Abstract
Fire is the predominant natural disturbance that influences the community structure as well as ecosystem function in forests. This study was conducted to examine the soil properties, loss of aboveground biomass, and understory plant community in response to an anthropogenic fire in a coniferous (Pinus massoniana Lamb.) and broadleaf (Quercus acutissima Carruth.) mixed forest in a subtropical–temperate climatic transition zone in Central China. The results showed that soil pH, NO3−-N concentration, and microbial biomass carbon (C) increased three months after the fire; however, there were no significant differences in soil organic C, total nitrogen (N), NH4+-N concentration, or microbial biomass N between the burned and unburned observed plots. The total aboveground biomass was 39.0% lower in the burned than unburned plots four weeks after fire. Direct biomass combustion (19.15 t ha−1, including understory shrubs and litters) was lower than dead wood biomass loss (23.69 t ha−1) caused by the fire. The declining trends of tree mortality with increasing diameter at breast height for both pine and oak trees suggest that small trees are more likely to die during and after fires due to the thinner bark of small trees and tree and branch fall. In addition, burning significantly stimulated the density of shrub (160.9%) and herb (88.0%), but it also affected the richness of shrub and herb compared with that in the unburned plots two months after the fire. The rapid recovery of understory plants after fires suggest that the diversity of understory species could benefit from low-severity fires. Our findings highlight that the decomposition of dead wood and understory community recovery should be considered for offsetting C emissions after fires for further research.
1. Introduction
Forest ecosystems are widely distributed across the land surface, contain two-thirds of all terrestrial plant species [1], and sequester approximately 12% of anthropogenic carbon (C) emissions [2]. Fire activity has been shifted by land use and change and population growth, and it is predicted to increase in forest ecosystems under future warmer and drier climate scenarios [3,4,5]. The widespread fire in forests can influence emissions of greenhouse gases and aerosols, vegetation dynamics, and global C budgets [6,7,8], with subsequent feedback to future climate change [9,10].
As the most critical threat across diverse forest types [7,11,12], fire has pervasive effects on the physical, chemical, and biological properties of soil [13,14,15]. During burning, plant biomass, litter layers, and soil organic matter are consumed, resulting in changes in soil acidity, water storage capacity, and nutrients concentration [13,16,17]. Fire can directly increase soil water repellency, leading to decreased permeability and stimulated runoff [18,19]. The total nutrient contents of soil are instantaneously reduced during the fire through losses from volatilization, smoke, ash transport, leaching, and erosion [20,21]. However, fire can elevate soil nutrient availability by ash deposition and enhanced mineralization in the short term [22,23,24]. Fire effects on soil properties depend on several factors including fire type, intensity, topography, and region, as well as fire weather conditions (e.g., temperature, precipitation, and drought) [13,15,25]. Changes in the soil physical and chemical properties induced by fire can alter soil microbial biomass and activity [15,26,27]. Fire directly influences the soil microbial community structure primarily due to heat-induced microbial mortality in the short term [28,29]. Fire may indirectly modify soil microbial communities by altering plant community composition and production in the long term [30].
Fire has substantial impacts on stand-scale C dynamics directly through converting plant biomass to atmospheric CO2 and indirectly by starting successional changes in the balance between deadwood decomposition and plant regeneration [31,32,33]. The emission of biomass C by combustion induced by fire ranges from 5% to 25% depending on ecosystem type and fire intensity [34,35]. Plant mortality induced by fire can decrease net primary productivity and create a large amount of plant debris [36]. Increased air temperature and elevated throughfall reaching the ground due to the removal of forest canopy can stimulate the decomposition of detritus, leading to consequent increases in soil C emissions [7,37]. However, the role of forest as a C source after fire would transform into a C sink as pioneer tree species and understory shrub grow vigorously in the long term [38].
Fire has been reported to affect the plant community structure and composition in forests [39,40,41,42,43]. Understory plant communities are a critical indicator of ecosystem health and can be affected by changing light, soil chemical, and physical properties after fires [44]. Herbaceous plants can immediately recover and grow, because the released nutrients from the ash deposited on the soil surface can be quickly absorbed by the abundant and shallow root of grasses [45,46]. For example, periodic fires have been reported to elevate understory plant species diversity and evenness in a longleaf pine wiregrass ecosystem [47]. However, high-intensity fire can reduce species diversity through destroying the propagules of herbaceous species and dormant seeds in a forest floor [48].
Beginning in May 2014, a field experiment was conducted to examine the potential impacts of fire on soil properties, biomass loss, and understory plant community in a coniferous (Pinus massoniana Lamb.) and broadleaf (Quercus acutissima Carruth.) mixed forest in Central China. In China, there are more than 13,000 mean annual forest fire events with approximately 650,000 ha forests being burned annually at the end of the 20th century [49]. Coniferous–broadleaf mixed forest covers approximately 23% of forested area and accounts for 10% of the forest biomass in China [50]. The forest is located in a transitional zone from subtropical to warm temperate region where plant growth and ecosystem functions are considered to be sensitive to climate change [51]. The main objectives of this study were to (1) assess impacts of fire on soil nutrients, microbial biomass C, and N in the short term, (2) quantify the instantaneous losses of C pools in aboveground live biomass (trees, shrubs, and herbs) and dead biomass (standing deadwood and debris), (3) and evaluate the initial effect of fire on the understory plant community.
2. Materials and Methods
2.1. Study Site
The study site is located at the Mountain Xian (32°06′ N, 114°01′ E, 204 m a.s.l.) of Nanwan Forest Service, Henan Province in Central China. Mean annual precipitation derived from the long-term (1951–2014) historical meteorological data (http://data.cma.gov.cn) is 1063 mm, of which approximately 66% occurs from May to September. The mean annual temperature is 15.2 °C, varying from 1.9 °C in January to 33.6 °C in July. The soil type is a Haplic Luvisols (FAO classification) and pH is 4.5 ± 0.03. The mean soil bulk density, litter depth, and litter mass are 0.99 g cm−3, 1.2 cm, and 774 g m−2, respectively. The experiment was carried out in a 25-year-old secondary forest, consisting mainly of German oak (Quercus acutissima Carruth.) and Masson pine (Pinus massoniana Lamb.). The understory shrub species are mainly composed of Vitex negundo L., Lindera glauca (Sieb. et Zucc.) BI, Rubus corchorifolius L. f., and Symplocos chinensis (Lour.) Druce. Herbs are dominated by perennial grass Carex rigescens (Franch.) and Lygodium japonicum (Thunb.) Sw [52].
