Effects of One-Year Simulated Nitrogen and Acid Deposition on Soil Respiration in a Subtropical Plantation in China
Abstract
1. Introduction
2. Materials and Methods
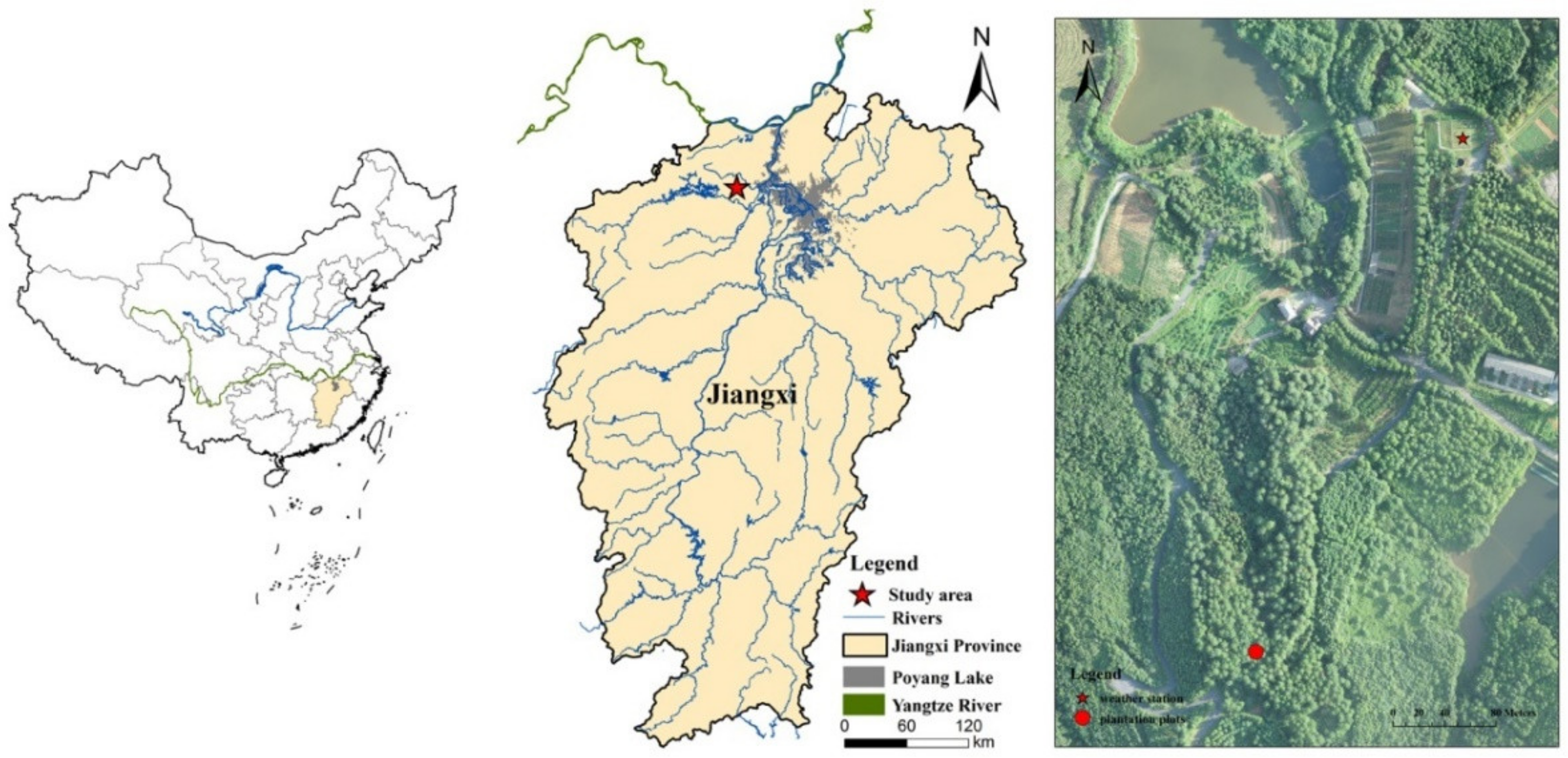
2.1. Site Description
2.2. Experimental Design
2.3. Soil Respiration Measurement
2.4. Soil Sample Collection and Determination
2.5. Statistical Analysis
3. Results
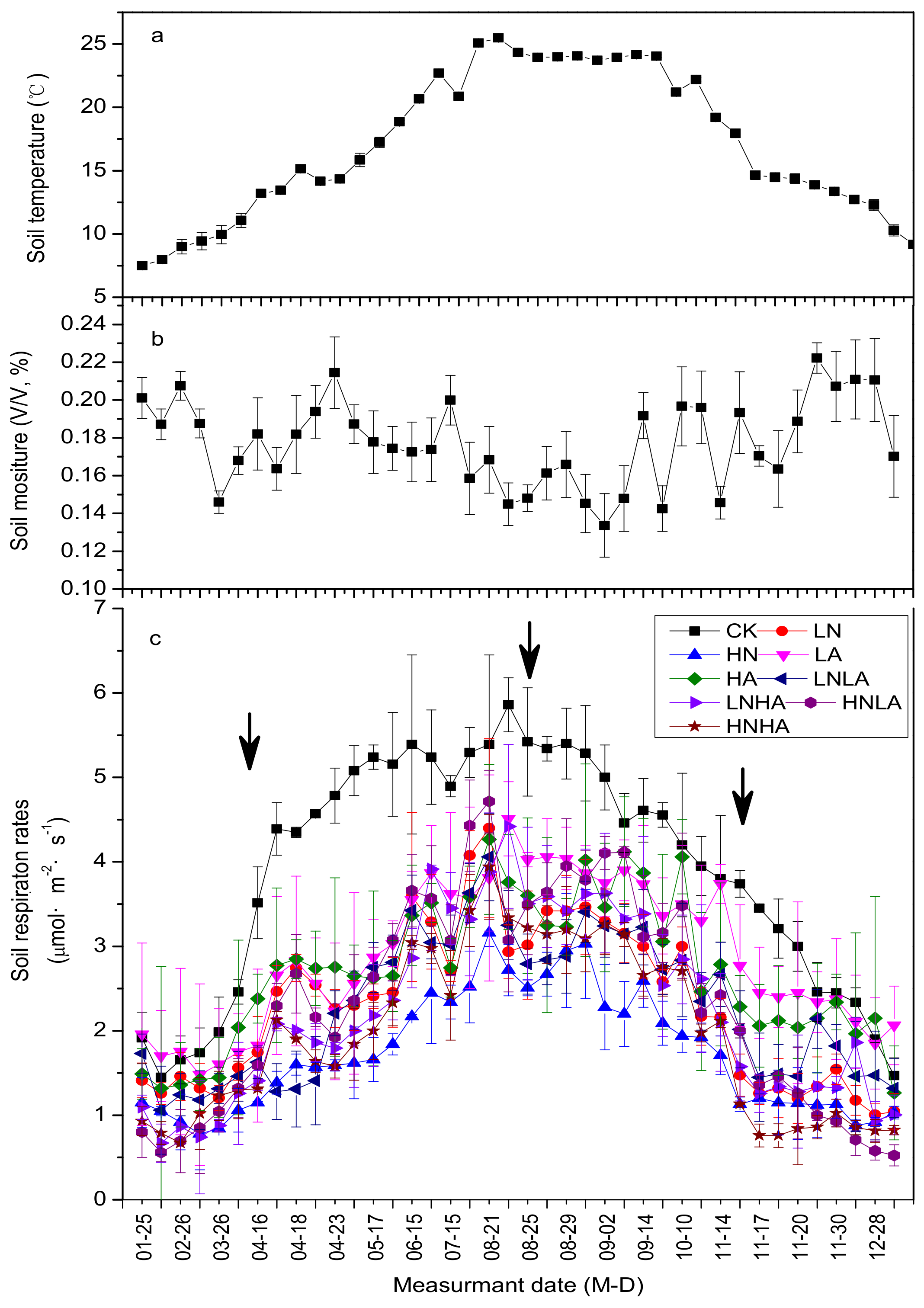
3.1. N and Acid Addition Effects on Rs Dynamics and Average Annual Efflux
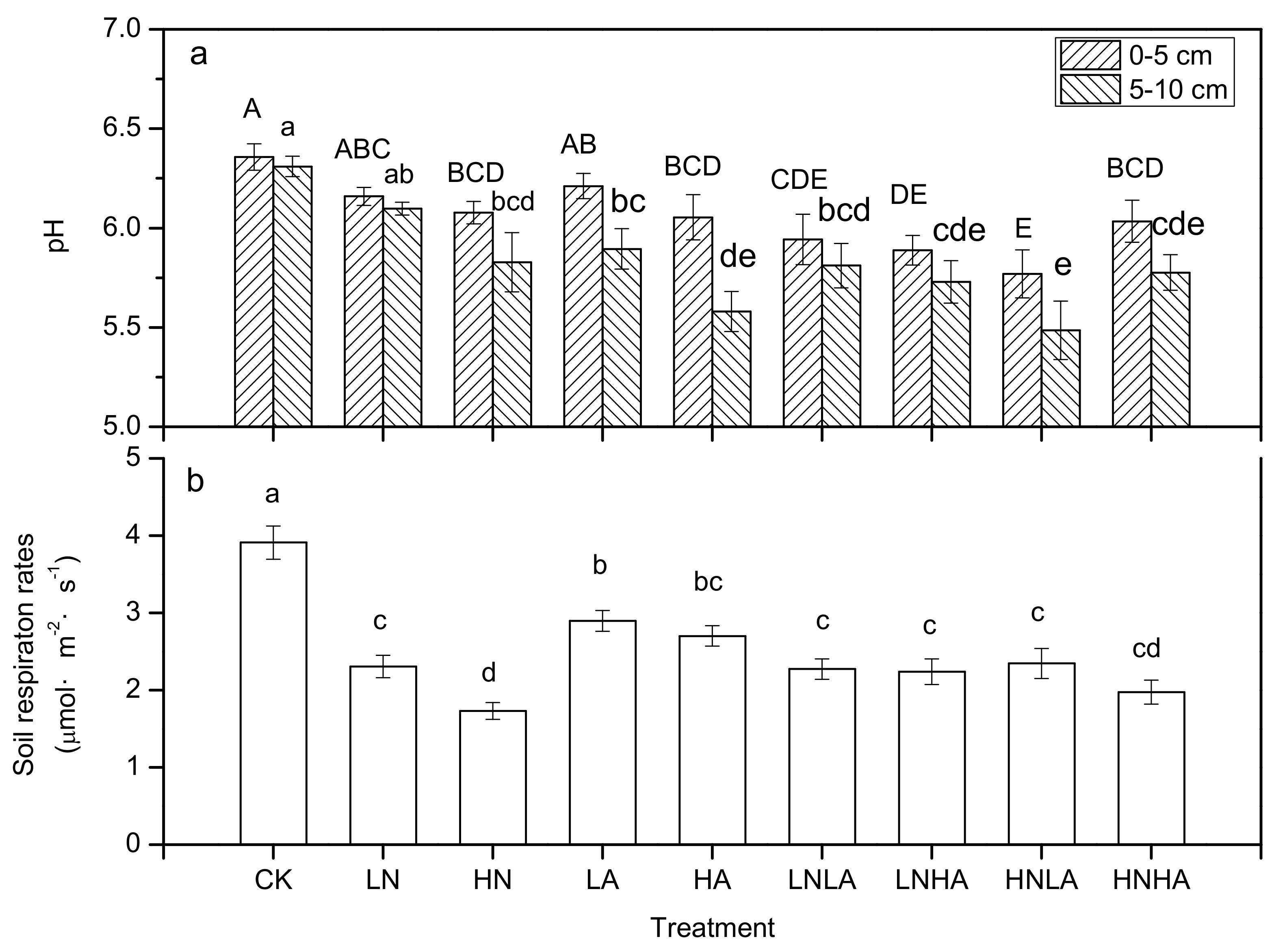
3.2. N and acid Addition Effects on Average Annual Efflux
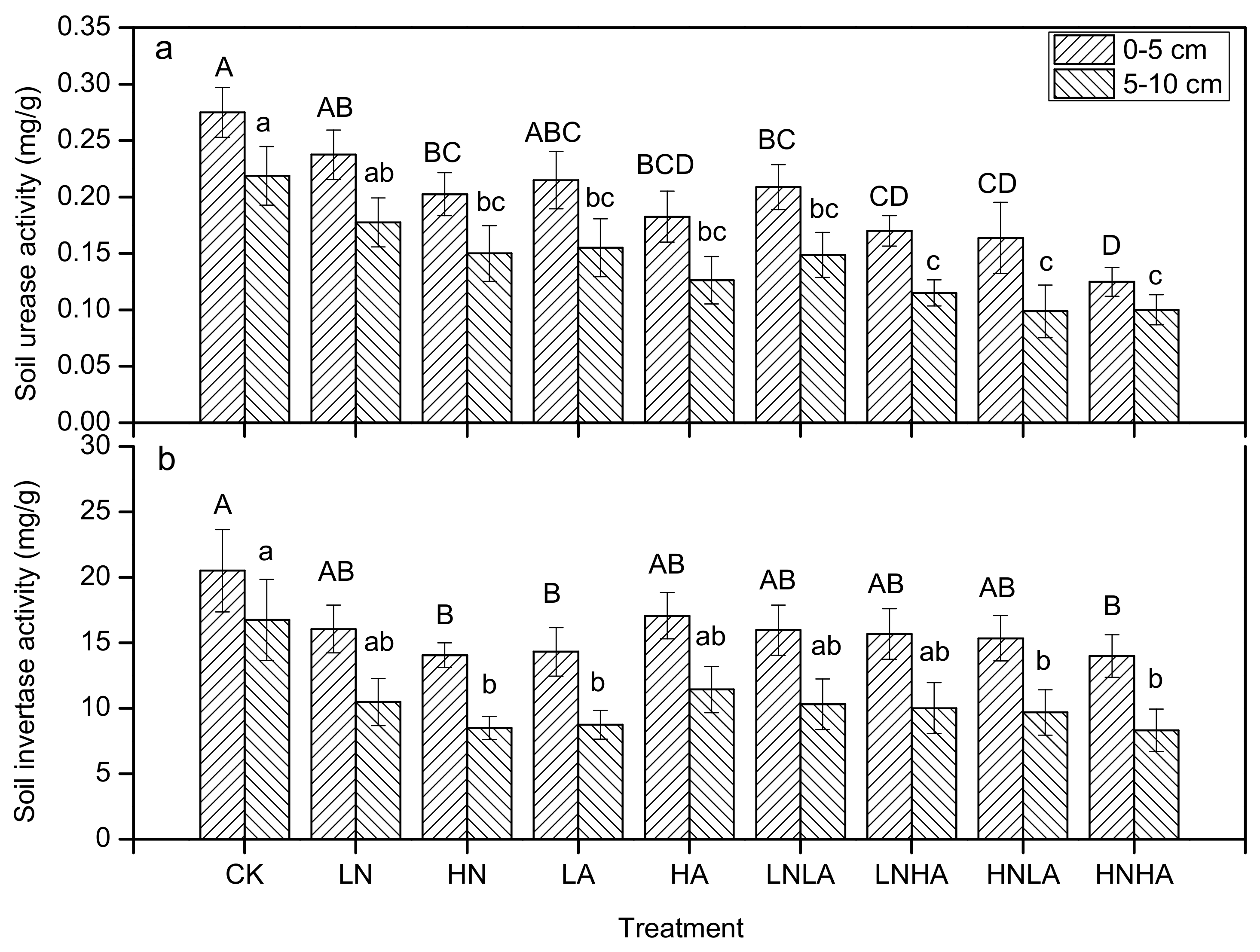
3.3. N and Acid Addition Effects on Soil Enzyme Activity and Soil Properties
3.4. Relationships Between Rs and Soil Properties
4. Discussion
4.1. Soil Respiration Response to N Addition
4.2. Effect of Acid Treatment on Soil Respiration
4.3. Interaction Between N Addition and Acid Addition on Soil Respiration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duan, L.; Yu, Q.; Zhang, Q.; Wang, Z.; Pan, Y.; Larssen, T.; Mulder, J. Acid deposition in Asia: Emissions, deposition, and ecosystem effects. Atmos. Environ. 2016, 146, 55–69. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Yu, H.L.; He, N.P.; Wang, Q.; Zhu, J.X.; Gao, Y.; Zhang, Y.; Jia, Y.; Yu, G. Development of atmospheric acid deposition in China from the 1990s to the 2010s. Environ. Pollut. 2017, 231, 182–190. [Google Scholar] [CrossRef]
- Gu, B.; Ju, X.; Chang, J.; Ge, Y.; Vitousek, P.M. Integrated reactive nitrogen budgets and future trends in China. PNAS 2015, 112, 8792–8797. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Huang, M.; Zhang, Y. Modeling the temporal-spatial patterns of atmospheric nitrogen deposition in China during 1961−2010. Acta Ecol. Sin. 2016, 36, 3591–3600, (Chinese with English abstract). [Google Scholar]
- Du, E.; De Vries, W.; Han, W. Imbalanced phosphorus and nitrogen deposition in China’s forests. Atmos. Chem. Phys. 2016, 16, 8571–8579. [Google Scholar] [CrossRef]
- Shen, J. Atmospheric dry and wet nitrogen deposition on three contrasting land use types of an agricultural catchment in subtropical central China. Atmos. Environ. 2013, 67, 415–424. [Google Scholar] [CrossRef]
- Jia, J.; Gao, Y. Acid deposition and assessment of its critical load for the environmental health of waterbodies in a subtropical watershed, China. J. Hydrol. 2017, 555, 155–168. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Xing, J.; Larssen, T.; Nielsen, C.P.; Hao, J.M. Soil Acidification in China: Is controlling SO2 emissions enough. Environ. Sci. Technol. 2009, 43, 8021–8026. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Mo, J.M.; Zhang, W.; Zhu, W.X. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Liang, G.H.; Wu, J.P.; Xiong, X.; Wu, X.Y.; Chu, G.W.; Zhou, G.Y.; Zeng, R.S.; Zhang, D.Q. Response of soil respiration to simulated acid rain in three successional subtropical forests in Southern China. Chin. J. Eco-Agric. 2016, 35, 125–134. [Google Scholar]
