Genome-Wide Identification and Characterization of Apple P3A-Type ATPase Genes, with Implications for Alkaline Stress Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Identification and Phylogenetic Analysis of P3A-Type ATPase Genes
2.3. Analysis of the Structure, Conserved Motifs, and Transmembrane Domains of P3A-Type ATPases
2.4. Estimation of Gene Duplication and Loss
2.5. Analysis of Positive Selection
2.6. RNA Extraction and qRT-PCR Analysis
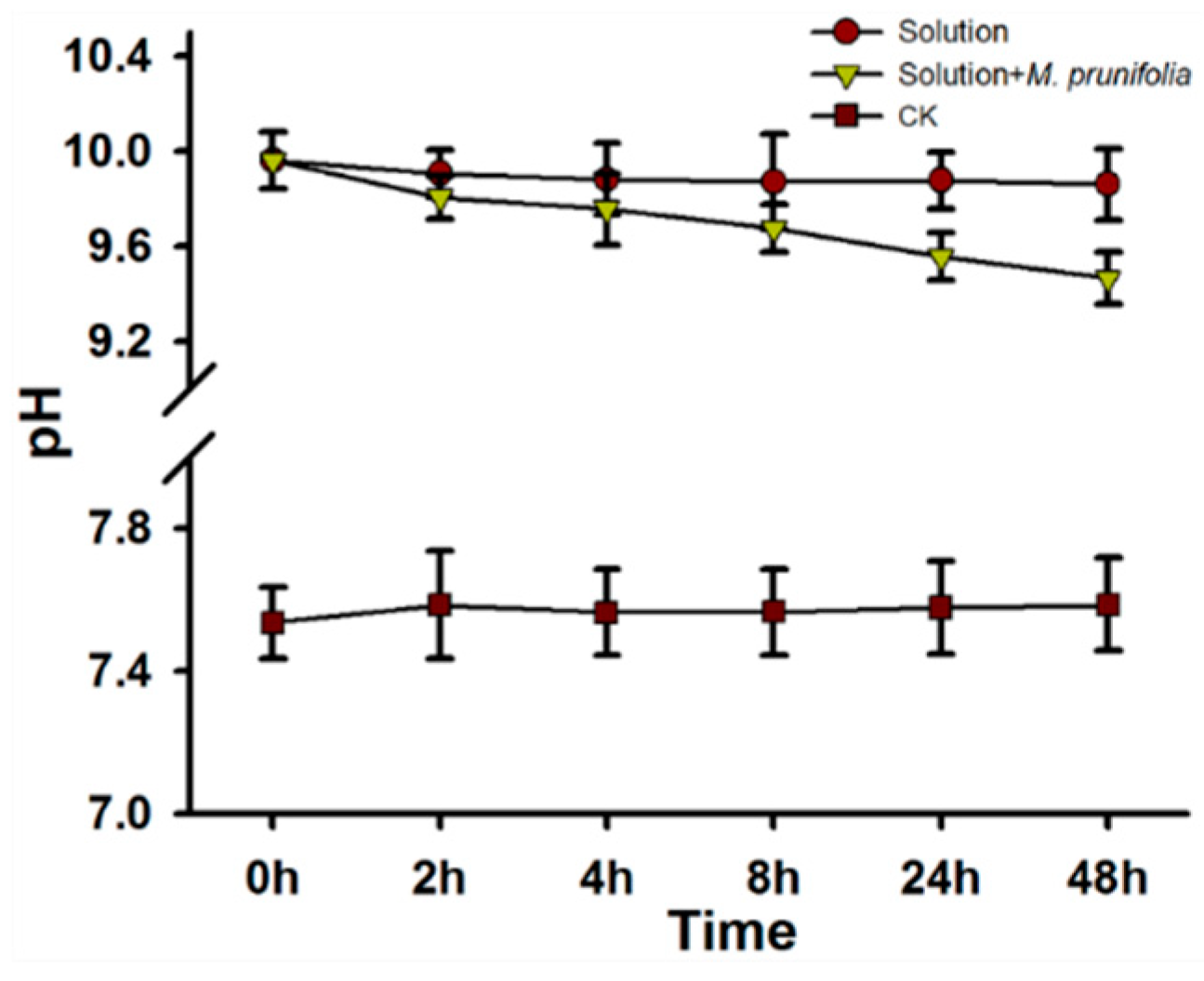
2.7. Measurement of the Solution pH and Analysis of the Transcriptome
2.8. Subcellular Localization of Apple P3A-Type ATPases in Arabidopsis thaliana Protoplasts
2.9. Data Analysis
3. Results
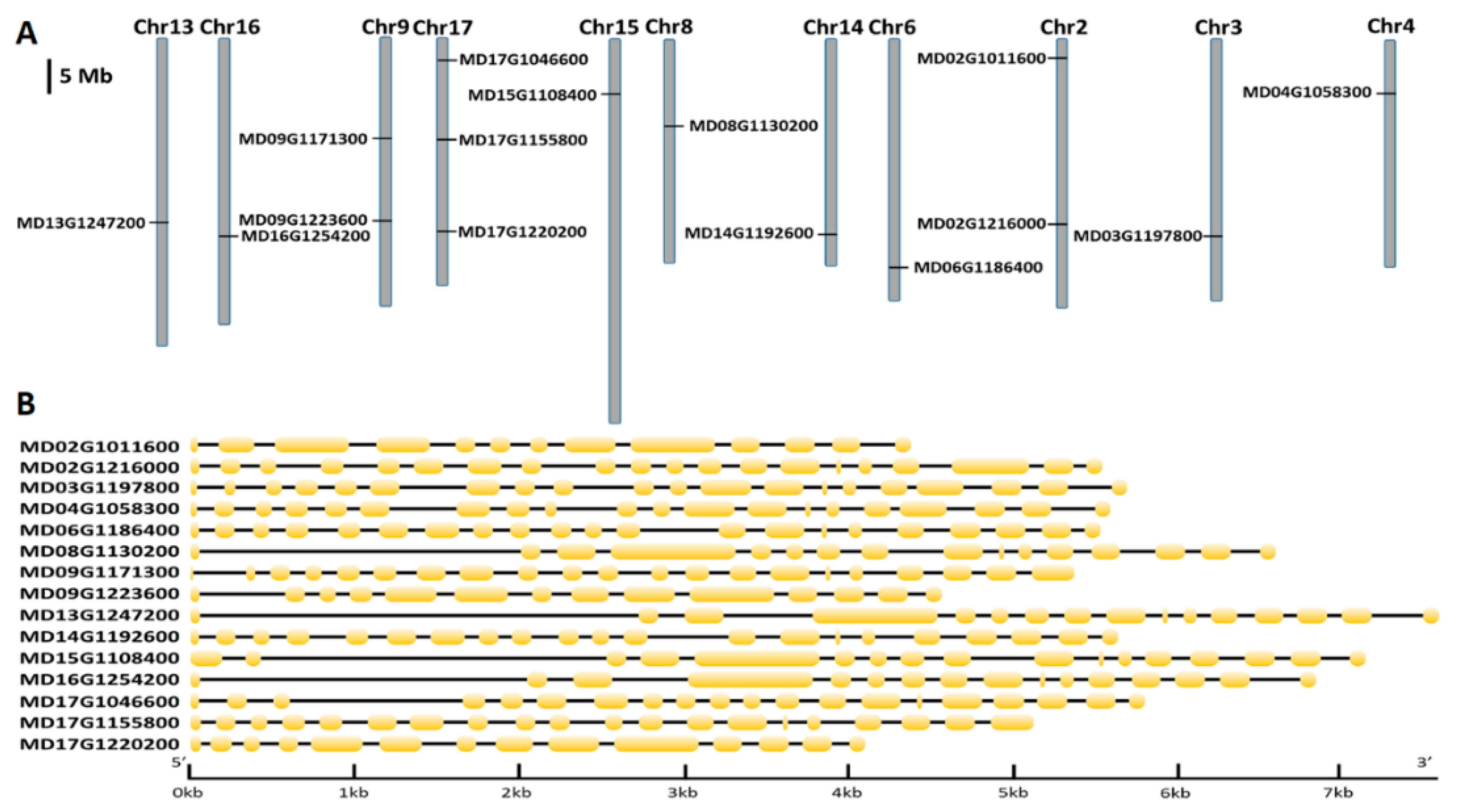
3.1. Identification, Characterization, and Genomic Distribution of Apple P3A-Type ATPase Genes
3.2. Phylogenetic Relationship Analysis, Gene Duplication, and Gene Loss of the P3A-Type ATPase Gene Family in Four Rosaceae Species
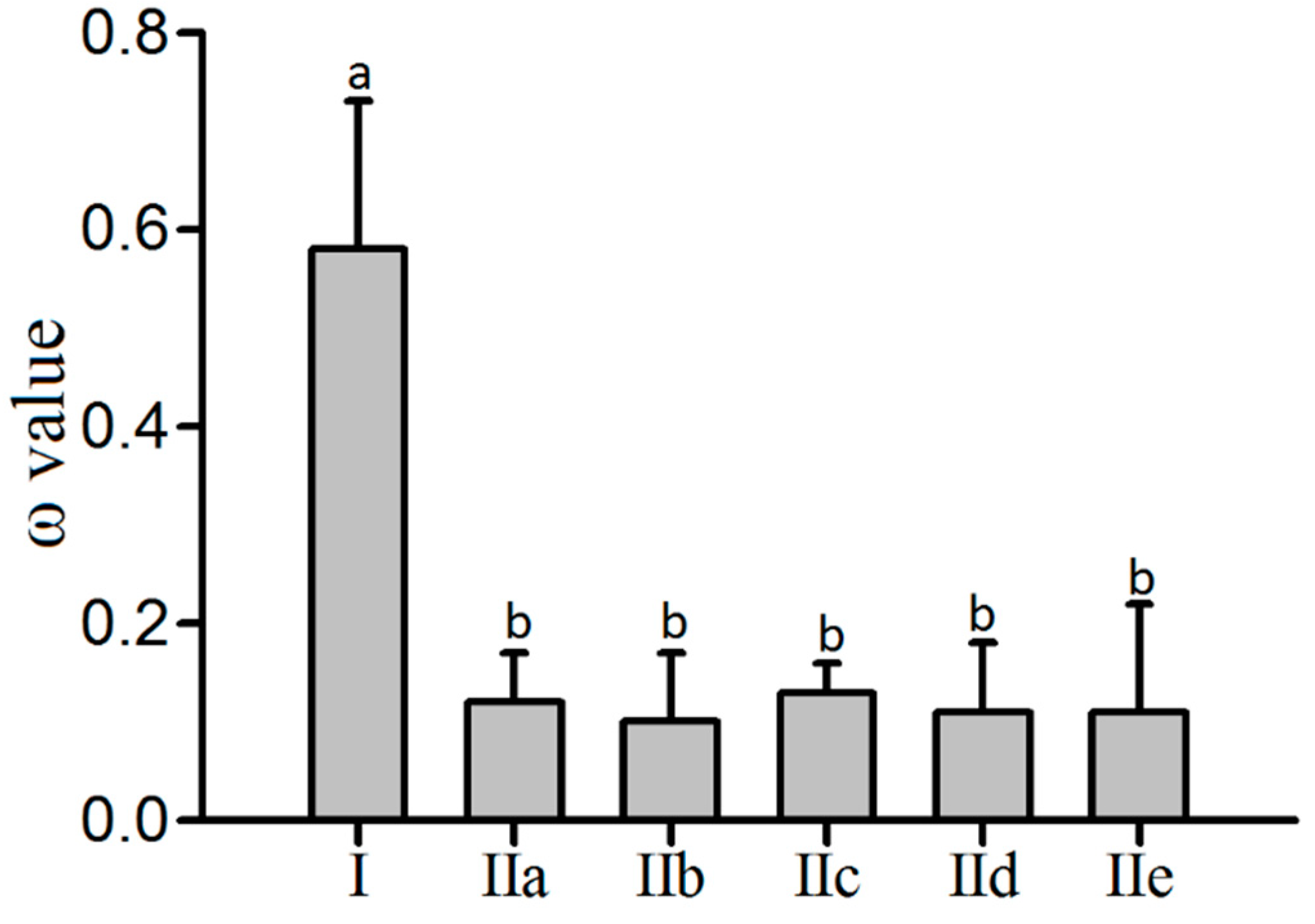
3.3. Estimation of Selection Pressures Affecting P3A-Type ATPase Genes in Four Rosaceae Species
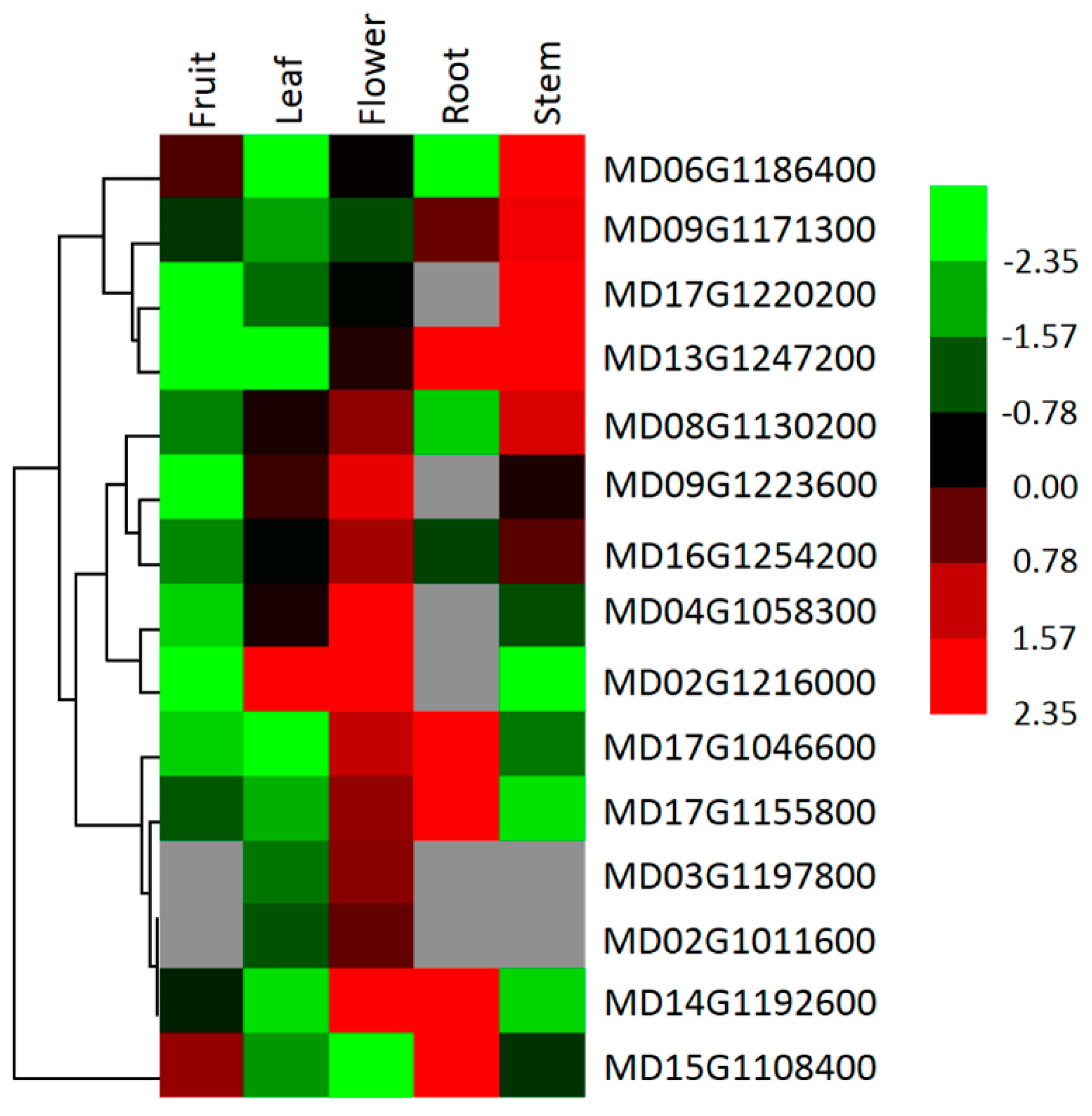
3.4. Analysis of P3A-Type ATPase Gene Expression in Various Apple Tree Tissues
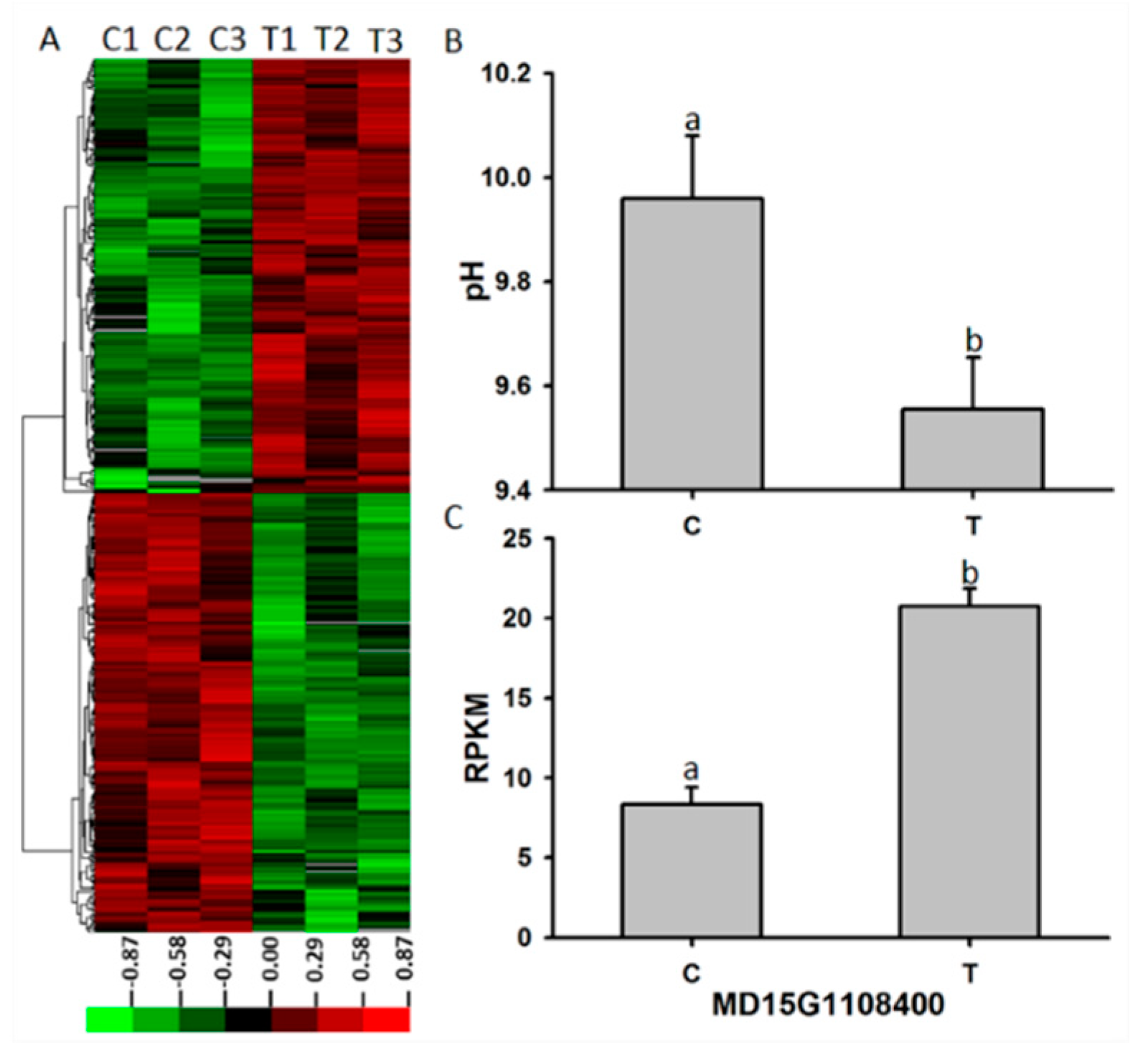
3.5. Transcriptome Analysis and Identification of Candidate Genes Responsive to Alkaline Stress
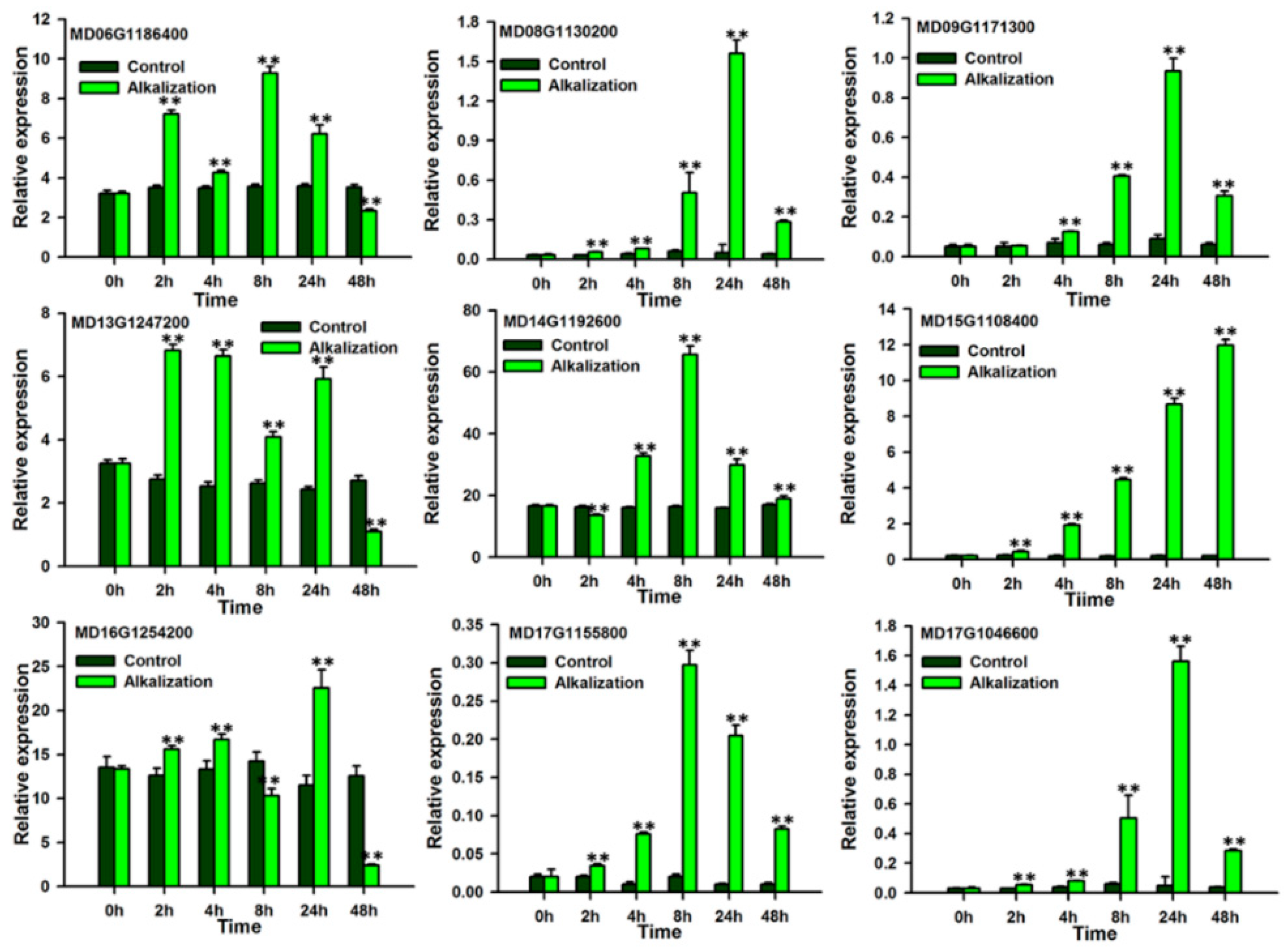
3.6. Expression Profiles of Apple P3A-Type ATPase Genes in the Roots Under Alkaline Conditions
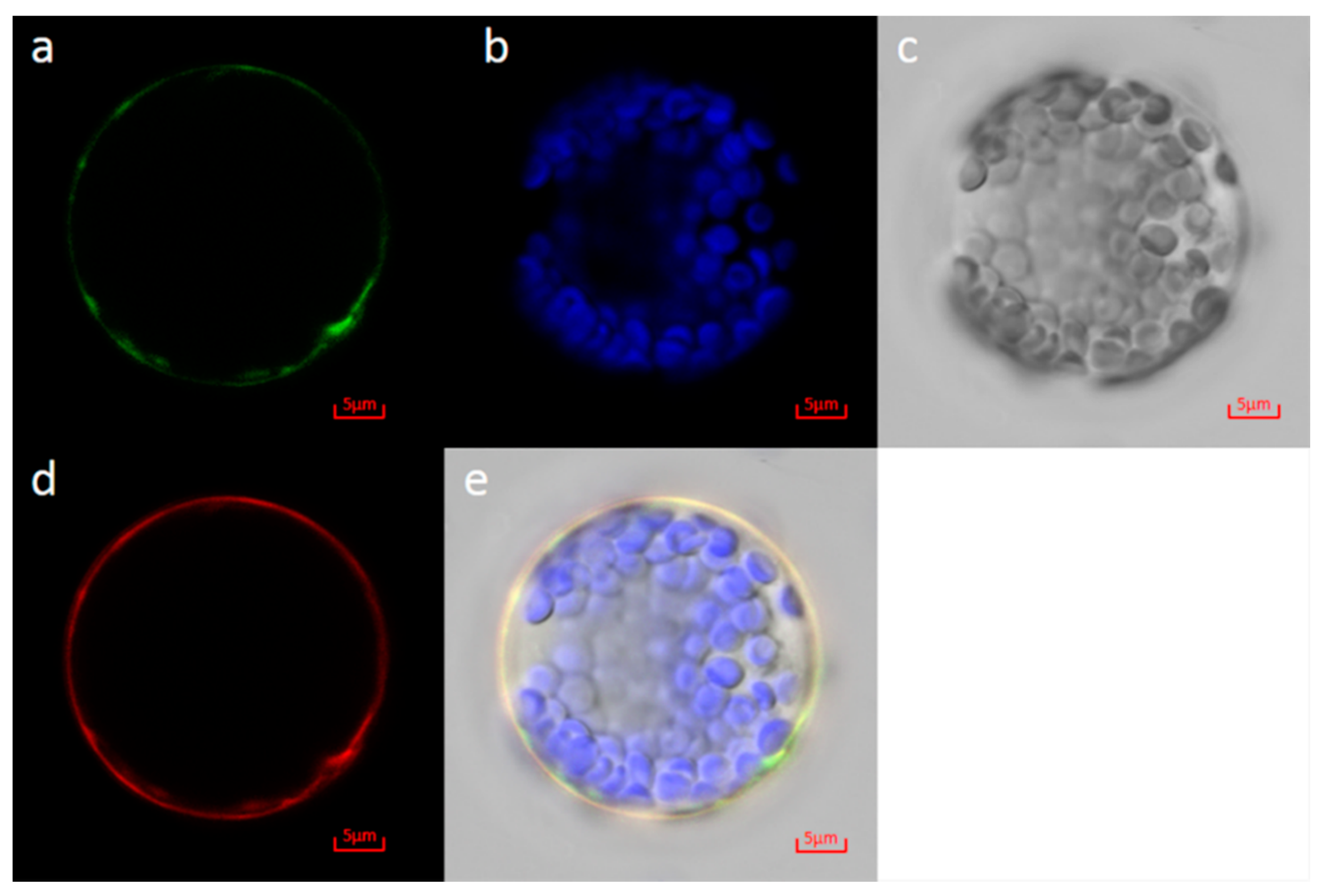
3.7. Subcellular Localization of MD15G1108400
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, T.; Dong, L.; Wang, L.; Ma, F.; Zou, Y.; Li, C. Changes in root architecture and endogenous hormone levels in two Malus rootstocks under alkali stress. Sci. Hortic. 2018, 235, 198–204. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Y.; Gao, M.; Qi, T.; Li, M.; Ma, F. Genome-Wide Identification, Molecular Evolution, and Expression Divergence of Aluminum-Activated Malate Transporters in Apples. Int. J. Mol. Sci. 2018, 19, 2807. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Y.; Gao, M.; Xing, L.; Li, C.; Li, M.; Ma, F. Genome-wide Identification, Classification, Molecular Evolution and Expression Analysis of Malate Dehydrogenases in Apple. Int. J. Mol. Sci. 2018, 19, 3312. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Plaha, P.; Park, J.Y.; Hong, C.P.; Lee, I.S.; Yang, Z.H.; Jiang, G.B.; Kwak, S.S.; Liu, S.K.; Lee, J.S.; et al. Comparative EST profiles of leaf and root of Leymus chinensis, a xerophilous grass adapted to high pH sodic soil. Plant Sci. 2006, 170, 1081–1086. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.H.; Liu, W.C.; Zhang, X.W.; Lu, Y.T. Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol. 2015, 168, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.C.; Sheng, Y.M. Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ. Exp. Bot. 2005, 54, 8–21. [Google Scholar] [CrossRef]
- Shi, D.C.; Wang, D.L. Effects of various salt-alkaline mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant Soil 2005, 271, 15–26. [Google Scholar] [CrossRef]
- Chen, W.C.; Cui, P.J.; Sun, H.Y.; Guo, W.Q.; Yang, C.W.; Jin, H.; Fang, B.; Shi, D.C. Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.). Ind. Crop. Prod. 2009, 30, 351–358. [Google Scholar] [CrossRef]
- Gévaudant, F.; Duby, G.; von Stedingk, E.; Zhao, R.; Morsomme, P.; Boutry, M. Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol. 2007, 144, 1763–1776. [Google Scholar] [CrossRef]
- Monneuse, J.M.; Sugano, M.; Becue, T.; Santoni, V.; Hem, S.; Rossignol, M. Towards the profiling of the Arabidopsis thaliana plasma membrane transportome by targeted proteomics. Proteomics 2011, 11, 1789–1797. [Google Scholar] [CrossRef]