2.2. Experimental Design
The experimental site was located on a south-facing hillslope (approximately 20% in slope) with the altitude ranging from 193 m at the bottom to 224 m at the top. On 5 April 2014, the west side of the hillslope was burned accidentally by an anthropogenic fire, whereas the east side was left intact due to the existence of a small trail between the two sides. The fire was classified as a surface fire, due to the flame length (1.7–2.5 m) matching the surface fire classification of Ryan. (2002) [53]. The experimental area had not been burned for at least 20 years when fire records started, and it had uniform plant composition and site characteristics. The fire consumed all the litter layer and most aboveground parts of the understory shrubs and herbs, leading to 30% overstory mortality. Fornwalt et al. (2010) reported that burned areas with <50% overstory mortality were categorized as low-severity fire, while moderate-severity burned areas had ≥50% overstory mortality but did not experience much crown consumption [54]. Consequently, the fire was subject to low-severity fire according to the fire’s direct effects on the overstory [54]. The stands in both sides are of comparable stand density, diameter at breast height (DBH), and species composition (Table 1).

Table 1.
General description of the unburned and burned plots in the coniferous–broadleaf mixed forest (mean ± standard error (SE)) (n = 3). DBH, diameter at breast height.
2.3. Soil Microclimate and Properties
Soil temperature at the depth of 10 cm was monitored using a thermocouple probe (LI-8100-201) attached to the LI-8100 at the same time of soil respiration measurement. Volumetric soil moisture content (0–10 cm) was measured using a time-domain reflectometer (TDR 200, Spectrum Scientific Inc., Lincoln, NE, USA). The soil temperature and moisture of each plot were measured from eight locations within this plot, and the measurement was conducted three times in June 2014.
Soil samples were collected from all the 10 plots from the end of June to the middle of July 2014. Two soil cores (0–10 cm in depth, 7 cm in diameter) were randomly collected in each plot. After homogeneous mixing and the removal of stones and roots through a 2-mm mesh, soil samples were maintained fresh at 4 °C for measuring microbial and chemical analysis (Table 2).

Table 2.
Laboratory methods to measure soil physical, chemical, and microbial properties.
2.4. Measurement of Aboveground Biomass
The aboveground biomass of overstory trees, shrubs, herbaceous plants, and litters were inventoried on 1 May 2014. Overstory trees were tagged and investigated, including species, DBH, height, and health status (living or dead) [58]. The aboveground biomass of Q. acutissima and P. massoniana was estimated using published allometric equations for oak and pine in this region [59]. The sum of all individual estimates was used as the tree layer biomass for each plot. Tree mortality was calculated as the ratio of dead trees/total trees in each 5 cm DBH class after fire.
Four 5 m × 5 m subplots were randomly established in each plot to investigate shrub biomass. The aboveground biomass of tree seedlings and shrubs (<5 cm DBH) was estimated by allometric equations [60]. Allometric equations of three dominant shrub species (V. negundo, L. glauca, and S. chinensis) were established through the destructive sampling method [61]. For each species, 8–10 individuals outside the subplot were destructively sampled and oven-dried at 70 °C for 48 hours to estimate aboveground biomass. The relationship between aboveground biomass and basal diameter for each species was determined by a power equation (B = a × D b, where B represents the aboveground biomass and D represents the basal diameter) [62]. The aboveground biomass of other sporadic shrub species (<5%) was assessed based on a general allometric equation established using data of all three shrub species (Table 3). The sum of all individual biomass was calculated as the shrub-layer biomass for each plot. The species richness and density of shrubs were recorded as the occurrence of the number of plant species and the number of individuals (stems), respectively.

Table 3.
Allometric relationships established for aboveground biomass estimation of three dominant shrub species in this study. AGB, aboveground biomass; D, basal diameter of each species.
2.5. Measurement of Understory Species Diversity
Shrub layer was monitored within the four permanent 5 m × 5 m subplot in each plot, and a 1 m × 1 m herbaceous quadrat was set up in each subplot on 15 June 2014. The species richness of shrub and herbaceous plants was recorded as the occurrence of the number of plant species in each subplot and quadrat, respectively. The number of individuals (stems) for all shrubs and herbaceous species was counted to calculate the density of shrubs and herbaceous species in each subplot and quadrat, respectively.
2.6. Data Analysis
The data were checked for normality and homogeneity of variance. A paired-samples T-test (one-tailed) was used to examine the differences in soil microclimate and properties as well as vegetation variables between the unburned and burned plots. The changes of biomass loss induced by fire were calculated as the difference of the partial aboveground biomass (including shrub, herbaceous, and litter layer) between the burned and unburned plots. The changes of dead wood loss (standing deadwood and debris) induced by fire were calculated as the difference between the total aboveground biomass and the live aboveground biomass in the burned plots. The analyses of data were conducted using SAS V.8.1 software (SAS Institute, Cary, NC, USA).
3. Results
3.1. Soil Microclimate and Properties
Burning significantly increased soil temperature (at the depth of 10 cm) by 1.64 °C (p < 0.01), but it did not affect soil moisture (p > 0.05) compared with that in the unburned plots two months after fire (Figure 1A,B). Soil pH was higher in the burned plots (4.84) than in the unburned plots three months after fire (4.48; Figure 1C; p < 0.05). No differences in soil organic C or total N between the burned and unburned observed plots (Figure 2A,B; both p > 0.05). In addition, burning enhanced soil NO3−-N (47.9%; Figure 2C; p < 0.05) but did not affect on NH4+-N (Figure 2D; p > 0.05). The burned plots had higher microbial biomass C (MBC) (249.87 versus 145.44 mg kg−1) than the unburned plots (Figure 2E; p < 0.05). Soil MBN did not change following the fire (Figure 2F; p > 0.05).
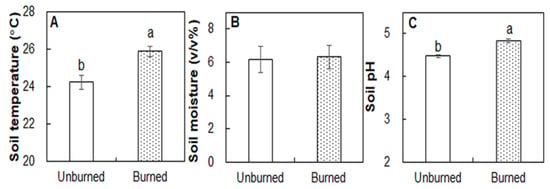
Figure 1.
Differences in soil temperature (A), moisture (B), and pH (C) between the unburned and burned plots. Different letters indicate significant differences between treatments at p < 0.05. Values are presented as the means ± standard error.