- Tu, L.H.; Hu, T.X.; Zhang, J. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Zheng, S.; Bian, H.; Quan, Q.; Xu, L.; Chen, Z.; He, N. Effect of nitrogen and acid deposition on soil respiration in a temperate forest in China. Geoderma 2018, 329, 82–90. [Google Scholar] [CrossRef]
- Luo, Y. Terrestrail carbon-cycle feedback to climate warming. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 683–712. [Google Scholar] [CrossRef]
- Metcalfe, D.; Fisher, R.; Wardle, D. Plant communities as drivers of soil respiration: Pathways, mechanisms, and significance for global change. Biogeosciences 2011, 8, 2047–2061. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, C.; Wang, F.; Zhao, G.; Pu, G.; Ma, X.; Tian, X. Effects of nitrogen addition on litter decomposition, soil microbial biomass, and enzyme activities between leguminous and non-leguminous forests. Ecol. Res. 2013, 28, 793–800. [Google Scholar] [CrossRef]
- Peng, Q.; Dong, Y.S.; Qi, Y.C.; Xiao, S.S.; He, Y.T.; Ma, T. Effects of nitrogen fertilization on soil respiration in temperate grassland in Inner Mongolia, China. Environ. Earth Sci. 2011, 62, 1163–1171. [Google Scholar] [CrossRef]
- Sun, H.F.; Zhu, J.G.; Xie, Z.B.; Liu, G.; Tang, H.Y. Effect of atmospheric CO2 enrichment on soil respiration in winter wheat growing seasons of a rice-wheat rotation system. Pedosphere 2013, 23, 752–766. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.A.; Li, R.H.; Dai, H.Z.; Luo, S.H. Short-term simulated nitrogen deposition increases carbon sequestration in a Pleioblastus amarus plantation. Plant Soil 2011, 340, 383–396. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Zhang, B.; Lu, M.; Luo, Y.; Liu, L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2332–2343. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Shao, J.; Nie, Y.; He, Y.; Jiang, L.; Bai, S.H. Interactive effects of global change factors on soil respiration and its components: A meta-analysis. Glob. Chang. Biol. 2016, 22, 3157–3169. [Google Scholar] [CrossRef]
- Chen, D.M.; Wang, Y.; Lan, Z.C.; Li, J.J.; Xing, W.; Hu, S.J.; Bai, Y.F. Biotic community shifts explain the contrasting responses of microbial and root respiration to experimental soil acidification. Soil Biol. Biochem. 2015, 90, 139–147. [Google Scholar] [CrossRef]
