Temperature Sensitivity in Individual Components of Ecosystem Respiration Increases along the Vertical Gradient of Leaf–Stem–Soil in Three Subtropical Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Leaf Respiration
2.4. Stem Respiration
2.5. Soil Respiration
2.6. Leaf, Stem and Soil Characteristics
2.7. Ecosystem Respiration
2.8. Air Temperature, Soil Temperature and Soil Moisture
2.9. Data Analysis
3. Results
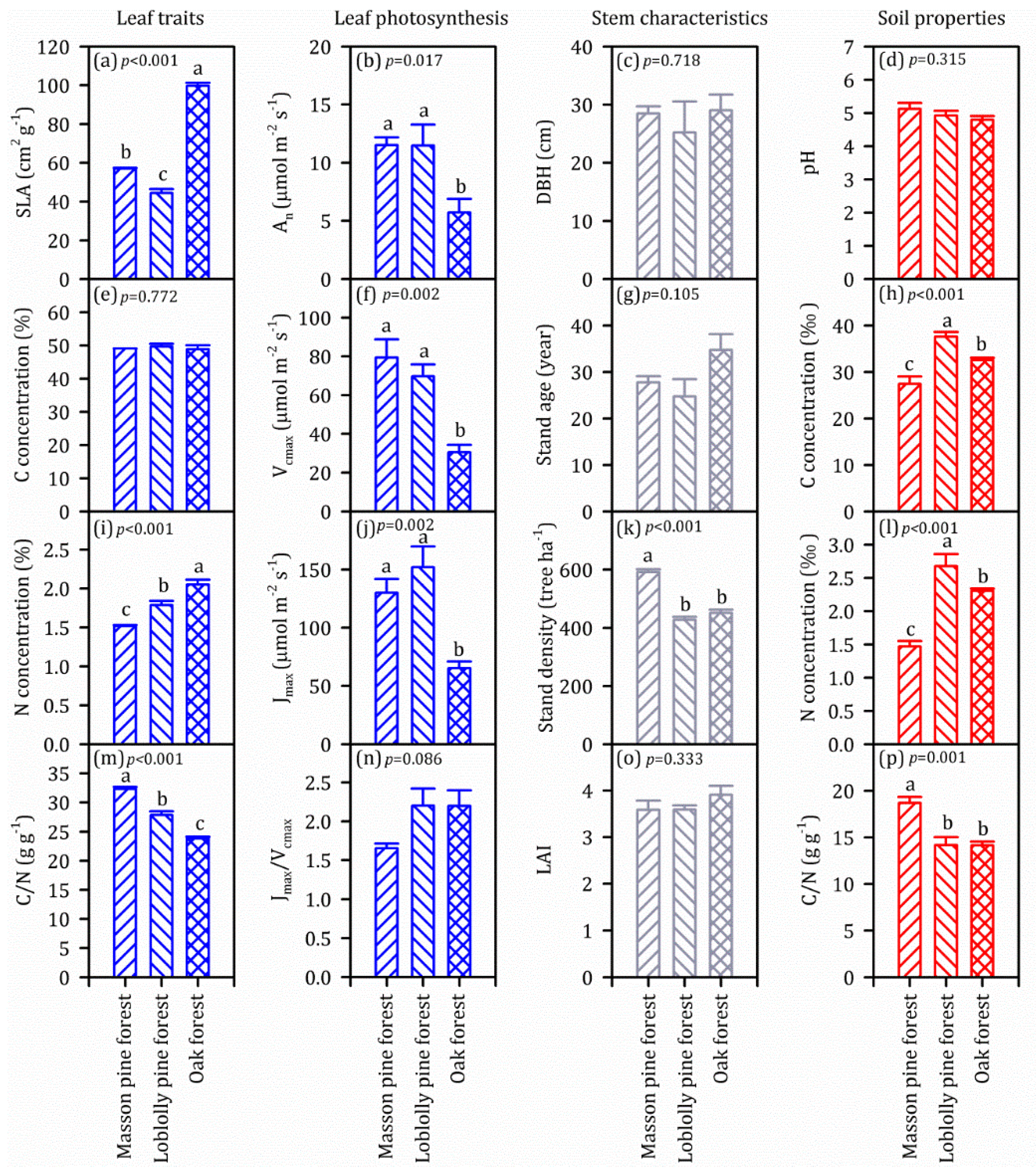
3.1. Leaf, Stem and Soil Characteristics of the Three Forest Stands
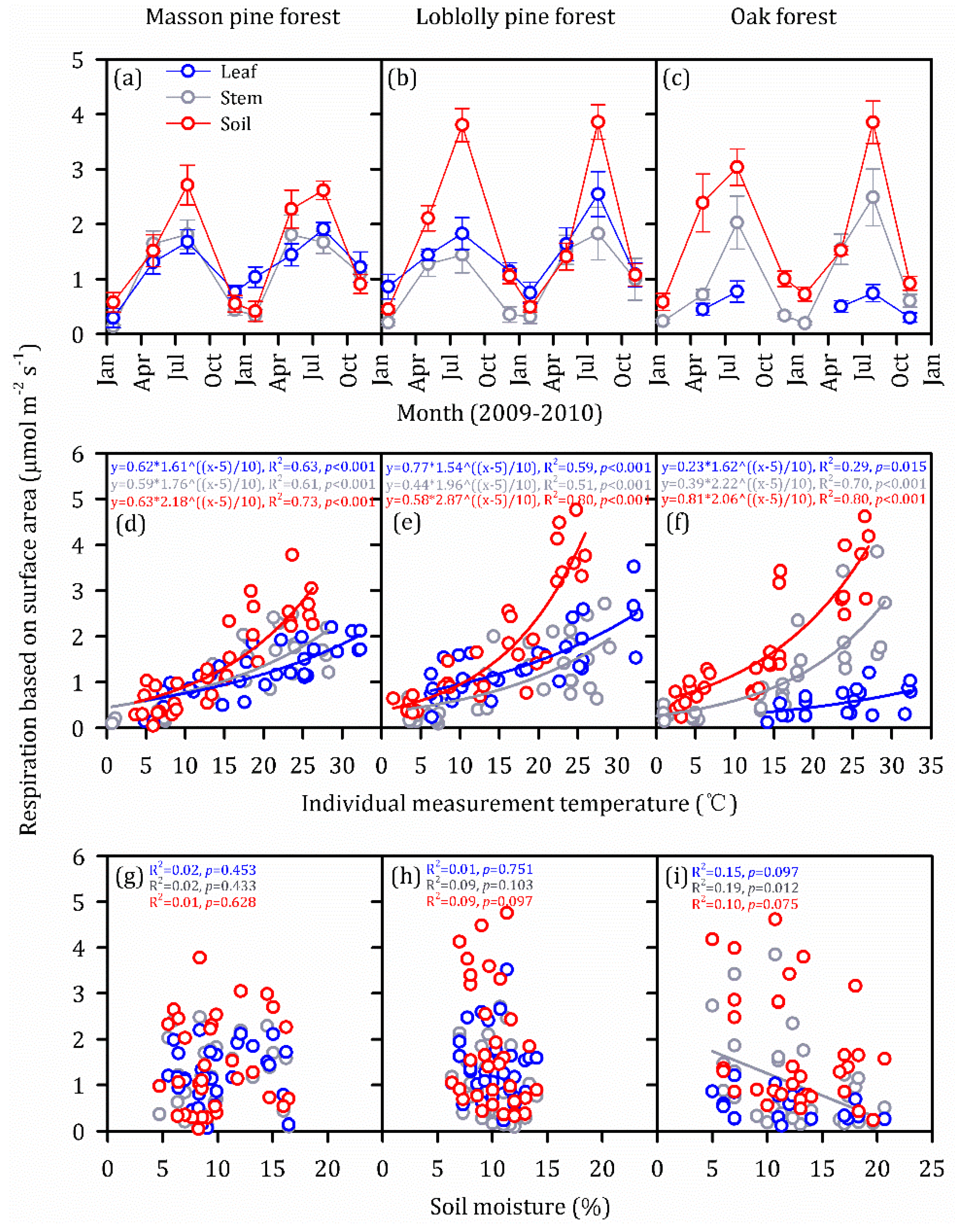
3.2. Vertical and Seasonal Variations in Individual Components of ER
3.3. Temperature Dependence and Soil Moisture Dependence in Individual Components of ER
3.4. Overall ER per Ground Area from Specific Components during the Temperatures Peak
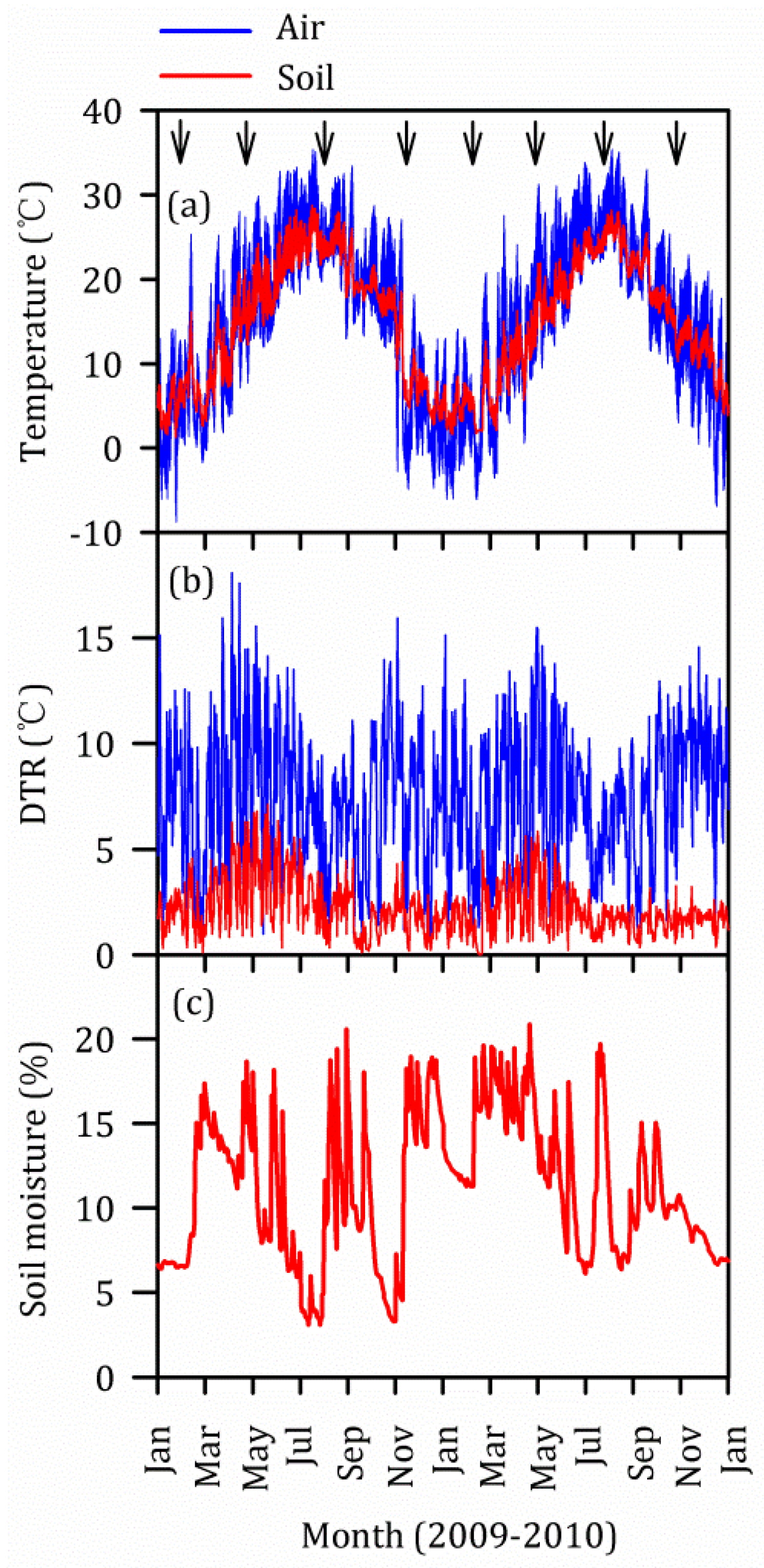
3.5. Vertical and Seasonal Variations of Temperature
4. Discussion
4.1. Q10 in Individual Components of ER Increased along the vertical gradient of Leaf–stem–soil
4.2. Mechanisms Underlying Differences among Q10 in Individual Components of ER
4.3. Uncertainty Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reichstein, M.; Ciais, P.; Papale, D.; Valentini, R.; Running, S.; Viovy, N.; Cramer, W.; Granier, A.; Ogee, J.; Allard, V.; et al. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: A joint flux tower, remote sensing and modelling analysis. Glob. Chang. Biol. 2007, 13, 634–651. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Cox, P.; Betts, R.; Bopp, L.; Von Bloh, W.; Brovkin, V.; Cadule, P.; Doney, S.; Eby, M.; Fung, I.; et al. Climate-carbon cycle feedback analysis: Results from the (CMIP)-M-4 model intercomparison. J. Clim. 2006, 19, 3337–3353. [Google Scholar] [CrossRef]
- Mahecha, M.D.; Reichstein, M.; Carvalhais, N.; Lasslop, G.; Lange, H.; Seneviratne, S.I.; Vargas, R.; Ammann, C.; Arain, M.A.; Cescatti, A.; et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science 2010, 329, 838–840. [Google Scholar] [CrossRef]