- Zhang, H.; Han, B.; Wang, T.; Chen, S.; Li, H.; Zhang, Y.; Dai, S. Mechanisms of plant salt response: Insights from proteomics. J. Proteome Res. 2012, 11, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, A.T.; Guo, Y.; Cuin, T.A.; Qiu, Q.; Song, C.; Kristiansen, K.A.; Bych, K.; Schulz, A.; Shabala, S.; Schumaker, K.S.; et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell. 2007, 19, 1617–1634. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Y.; Xie, C.; Zhao, F.; Zhao, J.; Liu, D.; Chen, S.; Fuglsang, A.T.; Palmgren, M.G.; Schumaker, K.S.; et al. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell. 2010, 22, 1313–1332. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Ma, L.; Yang, Z.; Dong, Q.; Li, Q.; Ni, X.; Kudla, J.; Song, C.; Guo, Y. The Ca2+ Sensor SCaBP3/CBL7 Modulates Plasma Membrane H+-ATPase Activity and Promotes Alkali Tolerance in Arabidopsis. Plant Cell. 2019, 31, 1367–1384. [Google Scholar] [CrossRef]
- Zhou, L.J.; Zhang, C.L.; Zhang, R.F.; Wang, G.L.; Li, Y.Y.; Hao, Y.J. The SUMO E3 Ligase MdSIZ1 Targets MdbHLH104 to Regulate Plasma Membrane H+-ATPase Activity and Iron Homeostasis. Plant Physiol. 2019, 179, 88–106. [Google Scholar] [CrossRef]
- Møller, J.V.; Juul, B.; leMaire, M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta 1996, 1286, 1–51. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Axelsen, K.B. Evolution of P-type ATPases. Biochim. Biophys. Acta 1998, 1365, 37–45. [Google Scholar] [CrossRef][Green Version]
- Pedersen, P.; Carafoli, E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 1987, 4, 146–150. [Google Scholar] [CrossRef]
- Serrano, R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim. Biophys. Acta 1989, 947, 1–28. [Google Scholar] [CrossRef]
- Axelsen, K.B.; Palmgren, M.G. Evolution of substrate specificities in the P-Type ATPase superfamily. J. Mol. Evol. 1998, 46, 84–101. [Google Scholar] [CrossRef]
- Pedersen, C.N.; Axelsen, K.B.; Harper, J.F.; Palmgren, M.G. Evolution of plant P-type ATPases. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef]