- Devaraju, N.; Bala, G.; Caldeira, K.; Nemani, R. A model based investigation of the relative importance of CO2-fertilization, climate warming, nitrogen deposition and land use change on the global terrestrial carbon uptake in the historical period. Clim. Dyn. 2016, 47, 173–190. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol. Biochem. 2010, 42, 2336–2338. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Chang. Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, L.; Mathew, R.; Stokes, T.; Feng, Y.C.; Aubrey, D.; Coleman, M. Soil and microbial respiration in a loblolly pine plantation in response to seven years of irrigation and fertilization. For. Ecol. Manag. 2009, 258, 2431–2438. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. The effects of nitrogen enrichment on soil CO2 fluxes depending on temperature and soil properties. Glob. Ecol. Biogeogr. 2016, 25, 475–488. [Google Scholar] [CrossRef]
- Bowden, R.D.; Davidson, E.; Savage, K.; Arabia, C.; Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For. Ecol. Manag. 2004, 196, 43–56. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Z.H.; Yu, J.B.; Wu, L.X.; Han, G.X.; Yang, L.Q.; Xing, Q.H.; Xie, B.H.; Mao, P.L.; Wang, G.M. Effect of nitrogen deposition on soil respiration in Phragmites australis wetland in the Yellow River Delta, China. J. Plant Ecol. 2013, 37, 517–529. [Google Scholar] [CrossRef]
- Fangueiro, D.; Surgy, S.; Coutinho, J. Impact of cattle slurry acidification on carbon and nitrogen dynamics during storage and after soil incorporation. J. Plant Nutr. Soil Sci. 2013, 176, 540–550. [Google Scholar] [CrossRef]
- Liang, G.H.; Liu, X.Z.; Chen, X.M. Response of soil respiration to acid rain in forests of different maturity in Southern China. PLoS ONE 2013, 8, e62207. [Google Scholar] [CrossRef] [PubMed]
- Oulehle, F.; Evans, C.D.; Hofmeister, J.; Krejci, R.; Tahovska, K.; Persson, T.; Cudlin, P.; Hruska, J. Major changes in forest carbon and nitrogen cycling caused by declining sulphur deposition. Glob. Chang. Biol. 2011, 17, 3115–3129. [Google Scholar] [CrossRef]
- Su, Y.G.; Huang, G.; Lin, Y.J.; Zhang, Y.M. No synergistic effects of water and nitrogen addition on soil microbial communities and soil respiration in a temperate desert. Catena 2016, 142, 126–133. [Google Scholar] [CrossRef]
- Vanhala, P.; Fritze, H.; Neuvonen, S. Prolonged simulated acid rain treatment in the subarctic: Effect on the soil respiration rate and microbial biomass. Biol. Fertil. Soils 1996, 23, 7–14. [Google Scholar] [CrossRef]
- Hutchinson, T.C.; Watmough, S.A.; Sager, E.P.S. The impact of simulated acid rain and fertilizer application on a mature sugar maple (Acer saccharum Marsh) forest in central Ontario, Canada. Water Air Soil Pollut. 1999, 109, 17–39. [Google Scholar] [CrossRef]
- Sitaula, B.K.; Bakken, L.R.; Abrahamsen, G. N-fertilization and soil acidification effects on N2O and CO2 emission from temperate pine forest soil. Soil Biol. Biochem. 1995, 27, 1401–1408. [Google Scholar] [CrossRef]