- Araki, M.G.; Gyokusen, K.; Kajimoto, T. Vertical and seasonal variations in temperature responses of leaf respiration in a Chamaecyparis obtusa canopy. Tree Physiolgy 2017, 37, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xia, J.; Shi, Z.; Huang, K.; Du, Z.; Lin, G.; Luo, Y. Variation of parameters in a Flux-Based Ecosystem Model across 12 sites of terrestrial ecosystems in the conterminous USA. Ecol. Model. 2016, 336, 57–69. [Google Scholar] [CrossRef]
- Drake, J.E.; Stoy, P.C.; Jackson, R.B.; DeLucia, E.H. Fine-root respiration in a loblolly pine (Pinus taeda L.) forest exposed to elevated CO2 and N fertilization. Plant Cell Environ. 2008, 31, 1663–1672. [Google Scholar]
- Li, Q.; Song, X.; Chang, S.X.; Peng, C.; Xiao, W.; Zhang, J.; Xiang, W.; Li, Y.; Wang, W. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Gruenwald, T.; et al. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Williams, C.A.; Gu, H.; MacLean, R.; Masek, J.G.; Collatz, G.J. Disturbance and the carbon balance of US forests: A quantitative review of impacts from harvests, fires, insects, and droughts. Glob. Planet. Chang. 2016, 143, 66–80. [Google Scholar] [CrossRef]
- Tang, J.; Bolstad, P.V.; Desai, A.R.; Martin, J.G.; Cook, B.D.; Davis, K.J.; Carey, E.V. Ecosystem respiration and its components in an old-growth forest in the Great Lakes region of the United States. Agric. For. Meteorol. 2008, 148, 171–185. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, Y.; Aubrey, D.P.; Zhuang, Q.; Teskey, R.O. Global patterns and predictors of stem CO2 efflux in forest ecosystems. Glob. Chang. Biol. 2016, 22, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Baldocchi, D.D. Spatial-temporal variation in soil respiration in an oak-grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 2005, 73, 183–207. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Amelung, W. The temperature sensitivity (Q10) of soil respiration: Controlling factors and spatial prediction at regional scale based on environmental soil classes. Glob. Biogeochem. Cycles 2018, 32, 306–323. [Google Scholar] [CrossRef]