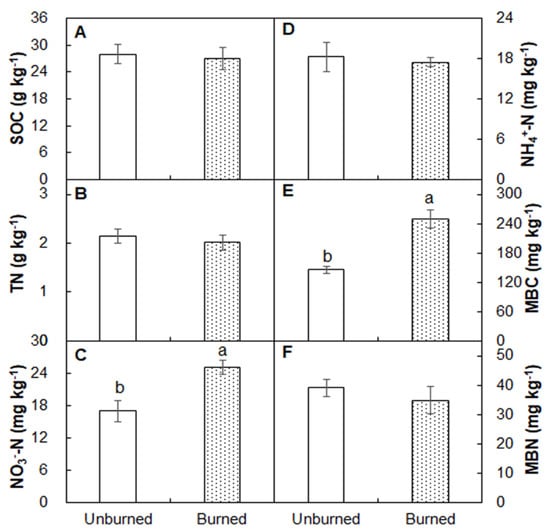
Figure 2.
Differences in soil organic C (SOC, A), total N (TN, B), NO3−-N (C), NH4+-N (D), microbial biomass C (MBC, E), and microbial biomass N (MBN, F) between the unburned and burned plots. Different letters indicate significant differences between treatments at p < 0.05. Values are presented as the means ± standard error.
3.2. Aboveground Biomass
Total aboveground biomass was 39.0% lower in the burned than unburned plots 4 weeks after fire (122.5 t ha−1; Figure 3A). The burned plots had lower biomass in tree (70.39 t ha−1), shrub (1.00 t ha−1), and herbaceous (0 t ha−1) layers than those in the unburned plots (98.98, 3.93, and 0.52 t ha-1; Figure 3B-D; all p < 0.05). In addition, forest floor litter (3.39 t ha−1) in the burned plot was 72.7% lower than that in the unburned plots (Figure 3E; p < 0.05). Direct biomass combustion loss (19.15 t ha−1) caused by the fire accounted for 25.6% of the total aboveground biomass in the burned plots (Figure 4). Dead wood biomass (23.69 t ha−1) accounted for 31.7% of the total aboveground biomass after fire (Figure 4).
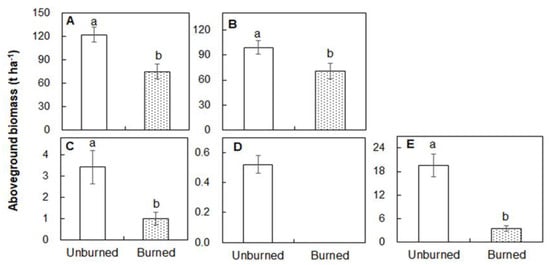
Figure 3.
Differences in total aboveground biomass (A, total; B, tree layer; C, shrub layer; D, herbaceous layer; E, litter) between the unburned and burned plots. Different letters indicate significant differences between treatments at p < 0.05. Values are presented as the means ± standard error.
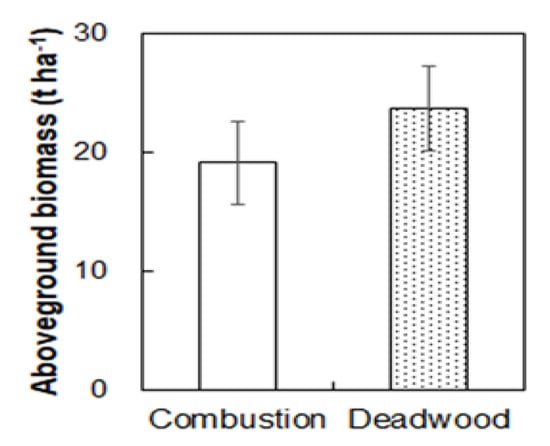
Figure 4.
Fire directly induced biomass combustion and indirectly induced deadwood biomass in the burned plots. Values are presented as the means ± standard error.
Burning significantly reduced the aboveground live biomass by 48.7% compared with that in the unburned plots (99.56 t ha−1; Figure 5A; p < 0.05). The aboveground live biomass of trees in the burned plots (51.09 t ha−1) was lower than that in the unburned plots (95.77 t ha−1; Figure 5B; p < 0.05). The understory shrubs and herbaceous plants were completely consumed (3.80 t ha−1) in the burned plots relative to the unburned plots (Figure 5C).
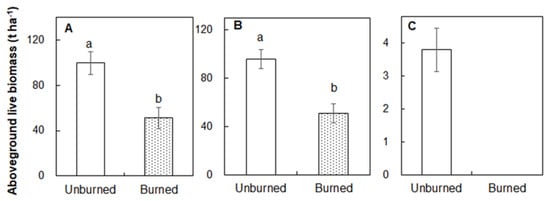
Figure 5.
Differences in aboveground live biomass (A, total; B, tree layer; C, shrub and herbaceous layer) between the unburned and burned plots. Different letters indicate significant differences between treatments at p < 0.05. Values are presented as the means ± standard error.
3.3. Tree Mortality
Tree density was decreased by 43.0% in the burned (90.8 stems plot−1; Figure 6; p < 0.05) than unburned plots 4 weeks after fire (159.2 stems plot−1). The density of pine and oak was reduced by 48.7% and 33.9% after fire, respectively (Figure 6). The tree mortality induced by fire declined with increasing DBH for both pine and oak trees (Figure 7A). The mean number of dead pine trees for 5–10 cm size class (25.8 stems plot−1) accounted for 53.0% of all dead pine trees, and it was significantly higher than other four classes (DBH < 5 cm (12.3%), 10–15 cm (27.2%), 15–20 cm (6.6%), >20 cm (0.8%); Figure 7B; p < 0.05). The percentage of dead oak trees in the 5–10 cm class (46.4%) was comparable to that of the <5 cm class (45.0%), which together accounted for more than 90% of all the dead oak trees (Figure 7B).
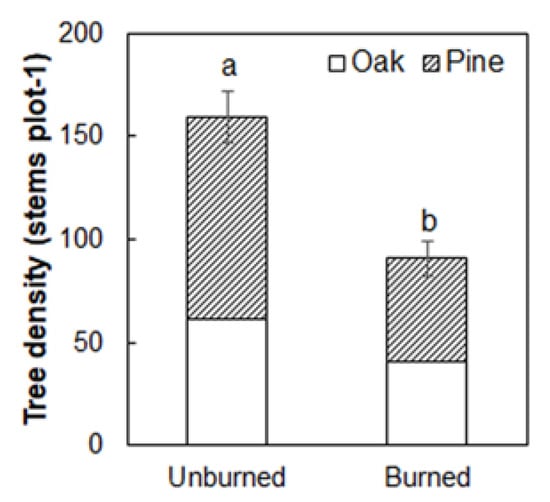
Figure 6.