- Chen, D.M.; Li, J.J.; Lan, Z.C.; Hu, S.J.; Bai, Y.F. Soil acidification exerts a greater control on soil respiration than soil nitrogen availability in grasslands subjected to long-term nitrogen enrichment. Funct. Ecol. 2016, 30, 658–669. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Tian, D.S.; Wang, J.S.; Ha, D.L.; Qu, Y.X.; Jing, G.W.; Niu, S.L. Soil acid cations induced reduction in soil respiration under nitrogen enrichment and soil acidification. Sci. Total Environ. 2018, 615, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Townsend, A.R.; Schmidt, S.K. Phosphorus limitation of microbial processes in moist tropical forests: Evidence from short-term laboratory incubations and field studies. Ecosystems 2002, 5, 680–691. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl. Acad. Sci. USA 2006, 103, 10316–10321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.S.; Wang, J.; Shi, Z. Responses of Soil Respiration and Its Main Components to Nitrogen Addition in a Subtropical Pinuselliottii Plantation. Res. Environ. Sci. 2018, 31, 1105–1113, (Chinese with English abstract). [Google Scholar]
- Lü, C.; Tian, H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res. Atmos. 2007, 112, 10–15. [Google Scholar] [CrossRef]
- Yu, H.L.; He, N.P.; Wang, Q.F.; Zhu, J.X.; Xu, L.; Zhu, Z.L.; Yu, G.R. Wet acid deposition in Chinese natural and agricultural ecosystems: Evidence from nationalscale monitoring. J. Geophys. Res. Atmos. 2016, 121, 10995–11005. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yang, J.; Hu, J.M.; Tang, C.J.; Zheng, H.J. Characteristics of the surface-subsurface flow generation and sediment yield to the rainfall regime and land-cover by long-term in-situ observation in the red soil region, Southern China. J. Hydrol. 2016, 539, 457–467. [Google Scholar] [CrossRef]
- Voroney, R.P.; Brookes, P.C.; Beyaert, R.P. Soil microbial biomass C, N, P, and S. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRS Press: Boca Raton, FL, USA, 2008; pp. 637–652. [Google Scholar]
- Sinsabaugh, R.L.; Carreiro, M.M.; Repert, D.A. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 2002, 60, 1–24. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W. Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest. Plant Soil 2014, 379, 361–371. [Google Scholar] [CrossRef]
- Du, Y.; Han, H.; Wang, Y.; Zhong, M.; Hui, D.; Niu, S.; Wan, S. Plant functional groups regulate soil respiration responses to nitrogen addition and mowing over a decade. Funct. Ecol. 2018, 32, 1117–1127. [Google Scholar] [CrossRef]
- Wu, J.P.; Liu, W.F.; Fan, H.B. A synchronous responses of soil microbial community and under story plant community to simulated nitrogen deposition in a subtropical forest. Ecol. Evol. 2013, 3, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, L.; Hu, S. Decoupling of soil microbes and plants with increasing anthropogenic nitrogen inputs in a temperate steppe. Soil Biol. Biochem. 2014, 72, 116–122. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Persson, H.; Ahlstom, K.; Clemensson-Lindell, A. Nitrogen addition and removal at nitrogen effects on fine-root growth and fine-root chemistry. For. Ecol. Manag. 1998, 101, 199–205. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, Ĺ.H.J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, M. Acid rain and its ecological consequences. J. Environ. Biol. 2008, 29, 15–24. [Google Scholar]