- Xu, M.; DeBiase, T.A.; Qi, Y.; Goldstein, A.; Liu, Z. Ecosystem respiration in a young ponderosa pine plantation in the Sierra Nevada Mountains, California. Tree Physiol. 2001, 21, 309–318. [Google Scholar] [CrossRef]
- Van Gestel, N.C.; Schwilk, D.W.; Tissue, D.T.; Zak, J.C. Reductions in daily soil temperature variability increase soil microbial biomass C and decrease soil N availability in the Chihuahuan Desert: Potential implications for ecosystem C and N fluxes. Glob. Chang. Biol. 2011, 17, 3564–3576. [Google Scholar] [CrossRef]
- van Gestel, N.C.; Dhungana, N.; Tissue, D.T.; Zak, J.C. Seasonal microbial and nutrient responses during a 5-year reduction in the daily temperature range of soil in a Chihuahuan Desert ecosystem. Oecologia 2016, 180, 265–277. [Google Scholar] [CrossRef]
- Sun, X.; Ren, G.; You, Q.; Ren, Y.; Xu, W.; Xue, X.; Zhan, Y.; Zhang, S.; Zhang, P. Global diurnal temperature range (DTR) changes since 1901. Clim. Dyn. 2019, 52, 3343–3356. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Lorenc-Plucinska, G.; Reich, P.B. Acclimation of respiratory temperature responses in northern and southern populations of Pinus banksiana. New Phytol. 2009, 181, 218–229. [Google Scholar] [CrossRef]
- He, M.; Zhang, K.; Tan, H.; Hu, R.; Su, J.; Wang, J.; Huang, L.; Zhang, Y.; Li, X. Nutrient levels within leaves, stems, and roots of the xeric species Reaumuria soongorica in relation to geographical, climatic, and soil conditions. Ecol. Evol. 2015, 5, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qi, L.; Zhou, W.; Liu, C.; Yu, D.; Dai, L. Carbon dynamics in the deciduous broadleaf tree Erman’s birch (Betula ermanii) at the subalpine treeline on Changbai Mountain, Northeast China. Am. J. Bot. 2018, 105, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Fierer, N.; McLauchlan, K.K.; Elmore, A.J. Reduction of the temperature sensitivity of soil organic matter decomposition with sustained temperature increase. Biogeochemistry 2013, 113, 359–368. [Google Scholar] [CrossRef]