Differences in tree density between the unburned and burned plots. Different letters indicate significant differences between treatments at p < 0.05. Values are presented as the means ± standard error.
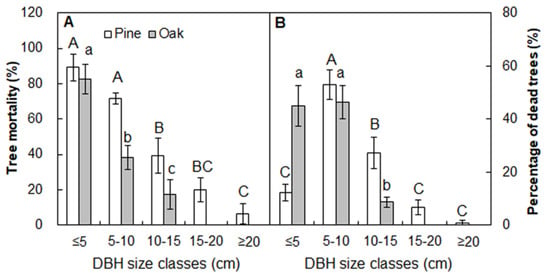
Figure 7.
Tree mortality (A) and the percentage of dead trees (B) in the burned plots by 5 cm diameter at breast height (DBH) class. Different letters indicate significant differences between treatments at p < 0.05. Values are presented as the means ± standard error.
3.4. Richness and Density of Understory Species
Two months after fire, there were no differences in the species richness of shrub or herb between the burned and unburned observed plots (Figure 8A,B; both p > 0.05). The shrub density was higher in the burned plots (6.0 stems m−2) than that in the unburned plots (2.3 stems m−2, Figure 8C; p < 0.05). Herb density was marginally increased by 88.0% under burning two months after fire (Figure 8D; p < 0.05).
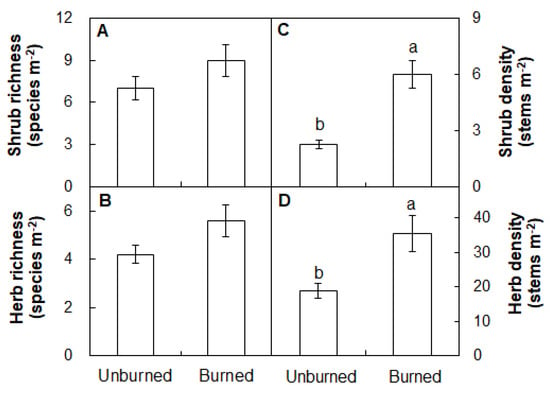
Figure 8.
Differences in richness (A,B) and density (C,D) of shrub and herbaceous species between the unburned and burned plots. Different letters indicate significant differences between treatments at p < 0.05. Values are presented as the means ± standard error.
4. Discussion
The fire season lasts from December to May, with fires occurring more frequently in January, February, and April. A total of approximate 214 fire events occur in this region, and an increasing trend in fire frequency and forest area has been observed from 2009 to 2018 [63,64,65]. Although understory plants are charred or consumed during the fire, this anthropogenic fire could be a low-severity fire due to overstory mortality <50% and overstory trees with green needles [54,66].
4.1. Effect of Fire on Soil Properties
Fire has profound impacts on soil properties such as the structure, infiltration, thermal regime, water storage, nutrient concentrations, C quality, and microbes [13,15,21,67,68], which are key factors in sustainability. Soil pH has been found to increase three months after fire due to the leaching of ash downwards into the soil profile [14,69] and the release of cations and oxides from the complete oxidation of the organic matter [13,19,70]. Fire showed neutral impacts on soil organic C and N in this mixed forest. Our observations are consistent with those reported in a Quercus frainetto forest [71] and a large-scale meta-analysis across temperate forests [67], which could be attributed to the low-fire severity and the formation of black C [72]. Nitrogen is most easily vaporized and lost after fire in forest ecosystems [73]. It has been demonstrated that fire increased soil NH4+ and NO3− by increased soil temperature [74], pH [75], and substrate supply [76]. However, burning increased soil NO3− but did not affect NH4+ at our experiment site (Figure 1). Nevertheless, the time after fire did affect soil NH4+ [22]. Soil NH4+ are direct products of fuel combustion, which generally persists for several months following fire and then declines to pre-fire levels [22]. In this study, the positive impacts of burning on soil microbial biomass are in line with those observed in a woodland savanna [77] and Mediterranean shrubs [78], and a grassland and pine forest of USA [79]. Two possible reasons could explain the positive response of soil microbial biomass to burning observed in this study. The low-severity fire only consumed aboveground litter and tree saplings, probably resulting in lesser mortality in microbial communities [80]. Moreover, burning increased soil temperature and some essential nutrients (e.g., N and P) from ash deposition after the combustion of aboveground vegetation and litter, which are the main limiting factors for the microbial growth and activity [28,80,81].
4.2. Effect of Fire on Aboveground Biomass and Tree Mortality
Fire severity influences the loss of forest aboveground biomass [82]. In this study, burning decreased the total aboveground biomass (39%) and aboveground live biomass (48.7%) right after fire. The loss of aboveground living biomass in this study is lower than that under high-severity fire (83%) but higher than that under moderate-severity fire (15%) in a mature pine forest [83]. The fire severity classification may contribute to the differential response. In our study, the surface fire was classified as a low-severity fire according to overstory mortality. However, many previous studies quantified fire severity depend on the depth of burned organic layer, percent of forest floor consumption, and percent of understory plants consumption [66,83]. Therefore, our results would overestimate the loss of aboveground biomass due to higher understory vegetation consumption.
Fire not only causes initial surface fuel consumption, but it also remains standing dead trees and down woody debris at the soil surface [36,84]. In this study, standing dead trees and down woody debris show the higher losses (23.69 t h−1) compared with the direct biomass combustion (19.15 t ha−1) right after fire. Trees are not complete combusted, but most of them would be dead due to the high temperature during the fire; thus, post-fire stands had higher deadwood biomass [36]. In addition to direct biomass loss through combustion, standing dead trees and down woody debris after fires can also affect ecosystem C cycling in the long term. For example, standing dead trees are drier and isolated from the soil, and they generally have slower decomposition rates than dead wood on the ground or forest floor [85]. As standing dead trees fall down with time, soil C storage could be increased due to the high charcoal content of standing dead trees [86]. In addition, fire can stimulate light, water, and soil nutrient availability, with consequent improvements in plant growth and primary productivity in forests. Therefore, the decomposition rates of dead wood and vegetation dynamics should be considered in models of postfire recovery of an ecosystem C pool in the long term, which could help to accurately predict the C balance of forest ecosystems in response to abrupt environmental perturbations.