- Baath, E.; Lundgren, B.; Soderstrom, B. Effects of artificial acid rain on microbial activity and biomass. Bull. Environ. Contam. Toxicol. 1979, 23, 737–740. [Google Scholar] [CrossRef]
- Kim, H.; Kang, H. The impacts of excessive nitrogen additions on enzyme activities and nutrient leaching in two contrasting forest soils. J. Microbiol. 2011, 49, 369–375. [Google Scholar] [CrossRef]
- Kotas, P.; Choma, M.; Santruckova, H.; Leps, J.; Triska, J.; Kastovska, E. Linking above and belowground responses to 16 years of fertilization, mowing, and removal of the dominant species in a temperate grassland. Ecosystems 2017, 20, 354–367. [Google Scholar] [CrossRef]
- Wang, Y.S.; Cheng, S.L.; Yu, G.R.; Fang, H.J.; Mo, J.M.; Xu, M.J.; Gao, W.L. Response of carbon utilization and enzymatic activities to nitrogen deposition in three forests of subtropical China. Can. J. For. Res. 2015, 45, 394–401. [Google Scholar] [CrossRef]
- Yang, X.H.; Nie, C.R.; Mai, Z.M. Effect of acid rain on the main microbial strains of peanut soil in South China. J. Peanut Sci. 2004, 33, 26–29. [Google Scholar]
- Bergkvist, B.; Folkeson, L. Soil acidification and element fluxes of a Fagus sylvatica forest as influenced by simulated nitrogen deposition. Water Air Soil Pollut. 1992, 65, 111–133. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Jiang, H.; Li, Y.H.; Yuan, H.Y. Short-term effects of acid rain on soil respiration in broadleaf tree sapling-soil systems in bubtropical China. Acta Pedol. Sin. 2011, 48, 563–569. [Google Scholar]
- Wang, J.S.; Li, Y.; Quan, Q. Nitrogen addition reduces soil respiration but increases the relative contribution of heterotrophic component in an alpine meadow. Funct. Ecol. 2019. [Google Scholar] [CrossRef]
| Indexes | Determination Method | References |
|---|---|---|
| pH | Using a pH meter (Orion, Thermo Fisher Scientific Inc., Beverly, MA, USA) at a 1-to-5 ratio of soil-to-deionized water | - |
| TN, NO3−, NH4+ | Soil samples were extracted using a 2 mol L−1 KCl solution first (at 1-to-5 ratio of soil-to-KCl solution) and then measured by a continuous flow analyzer (AA3 Bran + Luebbe, Germany) | - |
| TOC | potassium dichromate oxidation method | - |
| DOC | Water extraction first (at a 1-to-10 ratio of soil-to-deionized water), filtered through a membrane filter with 0.45 µm pores, followed by determination via aa TOC analyzer (Vario TOC Cube, Elementar, Germany) | [48] |
| DON | The difference between extractable N and available N (NO3− + NH4+) | - |
