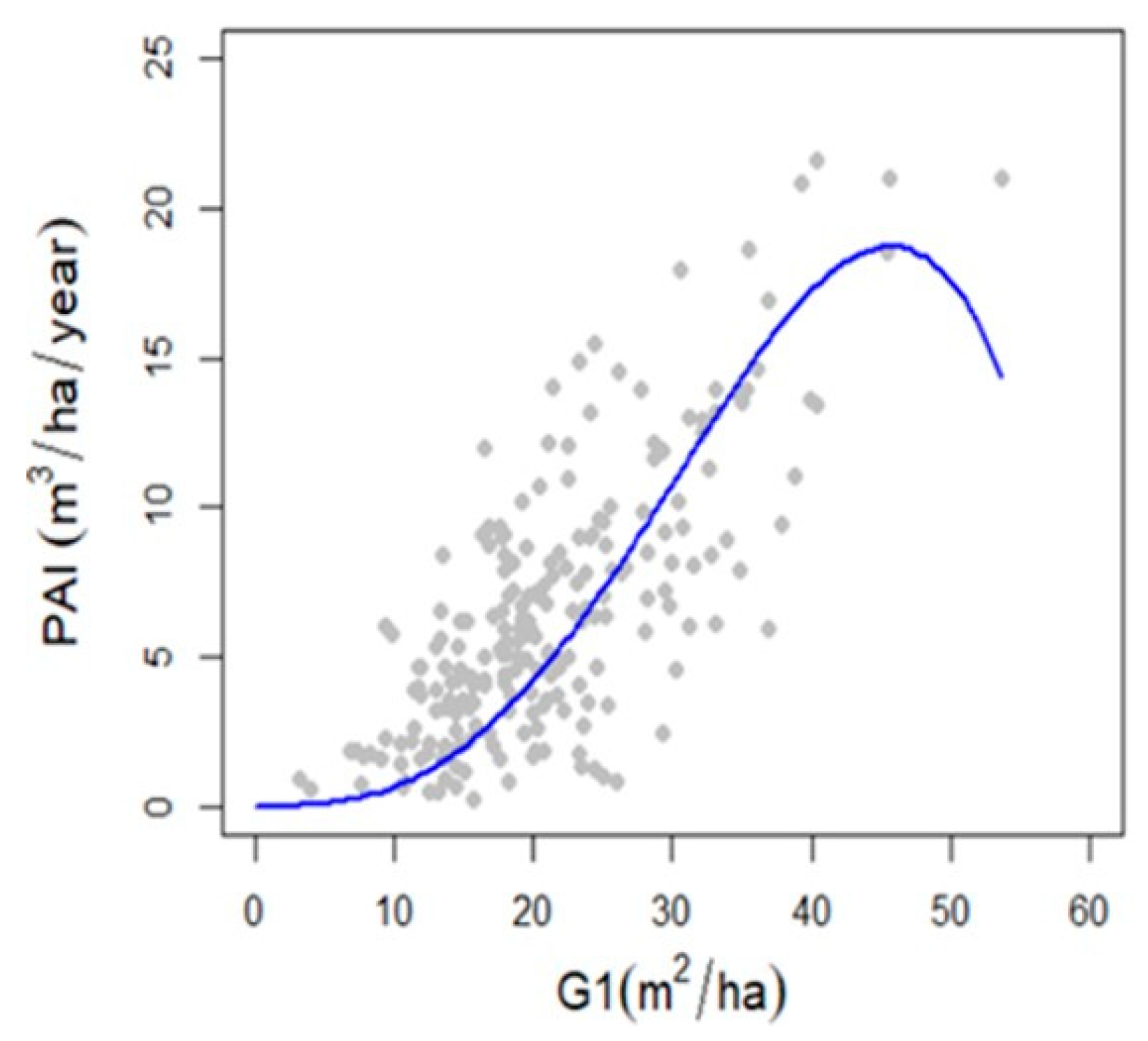
2.5. Modelling Forest Production
3. Results
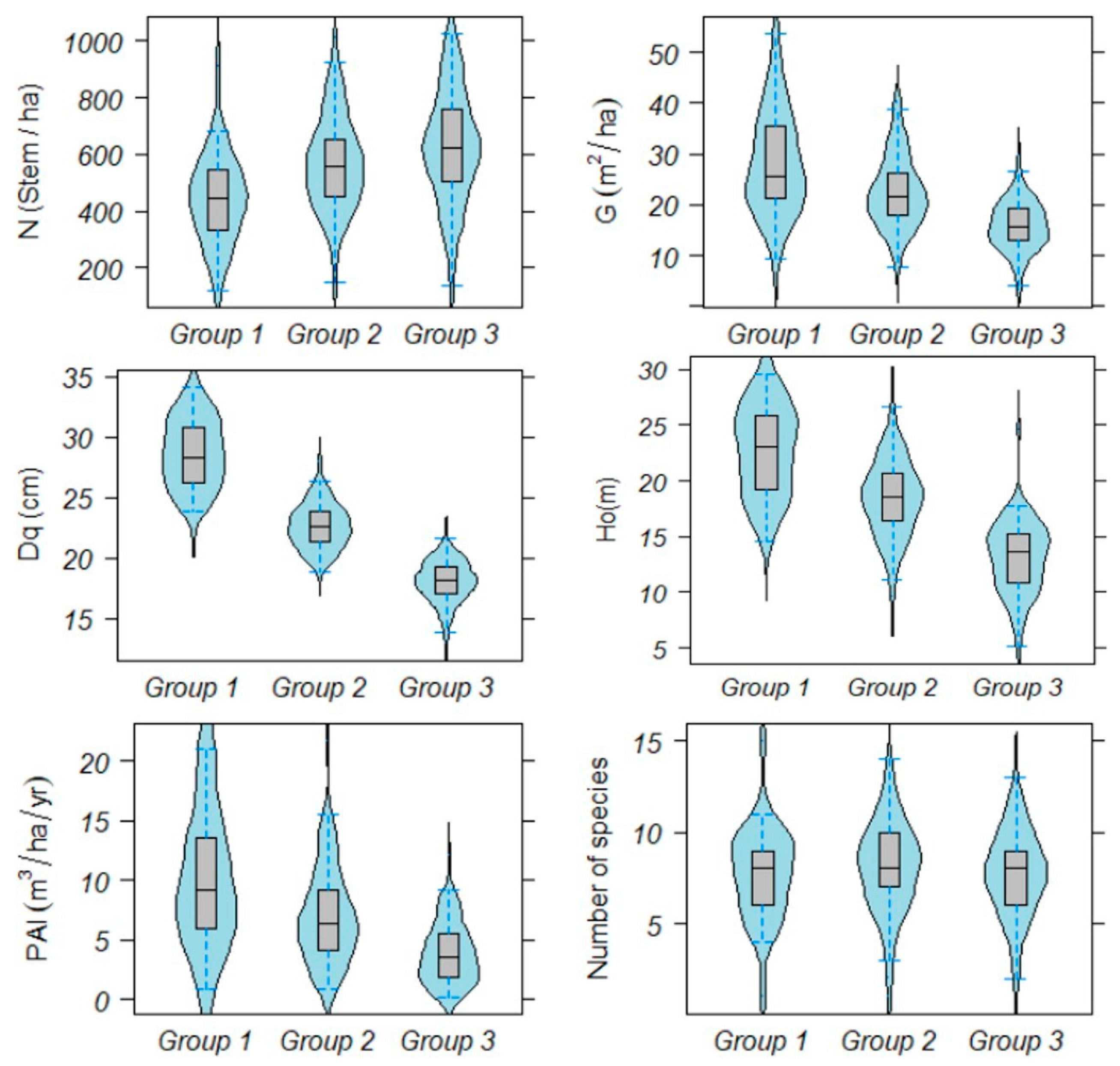
3.1. Stratification of Field Plots
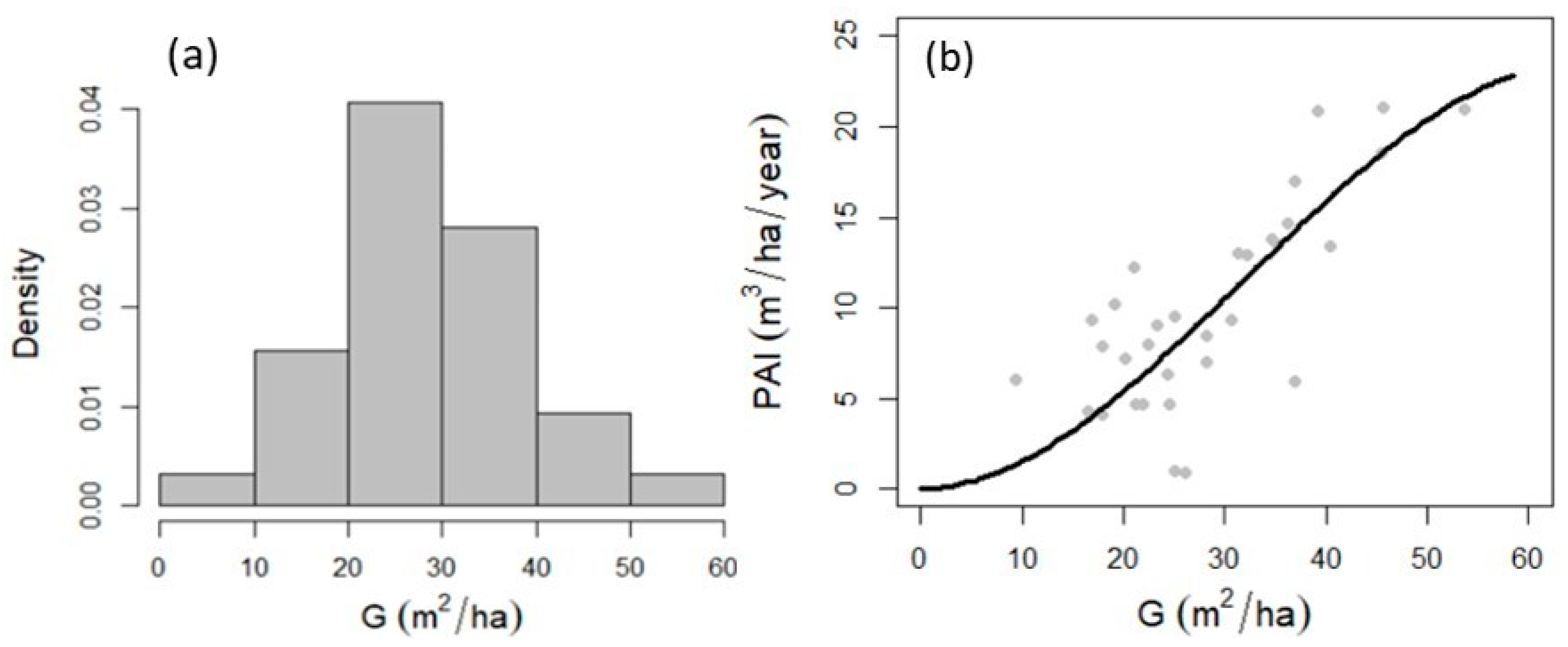
3.2. Maximum Forest Density
3.3. Relationship between Forest Density, Diversity and Production
4. Discussion
4.1. Groups of Field Plots and Maximun Forest Density
4.2. Maximun Forest Density
4.3. Relationship between Forest Density and Production
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Gadow, K.; Hui, G. Modelling Forest Development; Kluwer Academic: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Burkhart, H.E.; Tomé, M. Modelling Forest Trees and Stands; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Von Gadow, K.; Zhao, X.H.; Corral-Rivas, J.J. Retention Strategies for Multi-Species Forests. In Proceedings of the International Symposium for the 50th Anniversary of the Forestry Sector Planning in Turkey, Antalya, Turkey, 26–28 November 2013. [Google Scholar]
- Von Gadow, K.; Kotze, H. Tree survival and maximum density of planted forests—Observations from South African spacing studies. For. Ecosyst. 2014, 1, 21. [Google Scholar]