- Fierer, N.; Craine, J.; McLauchlan, K.; Schimel, J. Litter quality and the temperature sensitivity of decomposition. Ecology 2005, 85, 320–326. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, Y.; Liang, N.; Hsia, Y.; Zhang, Y.; Zhou, G.; Li, Y.; Juang, J.; Chu, H.; Yan, J. An observational study of the carbon-sink strength of East Asian subtropical evergreen forests. Environ. Res. Lett. 2012, 7, 044017. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, M.; Chi, Y.; Zheng, Y.; Shen, R.; Li, P.; Dai, H. Temporal and spatial variations of stem CO2 efflux of three species in subtropical China. J. Plant Ecol. 2012, 5, 229–237. [Google Scholar] [CrossRef]
- Li, R.; Zheng, W.; Yang, Q.; Zhang, W.; Chi, Y.; Wang, P.; Xu, M.; Guan, X.; Chen, L.; Wang, Q.; et al. The response of soil respiration to thinning was not affected by understory removal in a Chinese fir (Cunninghamia lanceolata) plantation. Geoderma 2019, 353, 47–54. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar]
- Xiang, W.; Zhou, J.; Ouyang, S.; Zhang, S.; Lei, P.; Li, J.; Deng, X.; Fang, X.; Forrester, D.I. Species-specific and general allometric equations for estimating tree biomass components of subtropical forests in southern China. Eur. J. For. Res. 2016, 135, 963–979. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.A.; Zhao, D.; Samuelson, L.J.; Martin, T.A.; Leduc, D.J.; Jack, S.B. Local and General Above-Ground Biomass Functions for Pinus palustris Trees. Forests 2018, 9, 310. [Google Scholar] [CrossRef]
- Ozdemir, E.; Makineci, E.; Yilmaz, E.; Kumbasli, M.; Caliskan, S.; Beskardes, V.; Keten, A.; Zengin, H.; Yilmaz, H. Biomass estimation of individual trees for coppice-riginated oak forests. Eur. J. For. Res. 2019, 138, 623–637. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, M.; Shen, R.; Yang, Q.; Huang, B.; Wan, S. Acclimation of foliar respiration and photosynthesis in response to experimental warming in a temperate steppe in northern China. PLoS ONE 2013, 8, e56482. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B. Modelling respiration of vegetation: Evidence for a general temperature-dependent Q10. Glob. Chang. Biol. 2001, 7, 223–230. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, M.; Shen, R.; Wan, S. Acclimation of leaf dark respiration to nocturnal and diurnal warming in a semiarid temperate steppe. Funct. Plant Biol. 2013, 40, 1159–1167. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Chang. 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Seebacher, F.; Holmes, S.; Roosen, N.J.; Nouvian, M.; Wilson, R.S.; Ward, A.J. Capacity for thermal acclimation differs between populations and phylogenetic lineages within a species. Funct. Ecol. 2012, 26, 1418–1428. [Google Scholar] [CrossRef]
- Carey, J.C.; Tang, J.; Templer, P.H.; Kroeger, K.D.; Crowther, T.W.; Burton, A.J.; Dukes, J.S.; Emmett, B.; Frey, S.D.; Heskel, M.A.; et al. Temperature response of soil respiration largely unaltered with experimental warming. Proc. Natl. Acad. Sci. USA 2016, 113, 13797–13802. [Google Scholar] [CrossRef]
- Heskel, M.A.; O’Sullivan, O.S.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Egerton, J.J.G.; Creek, D.; Bloomfield, K.J.; Xiang, J.; et al. Convergence in the temperature response of leaf respiration across biomes and plant functional types. Proc. Natl. Acad. Sci. USA 2016, 113, 3832–3837. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Fierer, N.; McLauchlan, K.K. Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat. Geosci. 2010, 3, 854–857. [Google Scholar] [CrossRef]
- Kruse, J.; Rennenberg, H.; Adams, M.A. Three physiological parameters capture variation in leaf respiration of Eucalyptus grandis, as elicited by short-term changes in ambient temperature, and differing nitrogen supply. Plant Cell Environ. 2018, 41, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.D.; Rothman, D.H.; Grant, K.E.; Rosengard, S.Z.; Eglinton, T.I.; Derry, L.A.; Galy, V.V. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 2019, 570, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liang, J.; Lu, W.; Chen, H.; Liu, F.; Lin, G.; Xu, F.; Luo, Y.; Lin, G. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Wen, X.; Yu, G.; Sun, X.; Li, Q.; Liu, Y.; Zhang, L.; Ren, C.; Fu, Y.; Li, Z. Soil moisture effect on the temperature dependence of ecosystem respiration in a subtropical Pinus plantation of southeastern china. Agric. For. Meteorol. 2006, 137, 166–175. [Google Scholar] [CrossRef]