| MBC, MBN | was determined by the chloroform fumigation-extraction method within a week after sampling. A 0.5 mol L−1 K2SO4 solution was used to extract C and N from fumigated and non-fumigated samples at a 1:10 (w/v) ratio, followed by determination via a TOC analyzer (Vario TOC Cube, Elementar, Germany) | [48] |
| soil invertase activity | Sodium phenol colorimetry | [49] |
| soil urease activity | 3,5-dinitrosalicylic acid colorimetry | [49] |
| Rs | 0–5 cm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Soil Urease Activity | Soil Invertase Activity | TOC | NH4+ | NO3− | DOC | DON | MBC | MBN | ||
| N | 0.000 ** | 0.003 ** | 0.004 ** | 0.209 | 0.373 | 0.898 | 0.055 | 0.999 | 0.260 | 0.849 | 0.756 |
| A | 0.031 * | 0.005 ** | 0.000 ** | 0.545 | 0.427 | 0.496 | 0.914 | 0.999 | 0.980 | 0.146 | 0.978 |
| N*A | 0.000 ** | 0.206 | 0.962 | 0.381 | 0.509 | 0.840 | 0.824 | 0.917 | 0.976 | 0.183 | 0.244 |
| 5–10 cm | |||||||||||
| pH | Soil Urease Activity | Soil Invertase Activity | TOC | NH4+ | NO3− | DOC | DON | MBC | MBN | ||
| N | 0.022 * | 0.018 * | 0.170 | 0.448 | 0.504 | 0.067 | 0.986 | 0.833 | 0.322 | 0.858 | |
| A | 0.000 ** | 0.001 ** | 0.476 | 0.609 | 0.515 | 0.375 | 0.995 | 0.963 | 0.022 * | 0.151 | |
| N*A | 0.018 * | 0.793 | 0.342 | 0.103 | 1.000 | 0.362 | 0.951 | 0.967 | 0.335 | 0.506 | |
| Soil Layer | Parameter | Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CK | LN | HN | LA | HA | LNLA | LNHA | HNLA | HNHA | ||
| 0~5 cm | TOC (g kg−1) | 32.1 ± 5.0 a | 30.8 ± 3.4 a | 33.5 ± 3.0 a | 30.9 ± 1.6 a | 27.3 ± 3.1 a | 33.0 ± 3.9 a | 32.5 ± 4.3 a | 25.0 ± 1.2 a | 26.2 ± 2.9 a |
| NH4+ (mg kg−1) | 4.86 ± 0.66 a | 5.96 ± 2.02 a | 5.98 ± 0.87 a | 5.15 ± 1.58 a | 6.87 ± 1.47 a | 4.87 ± 0.61 a | 7.69 ± 2.66 a | 5.79 ± 1.24 a | 5.48 ± 1.32 a | |
| NO3− (mg kg−1) | 17.39 ± 5.99 a | 28.42 ± 6.99 a | 34.10 ± 5.75 a | 21.23 ± 4.19 a | 19.39 ± 4.82 a | 28.18 ± 9.91 a | 31.73 ± 6.26 a | 25.18 ± 5.11 a | 28.51 ± 5.55 a | |
| DOC (mg kg−1) | 174.01 ± 51.90 a | 196.29 ± 48.89 a | 225.33 ± 53.96 a | 215.26 ± 53.14 a | 209.29 ± 59.13 a | 190.63 ± 48.99 a | 214.02 ± 55.99 a | 194.08 ± 50.69 a | 175.50 ± 44.18 a | |
| DON (mg kg−1) | 14.92 ± 3.23 a | 25.17 ± 8.24 a | 21.75 ± 6.90 a | 17.12 ± 3.42 a | 17.79 ± 3.71 a | 25.37 ± 9.78 a | 24.43 ± 8.54 a | 16.17 ± 3.80 a | 18.14 ± 6.28 a | |
| MBC (mg kg−1) | 165.62 ± 29.21 ab | 153.57 ± 36.96 ab | 230.01 ± 33.81 a | 172.93 ± 29.22 ab | 147.54 ± 28.28 ab | 129.16 ± 29.27 b | 160.79 ± 32.44 ab | 115.13 ± 20.29 b | 114.65 ± 32.81 b | |
| MBN (mg kg−1) | 52.99 ± 6.14 a | 64.11 ± 18.39 a | 80.75 ± 10.29 a | 75.65 ± 11.61 a | 76.49 ± 8.79 a | 58.76 ± 9.83 a | 62.67 ± 7.57 a | 58.96 ± 5.95 a | 59.54 ± 14.07 a | |
| 5~10 cm | TOC (g kg−1) | 22.6 ± 3.2 a | 17.5 ± 1.8 ab | 15.7 ± 1.9 b | 16.8 ± 1.7 ab | 15.9 ± 2.7 b | 17.9 ± 1.4 ab | 19.9 ± 2.6 ab | 19.0 ± 1.3 ab | 14.9 ± 1.1 b |