- Pretzsch, H.; Schütze, G. Effect of tree species mixing on the size structure, density, and yield of forest stands. Eur. J. For. Res. 2016, 135, 1–22. [Google Scholar] [CrossRef]
- Quiñonez-Barraza, G.; Tamarit-Urias, J.C.; Martínez-Salvador, M.; García-Cuevas, X.; de los Santos-Posadas, H.M.; Santiago-García, W. Maximum density and density management diagram for mixed-species forest in Durango, Mexico. Rev. Chapingo Ser. Cie. For. Ambiente 2017, 24, 73–90. [Google Scholar] [CrossRef]
- West, P.W. Comparison of stand density measures in even-aged regrowth eucalypt forest of southern Tasmania. Can. J. For. Res. 1983, 13, 22–31. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Diamantopoulou, M.J.; Özçelik, R.; Crecente-Campo, F.; Eler, Ü. Estimation of Weibull function parameters for modelling tree diameter distribution using least squares and artificial neural networks methods. Biosyst. Eng. 2015, 133, 33–45. [Google Scholar] [CrossRef]
- Arias-Rodil, M.; Diéguez-Aranda, U.; Álvarez-González, J.G.; Pérez-Cruzado, C.; Castedo-Dorado, F.; González-Ferreiro, E. Modeling diameter distributions in radiata pine plantations in Spain with existing countrywide LiDAR data. Ann. For. Sci. 2018, 75, 36. [Google Scholar] [CrossRef] [Green Version]