Burning instantaneously affects the tree mortality of pine and oak right after fire in this study. Burning-induced tree mortality patterns depend on fire characteristics and tree size [87]. In addition, burning-induced reductions in pine density (48.7%) were higher than in oak density (33.9%) in this study. Most previous studies focus on species-level or monoculture forests alone, which limits our ability to accurately predict changes in tree mortality across diverse forest types [88,89,90]. In fact, variations in mortality occur in coniferous and broadleaved species, but the survival of broadleaved trees is much higher than the survival of conifers in a coniferous–broadleaf mixed forest in the Mediterranean Basin [91]. This finding suggests that broadleaved species rather than coniferous species can survive the low-severity fire. Contrary to the findings of previous studies that mortality rates of tree depend on species and tree size [88,92], our results demonstrated a declining trend of mortality in both pine and oak with increasing DBH in this forest. The above observations suggest that small trees are more likely to die after fire, which is probably due to the tree and branch fall (personal observation) as well as thinner bark [93].
4.3. Effect of Fire on Understory Plants
In this study, burning did not affect the richness of shrub and herb, but it did increase the density of shrub and herb two months after fire. Our observations are consistent with those reported in a Quercus Pyrenaica forest [94] and a mixed conifer forest [95]. Given that dead standing trees fall down with time, higher understory species richness is expected in the following years. For example, fire has been demonstrated to enhance understory species richness in a jarrah forest peaked in 3–5 years after wildfire [96]. Two possible reasons could explain the rapid recovery of understory shrub and herb after fire observed in this climate transitional forest. First, light and nutrient availability play critical roles in the growth of understory shrub and herb [52,97,98]. Improved light conditions and nutrient availability from ash deposition could contribute to the rapid recovery of understory shrub and herb. Second, greater solar radiation at the soil surface due to the removal of vegetation after fires can promote seed germination and seedling growth [99], leading to accelerating the recovery of understory shrub and herb in this study.
5. Conclusions
In this study, we highlighted the short-term effects of fire on soil properties, losses of different C pools, and vegetation in a climate transitional forest. Burning stimulated soil NO3− and microbial biomass C, which was most likely due to the elevated inorganic nutrients from ash deposition. A higher loss of total aboveground biomass after the low-severity fire could have been attributed to the different fire severity classification. The finding that dead wood biomass was higher than the direct biomass combustion during the fire indicates that decomposition of dead wood may offset the biomass C loss of forest in the long-term scale. The rapid recovery of understory plants after fire suggest that the diversity of understory species could benefit from low-severity fires. Long-term research studies are needed in order to understand the fire effects on ecosystem components in diverse forests.
Author Contributions
Conceptualization, M.H. and S.W.; data collection and formal analysis, M.H., Y.L., T.W., Y.H., and Z.L.; writing—original draft preparation, M.H. and S.W.; writing—review and editing, M.H. and S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (31830012).
Acknowledgments
We wish to thank Zhenxing Zhou and Jingyi Ru for their editorial comments. The authors are grateful to two anonymous reviewers for their valuable and constructive comments, which greatly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pimm, S.L.; Raven, P. Biodiversity: Extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [PubMed]
- Knorr, W.; Arneth, A.; Jiang, L. Demographic controls of future global fire risk. Nat. Clim. Change 2016, 6, 781–785. [Google Scholar] [CrossRef]
- Andela, N.; Morton, D.C.; Giglio, L.; Chen, Y.; Van Der Werf, G.R.; Kasibhatla, P.S.; DeFries, R.S.; Collatz, G.J.; Hantson, S.; Kloster, S.; et al. A human-driven decline in global burned area. Science 2017, 356, 1356–1362. [Google Scholar] [CrossRef]
- Krawchuk, M.A.; Moritz, M.A.; Parisien, M.A.; Van Dorn, J.; Hayhoe, K. Global pyrogeography: The current and future distribution of wildfire. PLoS ONE 2009, 4, e5102. [Google Scholar] [CrossRef]
- Pellegrini, A.F.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 553, 194–198. [Google Scholar] [CrossRef]
- Walker, X.J.; Baltzer, J.L.; Cumming, S.G.; Day, N.J.; Ebert, C.; Goetz, S.; Johnstone, J.F.; Potter, S.; Rogers, B.M.; Schuur, E.A.G.; et al. Increasing wildfires threaten historic carbon sink of boreal forest soils. Nature 2019, 572, 520–523. [Google Scholar] [CrossRef]
- Bedia, J.; Herrera, S.; Gutiérrez, J.M.; Benali, A.; Brands, S.; Mota, B.; Moreno, J.M. Global patterns in the sensitivity of burned area to fire-weather: Implications for climate change. Agric. For. Meteorol. 2015, 214, 369–379. [Google Scholar] [CrossRef]
- Harris, R.M.; Remenyi, T.A.; Williamson, G.J.; Bindoff, N.L.; Bowman, D.M. Climate-vegetation-fire interactions and feedbacks: Trivial detail or major barrier to projecting the future of the Earth system? Wiley Interdiscip. Rev. Clim. Change 2016, 7, 910–931. [Google Scholar] [CrossRef]
- Harden, J.; Trumbore, S.; Stocks, B.; Hirsch, A.; Gower, S.; O’neill, K.; Kasischke, E. The role of fire in the boreal carbon budget. Glob. Change Biol. 2000, 6, 174–184. [Google Scholar] [CrossRef]