| NH4+ (mg kg−1) | 5.14 ± 1.09 a | 7.45 ± 1.79 a | 6.73 ± 2.47 a | 6.32 ± 1.98 a | 7.42 ± 1.89 a | 8.16 ± 1.79 a | 9.71 ± 3.41 a | 7.91 ± 2.37 a | 8.69 ± 3.00 a | |
| NO3− (mg kg−1) | 11.80 ± 2.04 a | 13.97 ± 4.15 a | 11.41 ± 4.26 a | 6.83 ± 2.41 ab | 1.43 ± 0.49 b | 9.91 ± 3.62 ab | 15.23 ± 5.19 a | 11.51 ± 2.96 a | 9.76 ± 2.35 ab | |
| DOC (mg kg−1) | 183.51 ± 50.05 a | 152.49 ± 40.35 a | 157.21 ± 38.12 a | 164.21 ± 37.58 a | 150.70 ± 39.29 a | 152.81 ± 35.20 a | 176.57 ± 42.97 a | 175.09 ± 40.63 a | 157.25 ± 35.39 a | |
| DON (mg kg−1) | 13.79 ± 3.45 a | 13.24 ± 3.16 a | 13.11 ± 2.63 a | 12.36 ± 2.23 a | 11.04 ± 2.34 a | 12.34 ± 2.44 a | 13.13 ± 3.34 a | 13.93 ± 2.60 a | 14.25 ± 2.77 a | |
| MBC (mg kg−1) | 140.12 ± 26.95 a | 94.64 ± 23.72 ab | 98.16 ± 18.11 ab | 64.47 ± 10.19 b | 97.57 ± 19.53 ab | 54.43 ± 16.22 b | 90.46 ± 17.10 ab | 84.03 ± 21.94 b | 60.84 ± 9.10 b | |
| MBN (mg kg−1) | 54.17 ± 9.11 a | 43.04 ± 9.42 ab | 39.99 ± 6.84 ab | 37.89 ± 7.88 ab | 29.86 ± 3.06 b | 30.70 ± 5.74 b | 38.20 ± 7.86 ab | 40.20 ± 6.69 ab | 36.31 ± 7.70 ab | |
| Rs | T5 | SWC | 0–5 cm | 5–10 cm | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Soil Urease Activity | Soil Invertase Activity | TOC | NH4+ | NO3− | DOC | DON | MBC | MBC | pH | Soil Urease Activity | Soil Invertase Activity | TOC | NH4+ | NO3− | DOC | DON | MBC | MBN | |||||
| Rs | 1.000 | 0.778 ** | −0.206 ** | 0.157 | 0.746 ** | 0.800 * | −0.302 * | −0.393 ** | −0.590 * | −0.430 * | −0.289 * | −0.209 | −0.141 | −0.205 | 0.726 ** | 0.793 ** | −0.080 | −0.463 ** | −0.226 | −0.330 ** | −0.199 | −0.058 | 0.025 | |
| T5 | 1.000 | −0.247 ** | −0.031 | 0.657 ** | 0.777 ** | −0.356 ** | −0.487 ** | −0.545 ** | −0.612 ** | −0.330 ** | −0.312 ** | −0.081 | −0.321 ** | 0.671 ** | 0.752 ** | −0.188 | −0.526 ** | −0.314 ** | −0.597 ** | −0.402** | −0.208 | −0.110 | ||
| SWC | 1.000 | −0.243 * | −0.501 ** | −0.294 * | −0.072 | 0.172 | 0.110 | 0.115 | −0.013 | −0.003 | −0.204 | −0.095 | −0.488 ** | −0.282 * | 0.158 | 0.138 | 0.129 | 0.202 | 0.196 | 0.164 | 0.147 | |||
| 0–5 cm | pH | 1.000 | 0.174 | 0.007 | 0.306 ** | −0.010 | −0.002 | 0.058 | 0.176 | 0.260* | 0.289 * | 0.562 ** | 0.179 | −0.003 | 0.176 | −0.206 | 0.125 | −0.001 | 0.089 | 0.278 * | 0.219 | |||
| soil urease activity | 1.000 | 0.713 ** | −0.205 | −0.308 ** | −0.490 ** | −0.353 ** | −0.203 | −0.178 | −0.161 | 0.017 | 0.939 ** | 0.694 ** | −0.125 | −0.277 * | −0.246 * | −0.306 ** | −0.249 * | −0.057 | −0.120 | |||||
| soil invertase activity | 1.000 | −0.321 ** | −0.486 ** | −0.491 ** | −0.412 ** | −0.223 | −0.351 ** | −0.071 | −0.174 | 0.744 ** | 0.986 ** | −0.177 | −0.326 ** | −0.256* | −0.367 ** | −0.294 * | −0.172 | −0.030 | ||||||
| TOC | 1.000 | 0.139 | 0.488 ** | 0.191 | 0.295 * | 0.475 ** | 0.313 ** | 0.326 ** | −0.233 * | −0.331 ** | 0.56 ** | −0.121 | 0.314 ** | 0.071 | 0.124 | 0.242 * | 0.221 | |||||||