- Gadow, K.V. Untersuchungen Zur Konstruktion Von Wuchsmodellen Für Schnellwüchsige Plantagenbaumarten; Universität München: München, Germany, 1987. [Google Scholar]
- Rubin, B.D.; Manion, P.D.; Faber-Langendoen, D. Diameter distributions and structural sustainability in forests. For. Ecol. Manag. 2006, 222, 427–438. [Google Scholar] [CrossRef]
- Calzado, C.A.; Torres, A.E. Modelling diameter distributions of Quercus suber L. stands, in “Los Alcornocales” natural park (Cádiz-Málaga, Spain) by using the two parameter Weibull functions. For. Syst. 2013, 22, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Biber, P. Tree species mixing can increase stand density. Can. J. For. Res. 2016, 46, 1179–1193. [Google Scholar] [CrossRef] [Green Version]
- Torres-Rojo, J.M. Exploring volume growth-density of mixed multiaged stands in northern Mexico. Agrociencia 2014, 48, 447–461. [Google Scholar]
- Corral-Rivas, J.J.; Torres-Rojo, J.M.; Lujan-Soto, J.E.; Nava-Miranda, M.G.; Aguirre-Calderón, O.A.; Gadow, K.V. Density and production in the natural forest of Durango/Mexico. Allg. Fort. Jagdztg. 2016, 187, 93–103. [Google Scholar]