- Baxter, I.; Tchieu, J.; Sussman, M.R.; Boutry, M.; Palmgren, M.G.; Gribskov, M.; Harper, J.F.; Axelsen, K.B. Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 2003, 132, 618–628. [Google Scholar] [CrossRef]
- Axelsen, K.B.; Palmgren, M.G. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 2001, 126, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Nissen, P. P-type ATPases. Annu. Rev. Biophys. 2011, 40, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, C.; Zlatic, S.A.; Wallin, M.; Vrailas-Mortimer, A.; Fahrni, C.J.; Faundez, V. Trafficking mechanisms of P-type ATPase copper transporters. Curr. Opin. Cell Biol. 2019, 59, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Verweij, W.; Spelt, C.; Di Sansebastiano, G.P.; Vermeer, J.; Reale, L.; Ferranti, F.; Koes, R.; Quattrocchio, F. An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat. Cell Boil. 2008, 10, 1456. [Google Scholar] [CrossRef]
- Faraco, M.; Spelt, C.; Bliek, M.; Verweij, W.; Hoshino, A.; Espen, L.; Prinsi, B.; Jaarsma, R.; Tarhan, E.; de Boer, A.H.; et al. Hyperacidification of vacuoles by the combined action of two different P-ATPases in the tonoplast determines flower color. Cell Rep. 2014, 6, 32–43. [Google Scholar] [CrossRef]
- Ma, B.; Liao, L.; Fang, T.; Peng, Q.; Ogutu, C.; Zhou, H.; Ma, F.; Han, Y. A Ma10 gene encoding P-type ATPase is involved in fruit organic acid accumulation in apple. Plant Biotechnol. J. 2019, 17, 674–686. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 2004, 5, 282–295. [Google Scholar] [CrossRef]
- Rensing, S.A. Gene duplication as a driver of plant morphogenetic evolution. Curr. Opin. Plant Biol. 2014, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of gene duplication in plant. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becke, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Wolfe, K.H. Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell 2004, 16, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stat. 1950, 347, 357–359. [Google Scholar]