| NH4+ | 1.000 | 0.335 ** | 0.442 ** | 0.302 ** | 0.241 * | 0.002 | 0.139 | −0.351 ** | −0.450 ** | 0.130 | 0.529 ** | 0.377 ** | 0.484 ** | 0.371 ** | 0.310 ** | 0.072 | ||||||||
| NO3− | 1.000 | 0.495 ** | 0.481 ** | 0.402 ** | 0.372 ** | 0.141 | −0.498 ** | −0.490 ** | 0.301 * | 0.368 ** | 0.527 ** | 0.382 ** | 0.438 ** | 0.213 ** | 0.219 | |||||||||
| DOC | 1.000 | 0.581 ** | 0.512 ** | 0.128 | 0.227 | −0.319 ** | −0.379 ** | 0.005 | 0.537 ** | 0.342 ** | 0.901 ** | 0.763 ** | 0.431 ** | 0.165 | ||||||||||
| DON | 1.000 | 0.346 ** | 0.218 | 0.310 ** | −0.166 | −0.213 | 0.151 | 0.257 * | 0.522 ** | 0.483 ** | 0.549 ** | 0.295 * | 0.199 | |||||||||||
| MBC | 1.000 | 0.272 * | 0.334 ** | −0.198 | −0.343 ** | 0.116 | 0.089 | 0.162 | 0.370 ** | 0.378 ** | 0.489 ** | 0.212 | ||||||||||||
| MBN | 1.000 | 0.131 | −0.171 | −0.084 | 0.233 * | −0.071 | 0.256 * | −0.011 | 0.109 | 0.172 | 0.372 ** | |||||||||||||
| 5−10 cm | pH | 1.000 | 0.033 | −0.156 | 0.219 | −0.100 | 0.406 ** | 0.219 | 0.289 * | 0.398 ** | 0.383 ** | |||||||||||||
| soil urease activity | 1.000 | 0.721 ** | −0.168 | −0.271 * | −0.258 * | −0.289 * | −0.222 | −0.086 | −0.128 | |||||||||||||||
| soil invertase activity | 1.000 | −0.168 | −0.308 ** | −0.239 * | −0.334 ** | −0.293 ** | −0.161 | −0.021 | ||||||||||||||||
| TOC | 1.000 | −0.202 | 0.436 ** | 0.057 | 0.114 | 0.365 ** | 0.556 ** | |||||||||||||||||
| NH4+ | 1.000 | −0.027 | 0.595 ** | 0.332 ** | 0.012 | −0.219 | ||||||||||||||||||
| NO3− | 1.000 | 0.382 ** | 0.486 ** | 0.408 ** | 0.542 ** | |||||||||||||||||||
| DOC | 1.000 | 0.825 ** | 0.497 ** | 0.224 | ||||||||||||||||||||
| DON | 1.000 | 0.521 ** | 0.271 * | |||||||||||||||||||||
| MBC | 1.000 | 0.482 ** | ||||||||||||||||||||||
| MBN | 1.000 | |||||||||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Wang, G.G.; Tang, C.; Fang, H.; Duan, J.; Yu, X. Effects of One-Year Simulated Nitrogen and Acid Deposition on Soil Respiration in a Subtropical Plantation in China. Forests 2020, 11, 235. https://doi.org/10.3390/f11020235
Xiao S, Wang GG, Tang C, Fang H, Duan J, Yu X. Effects of One-Year Simulated Nitrogen and Acid Deposition on Soil Respiration in a Subtropical Plantation in China. Forests. 2020; 11(2):235. https://doi.org/10.3390/f11020235
Chicago/Turabian StyleXiao, Shengsheng, G. Geoff Wang, Chongjun Tang, Huanying Fang, Jian Duan, and Xiaofang Yu. 2020. "Effects of One-Year Simulated Nitrogen and Acid Deposition on Soil Respiration in a Subtropical Plantation in China" Forests 11, no. 2: 235. https://doi.org/10.3390/f11020235
APA StyleXiao, S., Wang, G. G., Tang, C., Fang, H., Duan, J., & Yu, X. (2020). Effects of One-Year Simulated Nitrogen and Acid Deposition on Soil Respiration in a Subtropical Plantation in China. Forests, 11(2), 235. https://doi.org/10.3390/f11020235