- González-Elizondo, M.S.; González-Elizondo, M.; Tena-Flores, J.A.; Ruacho-González, L.; López-Enríquez, I.L. Vegetación de la Sierra Madre Occidental, México: Una síntesis. Acta Bot. Mex. 2012, 100, 351–403. [Google Scholar] [CrossRef] [Green Version]
- González-Elizondo, M.S.; González-Elizondo, M.; Ruacho-González, L.; López-Enríquez, I.L.; Tena-Flores, J.A. Ecosystems and diversity of the Sierra Madre Occidental. In Proceedings of the Merging Science and Management in a Rapidly Changing World: Biodiversity and Management of the Madrean Archipelago III and 7th Conference on Research and Resource Management in the Southwestern Deserts, Tucson, AZ, USA, 1–5 May 2012; Gottfried, G.J., Ffolliott, P.F., Gebow, B.S., Eskew, L.G., Collins, L.C., Eds.; RMRS-P. Department of Agriculture, Forest Service: Fort Collins, CO, USA, 2013; Volume 67, pp. 204–211. [Google Scholar]
- Corral-Rivas, J.J.; Reyes, R.I.; Wehenkel, C.; Aguirre-Calderón, O.A.; Gadow, K. A network of forest observational studies in Durango (Mexico). In Proceedings of the International Workshop at Beijing Forestry University, Beijing, China, 20–21 September 2012; Zhao, X.H., Zhang, C.Y., Gadow, K., Eds.; Beijing Forestry University: Beijing, China, 2012; pp. 125–138. [Google Scholar]
- Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 21 December 2019).