- Chagné, D.; Crowhurst, R.N.; Pindo, M.; Thrimawithana, A.; Deng, C.; Ireland, H.; Fiers, M.; Dzierzon, H.; Cestaro, A.; Fontana, P.; et al. The Draft Genome Sequence of European Pear (Pyrus communis L. ‘Bartlett’). PLoS ONE 2014, 9, 92644. [Google Scholar] [CrossRef]
- Verde, I.; Jenkins, J.; Dondini, L.; Micali, S.; Pagliarani, G.; Vendramin, E.; Paris, R.; Aramini, V.; Gazza, L.; Rossini, L.; et al. The Peach v2.0 release: High-resolution linkage mapping and deep resequencing improve chromosome-scale assembly and contiguity. BMC Genom. 2017, 18, 225. [Google Scholar] [CrossRef]
- VanBuren, R.; Bryant, D.; Bushakra, J.M.; Vining, K.J.; Edger, P.P.; Rowley, E.R.; Priest, H.D.; Michael, T.P.; Lyons, E.; Filichkin, S.A.; et al. The genome of black raspberry (Rubus occidentalis). Plant J. 2016, 87, 535–547. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: A program package for phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol. Boil. Evol. 1998, 15, 568–573. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ma, B.Q.; Liao, L.; Zheng, H.Y.; Chen, J.; Wu, B.H.; Ogutu, C.; Li, S.H.; Korban, S.S.; Han, Y.P. Genes encoding aluminum-activated malate transporter II and their association with fruit acidity in apple. Plant Genome 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Lou, Q.; Liu, Y.; Qi, Y.; Jiao, S.; Tian, F.; Jiang, L.; Wang, Y. Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower colour in grape hyacinth. J. Exp. Bot. 2014, 65, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef]
- Taylor, J.S.; Raes, J. Duplication and divergence: The evolution of new genes and old ideas. Annu. Rev. Genet. 2004, 38, 615–643. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Ma, B.Q.; Owiti, A.; Korban, S.S.; Han, Y.P. Divergent evolutionary pattern of sugar transporter genes is associated with the difference in sugar accumulation between grasses and eudicots. Sci. Rep. 2016, 6, 29153. [Google Scholar] [CrossRef] [PubMed]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant. 