- Schoennagel, T.; Balch, J.K.; Brenkert-Smith, H.; Dennison, P.E.; Harvey, B.J.; Krawchuk, M.A.; Mietkiewicz, N.; Morgan, P.; Moritz, M.A.; Rasker, R.; et al. Adapt to more wildfire in western North American forests as climate changes. Proc. Natl. Acad. Sci. USA 2017, 114, 4582–4590. [Google Scholar] [CrossRef] [PubMed]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boerner, R.E.; Huang, J.; Hart, S.C. Impacts of fire and fire surrogate treatments on forest soil properties: A meta-analytical approach. Ecol. Appl. 2009, 19, 338–358. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613, 944–957. [Google Scholar] [CrossRef]
- Hubbert, K.R.; Preisler, H.K.; Wohlgemuth, P.M.; Graham, R.C.; Narog, M.G. Prescribed burning effects on soil physical properties and soil water repellency in a steep chaparral watershed, southern California, USA. Geoderma 2006, 130, 284–298. [Google Scholar] [CrossRef]
- Hamman, S.T.; Burke, I.C.; Knapp, E.E. Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. For. Ecol. Manag. 2008, 256, 367–374. [Google Scholar] [CrossRef]
- DeBano, L.F. The role of fire and soil heating on water repellency in wildland environments: A review. J. Hydrol. 2000, 231, 195–206. [Google Scholar] [CrossRef]
- Imeson, A.C.; Verstraten, J.M.; Van Mulligen, E.J.; Sevink, J. The effects of fire and water repellency on infiltration and runoff under Mediterranean type forest. Catena 1992, 19, 345–361. [Google Scholar] [CrossRef]
- Arocena, J.M.; Opio, C. Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma 2003, 113, 1–16. [Google Scholar] [CrossRef]
- Wanthongchai, K.; Bauhus, J.; Goldammer, J.G. Nutrient losses through prescribed burning of aboveground litter and understorey in dry dipterocarp forests of different fire history. Catena 2008, 74, 321–332. [Google Scholar] [CrossRef]
- Wan, S.; Hui, D.; Luo, Y. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: A meta-analysis. Ecol. Appl. 2001, 11, 1349–1365. [Google Scholar] [CrossRef]
- Turner, M.G.; Smithwick, E.A.; Metzger, K.L.; Tinker, D.B.; Romme, W.H. Inorganic nitrogen availability after severe stand-replacing fire in the Greater Yellowstone ecosystem. Proc. Natl. Acad. Sci. USA 2007, 104, 4782–4789. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, Q.; Chen, H. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in South China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Fonseca, F.; de Figueiredo, T.; Nogueira, C.; Queirós, A. Effect of prescribed fire on soil properties and soil erosion in a Mediterranean mountain area. Geoderma 2017, 307, 172–180. [Google Scholar] [CrossRef]
- Dooley, S.R.; Treseder, K.K. The effect of fire on microbial biomass: A meta-analysis of field studies. Biogeochemistry 2012, 109, 49–61. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Wang, Y.; Rafique, R.; Ma, L.; Hu, L.; Luo, Y. Fire alters vegetation and soil microbial community in alpine meadow. Land Degrad. Dev. 2016, 27, 1379–1390. [Google Scholar] [CrossRef]
- Choromanska, U.; DeLuca, T.H. Microbial activity and nitrogen mineralization in forest mineral soils following heating: Evaluation of post-fire effects. Soil Biol. Biochem. 2002, 34, 263–271. [Google Scholar] [CrossRef]
- Singh, A.K.; Kushwaha, M.; Rai, A.; Singh, N. Changes in soil microbial response across year following a wildfire in tropical dry forest. For. Ecol. Manag. 2017, 391, 458–468. [Google Scholar] [CrossRef]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; MacKenzie, M.D.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2005, 220, 166–184. [Google Scholar] [CrossRef]
- Rothstein, D.E.; Yermakov, Z.; Buell, A.L. Loss and recovery of ecosystem carbon pools following stand-replacing wildfire in Michigan jack pine forests. Can. J. For. Res. 2004, 34, 1908–1918. [Google Scholar] [CrossRef]
- Sun, L.; Hu, T.X.; Kim, J.H.; Guo, F.T.; Song, H.; Lv, X.S.; Hu, H.Q. The effect of fire disturbance on short-term soil respiration in typical forest of Greater Xing’an Range, China. J. For. Res. 2014, 25, 613–620. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.; Samuelson, L.; Stokes, T.; Cropper, W.; Martin, T.; Johnsen, K. Understory plant biomass dynamics of prescribed burned Pinus palustris stands. For. Ecol. Manag. 2015, 344, 84–94. [Google Scholar] [CrossRef]
- Kasischke, E.S.; Stocks, B.J.; O’Neill, K.; French, N.H.; Bourgeau-Chavez, L.L. Direct Effects of Fire on the Boreal Forest Carbon Budget. In Biomass Burning and Its Inter-Relationships with the Climate System; Springer: Dordrecht, The Netherlands, 2000; pp. 51–68. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Kane, E.S.; Harden, J.W.; Ottmar, R.D.; Manies, K.L.; Hoy, E.; Kasischke, E.S. Recent acceleration of biomass burning and carbon losses in Alaskan forests and peatlands. Nat. Geosci. 2011, 4, 27–31. [Google Scholar] [CrossRef]
- Seedre, M.; Taylor, A.R.; Brassard, B.W.; Chen, H.Y.; Jõgiste, K. Recovery of ecosystem carbon stocks in young boreal forests: A comparison of harvesting and wildfire disturbance. Ecosystems 2014, 17, 851–863. [Google Scholar] [CrossRef]
- Kasischke, E.S.; O’neill, K.; Bourgeau-Chavez, L.L.; French, N.H. Indirect and Long-term Effects of Fire on the Boreal Forest Carbon Budget. In Biomass Burning and Its Inter-relationships with the Climate System; Springer: Dordrecht, The Netherlands, 2000; pp. 263–280. [Google Scholar] [CrossRef]
- Murphy, B.P.; Lehmann, C.E.; Russell-Smith, J.; Lawes, M.J. Fire regimes and woody biomass dynamics in Australian savannas. J. Biogeogr. 2014, 41, 133–144. [Google Scholar] [CrossRef]
- Harper, K.A.; Bergeron, Y.; Drapeau, P.; Gauthier, S.; De Grandpré, L. Structural development following fire in black spruce boreal forest. For. Ecol. Manag. 2005, 206, 293–306. [Google Scholar] [CrossRef]
- Maia, P.; Keizer, J.; Vasques, A.; Abrantes, N.; Roxo, L.; Fernandes, P.; Ferreira, A.; Moreira, F. Post-fire plant diversity and abundance in pine and eucalypt stands in Portugal: Effects of biogeography, topography, forest type and post-fire management. For. Ecol. Manag. 2014, 334, 154–162. [Google Scholar] [CrossRef]
- Moritz, M.A.; Batllori, E.; Bradstock, R.A.; Gill, A.M.; Handmer, J.; Hessburg, P.F.; Leonard, J.; McCaffrey, S.; Odion, D.C.; Schoennagel, T. Learning to coexist with wildfire. Nature 2014, 515, 58–66. [Google Scholar] [CrossRef]