- Martínez-Fernández, J.; Chuvieco, S.E. Tipologías de incidencia y causalidad de incendios forestales basadas en análisis multivariante. Ecología 2003, 17, 47–63. [Google Scholar]
- Corral-Rivas, J.J.; González-Elizondo, M.S.; Lujan-Soto, J.E.; Gadow, K.V. Effects of density and structure on production in the communal forests of the Mexican Sierra Madre Occidental. South. For. 2019, 81. [Google Scholar] [CrossRef]
- Reineke, L.H. Perfecting a stand-density index for even-aged forests. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics. Update Edition; Yale School of Forestry & Environmental Studies Other Publications: New York, NY, USA, 1996; Available online: https://elischolar.library.yale.edu/fes_pubs/1 (accessed on 21 December 2019).
- Zeide, B. Optimal stand density: A solution. Can. J. For. Res. 2004, 34, 846–854. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhao, X.H.; Gadow, K.V. Maximum density patterns in two natural forests: An analysis based on large observational field studies in China. For. Ecol. Manag. 2015, 346, 98–105. [Google Scholar] [CrossRef]
- Koenker, R. Quantreg: Quantile Regression. R Package Version 5.4. 2019. Available online: https://CRAN.R-project.org/package=quantreg (accessed on 21 December 2019).
- Edwards, B.L.; Allen, S.T.; Braud, D.H.; Keim, R.F. Stand density and carbon storage in cypress-tupelo wetland forests of the Mississippi River Delta. For. Ecol. Manag. 2019, 441, 106–114. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–374. [Google Scholar] [CrossRef]
- Aerts, R.; Honnay, O. Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, A.; Chun-Huo, C.; Lou, J. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef] [Green Version]
- Hill, M. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M. Marine science and management means tackling exogenic unmanaged pressures and endogenic managed pressures—A numbered guide. Mar. Pollt. Bull. 2011, 62, 651–655. [Google Scholar] [CrossRef]
- Leinster, T.; Cobbold, C.A. Measuring diversity: The importance of species similarity. Ecology 2012, 93, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Gadow, K.V.; Zhang, C.Y.; Wehenkel, C.; Pommerening, A.; Corral-Rivas, J.J.; Korol, M.; Myklush, S.; Hui, G.Y.; Kiviste, A.; Zhao, X.H. Forest Structure and Diversity. In Continuous Cover Forestry, 2nd ed.; Pukkala, T., Gadow, K.V., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 29–84. [Google Scholar]
- Assmann, E. Principles of Forest Yield Study; Pergamon Press: New York, NY, USA, 1970. [Google Scholar]
- Pretzsch, H.; del Río, M.; Ammer, C.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Dirnberger, G.; Drössler, L.; et al. Growth and yield of mixed versus pure stands of Scots pine (Pinus Sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. Eur. J. For. Res. 2015, 134, 927–947. [Google Scholar] [CrossRef] [Green Version]