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zheng, D.; Vimolmangkang, S.; Khan, M.A.; Beever, J.E.; Korban, S.S. Integration of physical and genetic maps in apple confirms whole-genome and segmental duplications in the apple genome. J. Exp. Bot. 2011, 62, 5117–5130. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.; Filatov, V.; Herzyk, P.; Krijger, G.C.; Axelsen, K.B.; Chen, S.; Green, B.J.; Li, Y.; Madagan, K.L.; Sánchez-Fernández, R.; et al. Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J. 2003, 35, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Yang, Y.; Yang, L.; Liu, H.; Wang, C.T.; Yu, M.M.; Gao, X.S.; Zhang, H.X. Poplar calcineurin B-like proteins PtCBL10A and PtCBL10B regulate shoot salt tolerance through interaction with PtSOS2 in the vacuolar membrane. Plant Cell Environ. 2014, 37, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Cha-Um, S.; Supaibulwattana, K.; Kirdmanee, C. Comparative effects of salt stress and extreme pH stress combined on glycinebetaine accumulation, photosynthetic abilities and growth characters of two rice genotypes. Rice Sci. 2009, 16, 274–282. [Google Scholar] [CrossRef]
- Liu, J.; Guo, W.Q.; Shi, D.C. Seed germination, seedling survival, and physiological response of sunflowers under saline and alkaline conditions. Photosynthetica 2010, 48, 278–286. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, P.; Ma, Y.; Geng, S.; Wang, S.; Shi, D.C. Physiological strategies of sunflower exposed to salt or alkali stresses: Restriction of ion transport in the cotyledon node zone and solute accumulation. Agron. J. 2015, 107, 2181. [Google Scholar] [CrossRef]
- Babgohari, M.Z.; Niazi, A.; Moghadam, A.A.; Deihimi, T.; Ebrahimie, E. Genome-wide analysis of key salinity-tolerance transporter (HKT1;5) in wheat and wild wheat relatives (A and D genomes). Vitro Celluar Dev. Biol. Plant 2013, 49, 97–106. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, Y.; Yoon, H.; Hwang, I.; Chang, Y.S. Iron nanoparticle-induced activation of plasma membrane H (+)-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 2015, 49, 1113–1119. [Google Scholar] [CrossRef]