- Pinno, B.; Errington, R. Burn severity dominates understory plant community response to fire in xeric jack pine forests. Forests 2016, 7, 83. [Google Scholar] [CrossRef]
- Foster, C.N.; Barton, P.S.; MacGregor, C.I.; Catford, J.A.; Blanchard, W.; Lindenmayer, D.B. Effects of fire regime on plant species richness and composition differ among forest, woodland and heath vegetation. Appl. Veg. Sci. 2018, 21, 132–143. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Pivello, V.R.; Oliveras, I.; Miranda, H.S.; Haridasan, M.; Sato, M.N.; Meirelles, S.T. Effect of fires on soil nutrient availability in an open savanna in Central Brazil. Plant Soil 2010, 337, 111–123. [Google Scholar] [CrossRef]
- Oliveras, I.; Meirelles, S.T.; Hirakuri, V.L.; Freitas, C.R.; Miranda, H.S.; Pivello, V.R. Effects of fire regimes on herbaceous biomass and nutrient dynamics in the Brazilian savanna. J. Wildland Fire 2013, 22, 368–380. [Google Scholar] [CrossRef]
- Brockway, D.G.; Lewis, C.E. Long-term effects of dormant-season prescribed fire on plant community diversity, structure and productivity in a longleaf pine wiregrass ecosystem. For. Ecol. Manag. 1997, 96, 167–183. [Google Scholar] [CrossRef]
- Roberts, M.R. Response of the herbaceous layer to natural disturbance in North American forests. Can. J. Bot. 2004, 82, 1273–1283. [Google Scholar] [CrossRef]
- Tian, X.R.; Shu, L.F.; Wang, M.Y. Direct carbon emissions from Chinese forest fires, 1991–2000. Fire Safety Sci. 2003, 12, 6–10. [Google Scholar] [CrossRef]
- Fang, J. Forest productivity in China and its response to global climate change. Acta Phyto. Sin. 2001, 24, 513–517. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, W.; Fu, C.; Wu, L. The influence of vegetation cover on summer precipitation in China: A statistical analysis of NDVI and climate data. Adv. Atmos. Sci. 2003, 20, 1002–1006. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; Sun, Z.; Zhang, K.; Liu, Y.; Miao, R.; Wan, S. Fire rather than nitrogen addition affects understory plant communities in the short term in a coniferous-broadleaf mixed forest. Ecol. Evol. 2018, 8, 8135–8148. [Google Scholar] [CrossRef]
- Ryan, K.C. Dynamic interactions between forest structure and fire behavior in boreal ecosystems. Silva Fenn. 2002, 36, 13–39. [Google Scholar] [CrossRef]
- Fornwalt, P.J.; Kaufmann, M.R.; Stohlgren, T.J. Impacts of mixed severity wildfire on exotic plants in a Colorado ponderosa pine—Douglas-fir forest. Biol. Invasions 2010, 12, 2683–2695. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Vance, E.D.; Brooks, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Berner, L.T.; Alexander, H.D.; Loranty, M.M.; Ganzlin, P.; Mack, M.C.; Davydov, S.P.; Goetz, S.J. Biomass allometry for alder, dwarf birch, and willow in boreal forest and tundra ecosystems of far northeastern Siberia and north-central Alaska. For. Ecol. Manag. 2015, 337, 110–118. [Google Scholar] [CrossRef]
- Yang, T. The investigating and study to the natural secondary mixed forest’s biomass and the root system’s spreading character of the oak and the horse-tail pine. J. Xinyang Agri. Coll. 2004, 14, 4–7. [Google Scholar]
- Zhang, Y.; Gu, F.; Liu, S.; Liu, Y.; Li, C. Variations of carbon stock with forest types in subalpine region of southwestern China. For. Ecol. Manag. 2013, 300, 88–95. [Google Scholar] [CrossRef]
- Conti, G.; Enrico, L.; Casanoves, F.; Díaz, S. Shrub biomass estimation in the semiarid Chaco forest: A contribution to the quantification of an underrated carbon stock. Ann. For. Sci. 2013, 70, 515–524. [Google Scholar] [CrossRef]
- Sah, J.P.; Ross, M.S.; Koptur, S.; Snyder, J.R. Estimating aboveground biomass of broadleaved woody plants in the understory of Florida Keys pine forests. For. Ecol. Manag. 2004, 203, 319–329. [Google Scholar] [CrossRef]
- Su, L.; He, Y.; Chen, S. Temporal and spatial characteristics and risk analysis of forest fires in China from 1950 to 2010. Sci. Silvae Sin. 2015, 51, 88–96. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, W.; Wang, J. An analysis report on major forest fires in Henan province in recent 10 years. J. Henan For. Sci. Technol. 2019, 39, 33–36. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, H.; Zhou, C.; Xue, L.; Zhao, X.; Liu, Z. Analysis of the laws of spatial and temporal distribution of remote sensing monitoring forest fires of in Henan province in recent years. Meteorol. Environ. Sci. 2009, 32, 29–32. [Google Scholar]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Fire effects on temperate forest soil C and N storage. Ecol. Appl. 2011, 21, 1189–1201. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, M.; Wang, S. A meta-analysis on the response of microbial biomass, dissolved organic matter, respiration, and N mineralization in mineral soil to fire in forest ecosystems. For. Ecol. Manag. 2012, 271, 91–97. [Google Scholar] [CrossRef]
- Pereira, P.; Úbeda, X.; Martin, D.; Mataix-Solera, J.; Cerdà, A.; Burguet, M. Wildfire effects on extractable elements in ash from a Pinus pinaster forest in Portugal. Hydrol. Process. 2014, 28, 3681–3690. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Goforth, B.R.; Hubbert, K.R. Fire effects on cation exchange capacity of California forest and woodland soils. Geoderma 2017, 286, 125–130. [Google Scholar] [CrossRef]
- Akburak, S.; Son, Y.; Makineci, E.; Çakir, M. Impacts of low-intensity prescribed fire on microbial and chemical soil properties in a Quercus frainetto forest. J. Forestry Res. 2018, 29, 687–696. [Google Scholar] [CrossRef]
- Moghaddas, E.E.; Stephens, S.L. Thinning, burning, and thin-burn fuel treatment effects on soil properties in a Sierra Nevada mixed-conifer forest. For. Ecol. Manag. 2007, 250, 156–166. [Google Scholar] [CrossRef]
- Rossiter-Rachor, N.A.; Setterfield, S.A.; Douglas, M.M.; Hutley, L.B.; Cook, G.D. Andropogon gayanus (gamba grass) invasion increases fire-mediated nitrogen losses in the tropical savannas of northern Australia. Ecosystems 2008, 11, 77–88. [Google Scholar] [CrossRef]