- Vanclay, J.K. Modelling Forest Growth and Yield; Applications to Mixed Tropical Forest; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Marziliano, P.A.; Menguzzato, G.; Scuderi, A.; Corona, P. Simplified methods to inventory the current annual increment of forest standing volume. Iforest 2012, 5, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.F.; Cheng, C.H. GMADM-based attributes selection method in developing prediction model. Qual. Quant. 2012, 47, 3335–3347. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance Test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Zhao, X.H.; Corral-Rivas, J.J.; Zhang, C.Y.; Temesgen, H.; Gadow, K.V. Forest observational studies-an essential infrastructure for sustainable use of natural resources. For. Ecosyst. 2014, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Vallet, P.; Perot, T. Tree diversity effect on dominant height in temperate forest. For. Ecol. Manag. 2016, 381, 106–114. [Google Scholar] [CrossRef]
- Ouyang, S.; Xiang, W.; Wang, X.; Xiao, W.; Chen, L.; Li, S.; Sun, H.; Deng, X.; Forrester, D.I.; Zeng, L.; et al. Effects of stand age, richness and density on productivity in subtropical forests in China. J. Ecol. 2019, 107, 2266–2277. [Google Scholar] [CrossRef]
- Kelty, M.J. Comparative productivity of monocultures and mixed species stands. In The Ecology and Silviculture of Mixed-Species Forests; Kelty, M.J., Larson, B.C., Oliver, C.D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Garber, S.M.; Maguire, D.A. Stand productivity and development in two mixed-species spacing trials in the Central Oregon Cascades. For. Sci. 2004, 50, 92–105. [Google Scholar]
- Jacob, M.; Leuschner, C.; Thomas, F.M. Productivity of temperate broad-leaved forest stands differing in tree species diversity. Ann. For. Sci. 2010, 67, 503. [Google Scholar] [CrossRef] [Green Version]
- Hernández, R.J.; García, M.; Muíoz, F.; García-Cuevas, J.; Sáenz, R.T.; Flores, L.C.; Hernández, R.A. Density management guide for natural Pinus teocote Schecht Cham Forest in Hidalgo. Rev. Mex. Cienc. For. 2013, 4, 62–76. [Google Scholar]
- VanderSchaaf, C.L.; Burkhart, H.E. Using segmented regression to estimate stages and phases of stand development. For. Sci. 2008, 54, 167–175. [Google Scholar]
- Nyland, R.D. Silviculture Concepts una Applications; Waveland Press: Long Grove, IL, USA, 2016; p. 680. [Google Scholar]
- Allen, M.G.; Burkhart, H.E. Growth-density relationships in Loblolly Pine plantations. For. Sci. 2018, 65, 250–264. [Google Scholar] [CrossRef]
- Harrington, T.B. Silvicultural Approaches for Thinning Southern Pines: Method, Intensity, and Timing. Available online: http://www.gfc.state.ga.us/resources/publications/SilviculturalApproaches.pdf (accessed on 21 December 2019).