- Yu, G.; Wen, X.; Li, Q.; Zhang, L.; Ren, C.; Liu, Y.; Guan, D. The seasonal patterns of ecosystem respiration and its response to environmental conditions for subtropical and temperate typical ecosystems. Sci. China Ser. D-Earth Sci. 2004, 34, 84–94. [Google Scholar]


| Species | Stem | Branch | References | ||
|---|---|---|---|---|---|
| a | b | a | b | ||
| Masson pine | 0.1730 | 2.1540 | 0.0630 | 2.5690 | Xiang et al. 2016 [30] |
| Loblolly pine | 0.0725 | 2.5074 | 0.0016 | 3.0786 | Gonzalez-Benecke et al. 2018 [31] |
| Oak | 0.2486 | 2.1350 | 0.0112 | 2.7353 | Ozdemir et al. 2019 [32] |
| d.f. | F | P | |
|---|---|---|---|
| Vertical position | 2 | 13.03 | <0.001 |
| Forest stand | 2 | 2.62 | 0.091 |
| Vertical position × Forest stand | 4 | 3.62 | 0.017 |
| Sampling date | 7 | 178.81 | <0.001 |
| Sampling date × Vertical position | 14 | 17.40 | <0.001 |
| Sampling date × Forest stand | 14 | 3.36 | <0.001 |
| Sampling date × Vertical position × Forest stand | 28 | 3.64 | <0.001 |
| Q10 | ΔHa | R5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | d.f. | F | p | |
| Vertical position | 2 | 12.29 | <0.001 | 2 | 14.23 | <0.001 | 2 | 2.38 | 0.112 |
| Forest stand | 2 | 1.55 | 0.230 | 2 | 1.13 | 0.338 | 2 | 1.20 | 0.316 |
| Vertical position×Forest stand | 4 | 2.57 | 0.060 | 4 | 2.36 | 0.079 | 4 | 3.34 | 0.024 |
| Respiration | Masson Pine | Loblolly Pine | Oak | Average | |||
|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2009 | 2010 | 2009 | 2010 | ||
| Leaf respiration | 6.03 (55%) | 6.86 (60%) | 6.58 (60%) | 9.17 (66%) | 3.03 (44%) | 2.91 (37%) | 5.76 (54%) |
| Stem respiration | 2.18 (20%) | 2.01 (17%) | 0.66 (6%) | 0.84 (6%) | 0.89 (13%) | 1.09 (14%) | 1.28 (13%) |
| Soil respiration | 2.71 (25%) | 2.62 (23%) | 3.80 (34%) | 3.86 (28%) | 3.04 (44%) | 3.86 (49%) | 3.32 (34%) |