- Ohwaki, Y.; Sugahara, K. Active extrusion of protons and exudation of carboxylic acids in response to iron deficiency by roots of chickpea (Cicer arietinum L.). Plant Soil 1997, 189, 49–55. [Google Scholar] [CrossRef]
- Wang, Y.; Noguchi, K.; Ono, N.; Inoue, S.I.; Terashima, I.; Kinoshita, T. Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl. Acad. Sci. USA 2014, 111, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.P.; Buch-Pedersen, M.J.; Morth, J.P.; Palmgren, M.G.; Nissen, P. Crystal structure of the plasma membrane proton pump. Nature 2007, 450, 1111. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, E.; Di Mambro, R.; Dello Ioio, R.; Costantino, P.; Sabatini, S. Acidic cell elongation drives cell differentiation in the Arabidopsis root. EMBO J. 2018, 37, 99134. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | Animo Acids | MW (kDa) | pI | GRAVY | Instability Index | Aliphatic Index | TMD |
|---|---|---|---|---|---|---|---|
| MD17G1155800 | 950 | 104.38 | 5.37 | 0.15 | 38.08 | 106.37 | 8 |
| MD09G1171300 | 948 | 104.45 | 5.50 | 0.15 | 36.68 | 107.10 | 9 |
| MD15G1108400 | 1032 | 113.75 | 6.86 | 0.02 | 40.21 | 100.40 | 8 |
| MD08G1130200 | 954 | 105.03 | 7.24 | 0.04 | 34.55 | 101.45 | 10 |
| MD13G1247200 | 955 | 105.21 | 6.51 | 0.09 | 36.47 | 101.27 | 8 |
| MD06G1186400 | 956 | 105.26 | 5.99 | 0.12 | 38.33 | 105.33 | 8 |
| MD16G1254200 | 955 | 105.01 | 6.13 | 0.09 | 36.11 | 103.11 | 9 |
| MD14G1192600 | 956 | 105.18 | 6.10 | 0.11 | 38.51 | 104.62 | 8 |
| MD02G1011600 | 947 | 103.68 | 6.05 | 0.09 | 34.82 | 101.44 | 9 |
| MD09G1223600 | 954 | 104.81 | 6.17 | 0.07 | 32.68 | 103.14 | 9 |
| MD17G1046600 | 956 | 105.31 | 6.45 | 0.12 | 35.64 | 104.01 | 8 |
| MD02G1216000 | 991 | 108.87 | 7.06 | 0.08 | 34.10 | 101.40 | 8 |