- Tewari, V.P.; Gadow, K.V. Modelling potential density limiting survival, stand density and basal area growth for pure even-aged Dalbergia sissoo stands in a hot arid region of India. For. Trees Livelihoods 2008, 18, 133–150. [Google Scholar] [CrossRef]
- Dickel, M.; Kotze, H.; Gadow, K.V.; Zucchini, W. Growth and Survival of Eucalyptus grandis—A Study based on Modelling Lifetime Distributions. MCFNS 2010, 2, 20–30. [Google Scholar]
- Thomasius, H.O.; Thomasius, H. Ableitung eines Verfahrens zur Berechnung der ertragskundlich optimalen Bestandesdichte. Beiträge F. D. Forstwirtsch. 1978, 12, 79. [Google Scholar]
- Schütz, J.P.; Saniga, M.; Diaci, J.; Vrška, T. Comparing close-to-nature silviculture with processes in prime forests: Lessons from Central Europe. Ann. For. Sci. 2016, 73, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Torres-Rojo, J.M. Sostenibilidad del volumen de cosecha calculado con el método mexicano de montes. Madera Bosques 2000, 6, 57–72. [Google Scholar] [CrossRef] [Green Version]

| Plot | First Inventory | Second Inventory | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | Max | Min | Sd | Mean | Max | Min | Sd |
| N | 623.78 | 2264.00 | 120.00 | 281.30 | 624.18 | 2152.00 | 144.00 | 275.91 |
| G (m2/ha) | 21.16 | 53.63 | 3.15 | 8.20 | 23.31 | 58.52 | 3.92 | 8.98 |
| Dq (cm) | 21.32 | 34.10 | 12.40 | 4.28 | 22.34 | 36.00 | 13.40 | 4.44 |
| V (m3/ha) | 188.55 | 604.21 | 12.04 | 106.19 | 221.04 | 709.20 | 16.57 | 124.28 |
| Ho (m) | 16.82 | 29.50 | 5.20 | 4.80 | 18.18 | 31.60 | 5.80 | 5.19 |
| S | 7.91 | 15.00 | 1.00 | 2.41 | 8.17 | 16.00 | 1.00 | 2.48 |
| W (ton/ha) | 104.88 | 306.67 | 6.30 | 62.53 | 123.92 | 374.75 | 8.90 | 73.15 |
| PAI (m3/ha/year) | 6.50 | 21.66 | 0.24 | 4.40 | ||||
| Parameter | Estimate | Standard Error | T Value | p-Value | RSE | R2 |
|---|---|---|---|---|---|---|
| a | −0.004465 | 0.001119 | −3.991 | 9.06 × 10−5 | 0.12 | 0.85 |
| b | 0.018327 | 0.002162 | 8.477 | 3.85 × 10−15 | ||
| c | 0.226110 | 0.053600 | 4.218 | 3.64 × 10−5 | ||
| d | −0.374981 | 0.074914 | −5.005 | 1.17 × 10−6 |
| G (m2/ha) | ∆G (m2/ha/year) | Time Required (years) |
|---|---|---|
| 15.00 | 0.0000 | 0 |
| 15.96 | 0.9595 | 5 |
| 17.03 | 1.0696 | 10 |
| 18.23 | 1.1966 | 15 |
| 19.57 | 1.3436 | 20 |
| 21.08 | 1.5139 | 25 |
| 22.79 | 1.7113 | 30 |
| 24.73 | 1.9398 | 35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Martínez, J.R.; Corral-Rivas, J.J.; Briseño-Reyes, J.; Paul, C.; López-Serrano, P.M.; v. Gadow, K. Patterns of Density and Production in the Community Forests of the Sierra Madre Occidental, Mexico. Forests 2020, 11, 307. https://doi.org/10.3390/f11030307
Padilla-Martínez JR, Corral-Rivas JJ, Briseño-Reyes J, Paul C, López-Serrano PM, v. Gadow K. Patterns of Density and Production in the Community Forests of the Sierra Madre Occidental, Mexico. Forests. 2020; 11(3):307. https://doi.org/10.3390/f11030307
Chicago/Turabian StylePadilla-Martínez, Jaime Roberto, José Javier Corral-Rivas, Jaime Briseño-Reyes, Carola Paul, Pablito Marcelo López-Serrano, and Klaus v. Gadow. 2020. "Patterns of Density and Production in the Community Forests of the Sierra Madre Occidental, Mexico" Forests 11, no. 3: 307. https://doi.org/10.3390/f11030307
APA StylePadilla-Martínez, J. R., Corral-Rivas, J. J., Briseño-Reyes, J., Paul, C., López-Serrano, P. M., & v. Gadow, K. (2020). Patterns of Density and Production in the Community Forests of the Sierra Madre Occidental, Mexico. Forests, 11(3), 307. https://doi.org/10.3390/f11030307