- Augustine, D.J.; Brewer, P.; Blumenthal, D.M.; Derner, J.D.; von Fischer, J.C. Prescribed fire, soil inorganic nitrogen dynamics, and plant responses in a semiarid grassland. J. Arid Environ. 2014, 104, 59–66. [Google Scholar] [CrossRef]
- Hanan, E.J.; D’Antonio, C.M.; Roberts, D.A.; Schimel, J.P. Factors regulating nitrogen retention during the early stages of recovery from fire in coastal chaparral ecosystems. Ecosystems 2016, 19, 910–926. [Google Scholar] [CrossRef]
- Homyak, P.M.; Sickman, J.O.; Miller, A.E.; Melack, J.M.; Meixner, T.; Schimel, J.P. Assessing nitrogen-saturation in a seasonally dry chaparral watershed: Limitations of traditional indicators of N-saturation. Ecosystems 2014, 17, 1286–1305. [Google Scholar] [CrossRef]
- Jensen, M.; Michelsen, A.; Gashaw, M. Responses in plant, soil inorganic and microbial nutrient pools to experimental fire, ash and biomass addition in a woodland savanna. Oecologia 2001, 128, 85–93. [Google Scholar] [CrossRef]
- Goberna, M.; García, C.; Insam, H.; Hernández, M.T.; Verdú, M. Burning fire-prone Mediterranean shrublands: Immediate changes in soil microbial community structure and ecosystem functions. Microb. Ecol. 2012, 64, 242–255. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Dathe, J.; Davinic, M.; Perry, G.; Wester, D.; Schwilk, D.W.; Rideout-Hanzak, S. Forest wildfire and grassland prescribed fire effects on soil biogeochemical processes and microbial communities: Two case studies in the semi-arid Southwest. Appl. Soil. Ecol. 2016, 99, 118–128. [Google Scholar] [CrossRef]
- Huang, W.; Xu, Z.; Chen, C.; Zhou, G.; Liu, J.; Abdullah, K.M.; Reverchon, F.; Liu, X. Short-term effects of prescribed burning on phosphorus availability in a suburban native forest of subtropical Australia. J. Soil Sediment. 2013, 13, 869–876. [Google Scholar] [CrossRef]
- Ando, K.; Shinjo, H.; Noro, Y.; Takenaka, S.; Miura, R.; Sokotela, S.B.; Funakawa, S. Short-term effects of fire intensity on soil organic matter and nutrient release after slash-and-burn in Eastern Province, Zambia. Soil Sci. Plant Nutr. 2014, 60, 173–182. [Google Scholar] [CrossRef]
- Shenoy, A.; Johnstone, J.F.; Kasischke, E.S.; Kielland, K. Persistent effects of fire severity on early successional forests in interior Alaska. For. Ecol. Manag. 2011, 261, 381–390. [Google Scholar] [CrossRef]
- Kukavskaya, E.A.; Ivanova, G.A.; Conard, S.G.; McRae, D.J.; Ivanov, V.A. Biomass dynamics of central Siberian Scots pine forests following surface fires of varying severity. J. Wildland Fire 2014, 23, 872–886. [Google Scholar] [CrossRef]
- Bradford, J.B.; Fraver, S.; Milo, A.M.; D’Amato, A.W.; Palik, B.; Shinneman, D.J. Effects of multiple interacting disturbances and salvage logging on forest carbon stocks. For. Ecol. Manag. 2012, 267, 209–214. [Google Scholar] [CrossRef]
- Mackensen, J.; Bauhus, J.; Webber, E. Decomposition rates of coarse woody debris-a review with particular emphasis on Australian tree species. Aust. J. Bot. 2003, 51, 27–37. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Aplet, G.H. Charcoal and carbon storage in forest soils of the Rocky Mountain West. Front. Ecol. Environ. 2008, 6, 18–24. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Vega, J.A.; Jimenez, E.; Rigolot, E. Fire resistance of European pines. For. Ecol. Manag. 2008, 256, 246–255. [Google Scholar] [CrossRef]
- Grayson, L.M.; Progar, R.A.; Hood, S.M. Predicting post-fire tree mortality for 14 conifers in the Pacific Northwest, USA: Model evaluation, development, and thresholds. For. Ecol. Manag. 2017, 399, 213–226. [Google Scholar] [CrossRef]
- Dey, D.; Schweitzer, C. A review on the dynamics of prescribed fire, tree mortality, and injury in managing oak natural communities to minimize economic loss in North America. Forests 2018, 9, 461. [Google Scholar] [CrossRef]
- Engber, E.A.; Varner, J.M. Predicting Douglas-fir sapling mortality following prescribed fire in an encroached grassland. Restor. Ecol. 2012, 20, 665–668. [Google Scholar] [CrossRef]
- Catry, F.X.; Rego, F.; Moreira, F.; Fernandes, P.M.; Pausas, J.G. Post-fire tree mortality in mixed forests of central Portugal. For. Ecol. Manag. 2010, 260, 1184–1192. [Google Scholar] [CrossRef]
- van Mantgem, P.J.; Nesmith, J.C.; Keifer, M.; Brooks, M. Tree mortality patterns following prescribed fire for Pinus and Abies across the southwestern United States. For. Ecol. Manag. 2013, 289, 463–469. [Google Scholar] [CrossRef]
- Brando, P.M.; Nepstad, D.C.; Balch, J.K.; Bolker, B.; Christman, M.C.; Coe, M.; Putz, F.E. Fire-induced tree mortality in a neotropical forest: The roles of bark traits, tree size, wood density and fire behavior. Glob. Change Biol. 2012, 18, 630–641. [Google Scholar] [CrossRef]
- Calvo, L.; Tarrega, R.; Luis, E. Regeneration in Quercus pyrenaica ecosystems after surface fires. Int. J. Wildland Fire 1991, 1, 205–210. [Google Scholar] [CrossRef]
- Knapp, E.E.; Schwilk, D.W.; Kane, J.M.; Keeley, J.E. Role of burning season on initial understory vegetation response to prescribed fire in a mixed conifer forest. Can. J. For. Res. 2007, 37, 11–22. [Google Scholar] [CrossRef]
- Bell, D.T.; Koch, J.M. Post-fire succession in the northern jarrah forest of Western Australia. Aust. J. Ecol. 1980, 5, 9–14. [Google Scholar] [CrossRef]
- Schafer, J.L.; Mack, M.C. Short-term effects of fire on soil and plant nutrients in palmetto flatwoods. Plant Soil 2010, 334, 433–447. [Google Scholar] [CrossRef]
- Dewan, S.; Vander Mijnsbrugge, K.; De Frenne, P.; Steenackers, M.; Michiels, B.; Verheyen, K. Maternal temperature during seed maturation affects seed germination and timing of bud set in seedlings of European black poplar. For. Ecol. Manag. 2018, 410, 126–135. [Google Scholar] [CrossRef]
- Santana, V.M.; Bradstock, R.A.; Ooi, M.K.; Denham, A.J.; Auld, T.D.; Baeza, M.J. Effects of soil temperature regimes after fire on seed dormancy and germination in six Australian Fabaceae species. Aust. J. Bot. 2010, 58, 539–545. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).