| MD04G1058300 | 973 | 106.86 | 6.25 | 0.17 | 35.45 | 107.07 | 9 |
| MD03G1197800 | 966 | 105.84 | 6.17 | 0.19 | 34.59 | 107.25 | 7 |
| MD17G1220200 | 930 | 102.12 | 8.64 | 0.07 | 33.80 | 103.84 | 8 |
| Clades | Null Hypothesis | Alternative Hypothesis | LRT | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | −In L | ω | −In L | ω1 | ω2 | Statistic | p |
| IId | IIe | 6436.65 | 0.14 | 6435.15 | 0.15 | 0.12 | 3.00 | >0.05 |
| IIc | (IId+IIe) | 8149.43 | 0.14 | 8148.49 | 0.17 | 0.14 | 1.88 | >0.05 |
| IIb | (IIc+IId+IIe) | 10,632.66 | 0.13 | 10,624.97 | 0.15 | 0.09 | 15.38 | <0.01 |
| IIa | (IIb+IIc+IId+IIe) | 10,466.18 | 0.13 | 10,458.89 | 0.15 | 0.10 | 14.58 | <0.01 |
| I | (IIa+IIb+IIc+IId+IIe) | 10,176.17 | 0.18 | 10,145.07 | 0.51 | 0.15 | 62.20 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Gao, M.; Zhang, L.; Zhao, H.; Zhu, L.; Su, J.; Li, C.; Li, M.; Ma, F.; Yuan, Y. Genome-Wide Identification and Characterization of Apple P3A-Type ATPase Genes, with Implications for Alkaline Stress Responses. Forests 2020, 11, 292. https://doi.org/10.3390/f11030292
Ma B, Gao M, Zhang L, Zhao H, Zhu L, Su J, Li C, Li M, Ma F, Yuan Y. Genome-Wide Identification and Characterization of Apple P3A-Type ATPase Genes, with Implications for Alkaline Stress Responses. Forests. 2020; 11(3):292. https://doi.org/10.3390/f11030292
Chicago/Turabian StyleMa, Baiquan, Meng Gao, Lihua Zhang, Haiyan Zhao, Lingcheng Zhu, Jing Su, Cuiying Li, Mingjun Li, Fengwang Ma, and Yangyang Yuan. 2020. "Genome-Wide Identification and Characterization of Apple P3A-Type ATPase Genes, with Implications for Alkaline Stress Responses" Forests 11, no. 3: 292. https://doi.org/10.3390/f11030292
APA StyleMa, B., Gao, M., Zhang, L., Zhao, H., Zhu, L., Su, J., Li, C., Li, M., Ma, F., & Yuan, Y. (2020). Genome-Wide Identification and Characterization of Apple P3A-Type ATPase Genes, with Implications for Alkaline Stress Responses. Forests, 11(3), 292. https://doi.org/10.3390/f11030292