| Ecosystem respiration | 10.92 (100%) | 11.49 (100%) | 11.04 (100%) | 13.87 (100%) | 6.96 (100%) | 7.86 (100%) | 10.36 (100%) |
| Site | Longitude | Latitude | Q10 | Forest Type | References |
|---|---|---|---|---|---|
| Dinghushan | 112°34′ E | 23°10′ N | 1.25 | Evergreen broad-leave forest | Cui et al. 2018 [45] |
| Qianyanzhou | 115°03′ E | 26°44′ N | 1.26 | Evergreen coniferous forest | Cui et al. 2018 [45] |
| Qianyanzhou | 115°03′ E | 26°44′ N | 1.54 | Evergreen coniferous forest | Wen et al. 2006 [46] |
| Qianyanzhou | 115°03′ E | 26°44′ N | 1.57 | Evergreen coniferous forest | Yu et al. 2004 [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Y.; Yang, Q.; Zhou, L.; Shen, R.; Zheng, S.; Zhang, Z.; Zhang, Z.; Xu, M.; Wu, C.; Lin, X.; et al. Temperature Sensitivity in Individual Components of Ecosystem Respiration Increases along the Vertical Gradient of Leaf–Stem–Soil in Three Subtropical Forests. Forests 2020, 11, 140. https://doi.org/10.3390/f11020140
Chi Y, Yang Q, Zhou L, Shen R, Zheng S, Zhang Z, Zhang Z, Xu M, Wu C, Lin X, et al. Temperature Sensitivity in Individual Components of Ecosystem Respiration Increases along the Vertical Gradient of Leaf–Stem–Soil in Three Subtropical Forests. Forests. 2020; 11(2):140. https://doi.org/10.3390/f11020140
Chicago/Turabian StyleChi, Yonggang, Qingpeng Yang, Lei Zhou, Ruichang Shen, Shuxia Zheng, Zhaoyang Zhang, Zhenzhen Zhang, Ming Xu, Chaofan Wu, Xingwen Lin, and et al. 2020. "Temperature Sensitivity in Individual Components of Ecosystem Respiration Increases along the Vertical Gradient of Leaf–Stem–Soil in Three Subtropical Forests" Forests 11, no. 2: 140. https://doi.org/10.3390/f11020140
APA StyleChi, Y., Yang, Q., Zhou, L., Shen, R., Zheng, S., Zhang, Z., Zhang, Z., Xu, M., Wu, C., Lin, X., & Jin, J. (2020). Temperature Sensitivity in Individual Components of Ecosystem Respiration Increases along the Vertical Gradient of Leaf–Stem–Soil in Three Subtropical Forests. Forests, 11(2), 140. https://doi.org/10.3390/f11020140






