Drivers of Spruce Bark Beetle (Ips typographus) Infestations on Downed Trees after Severe Windthrow
Abstract
1. Introduction
2. Materials and Methods
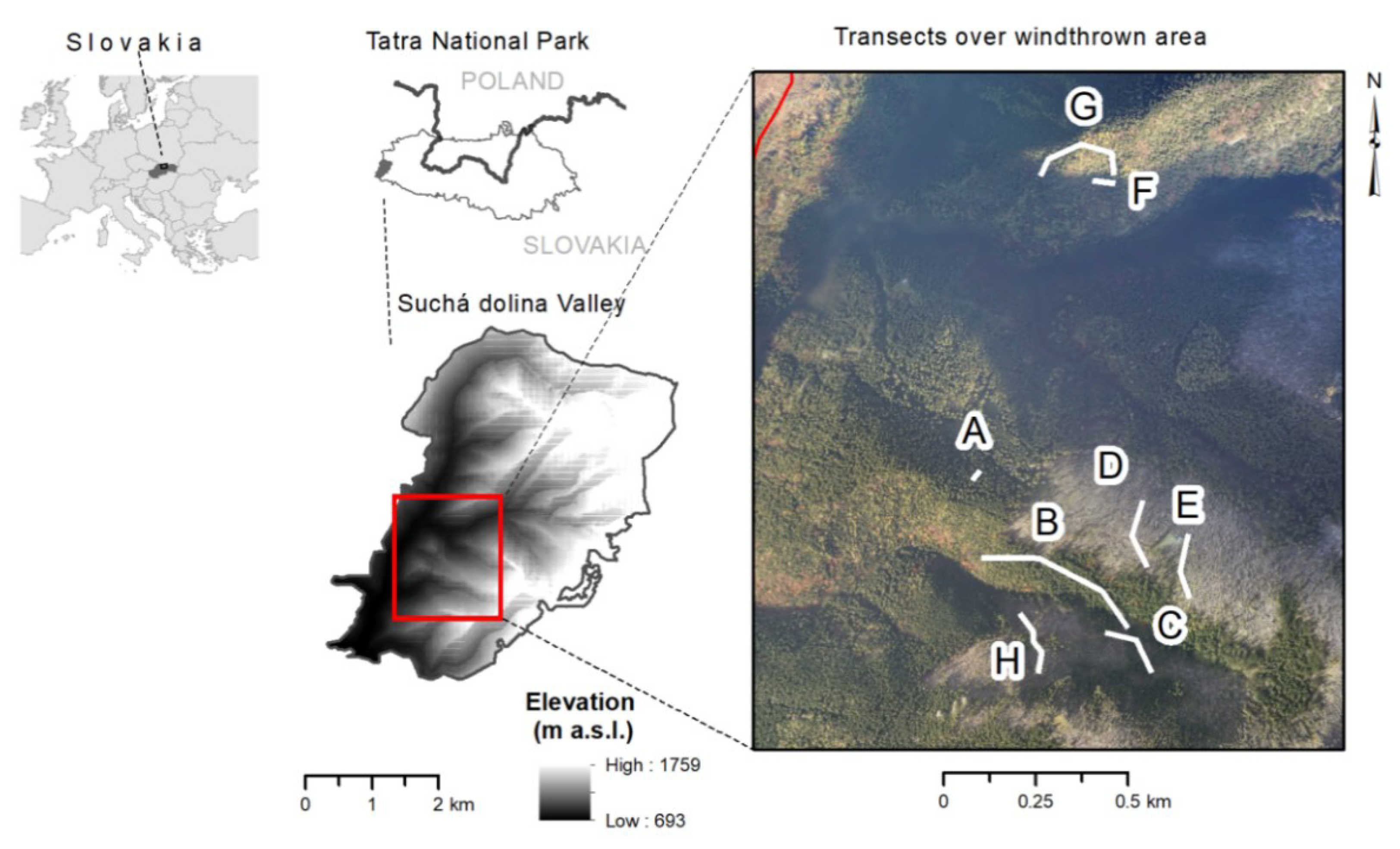
2.1. Study Area
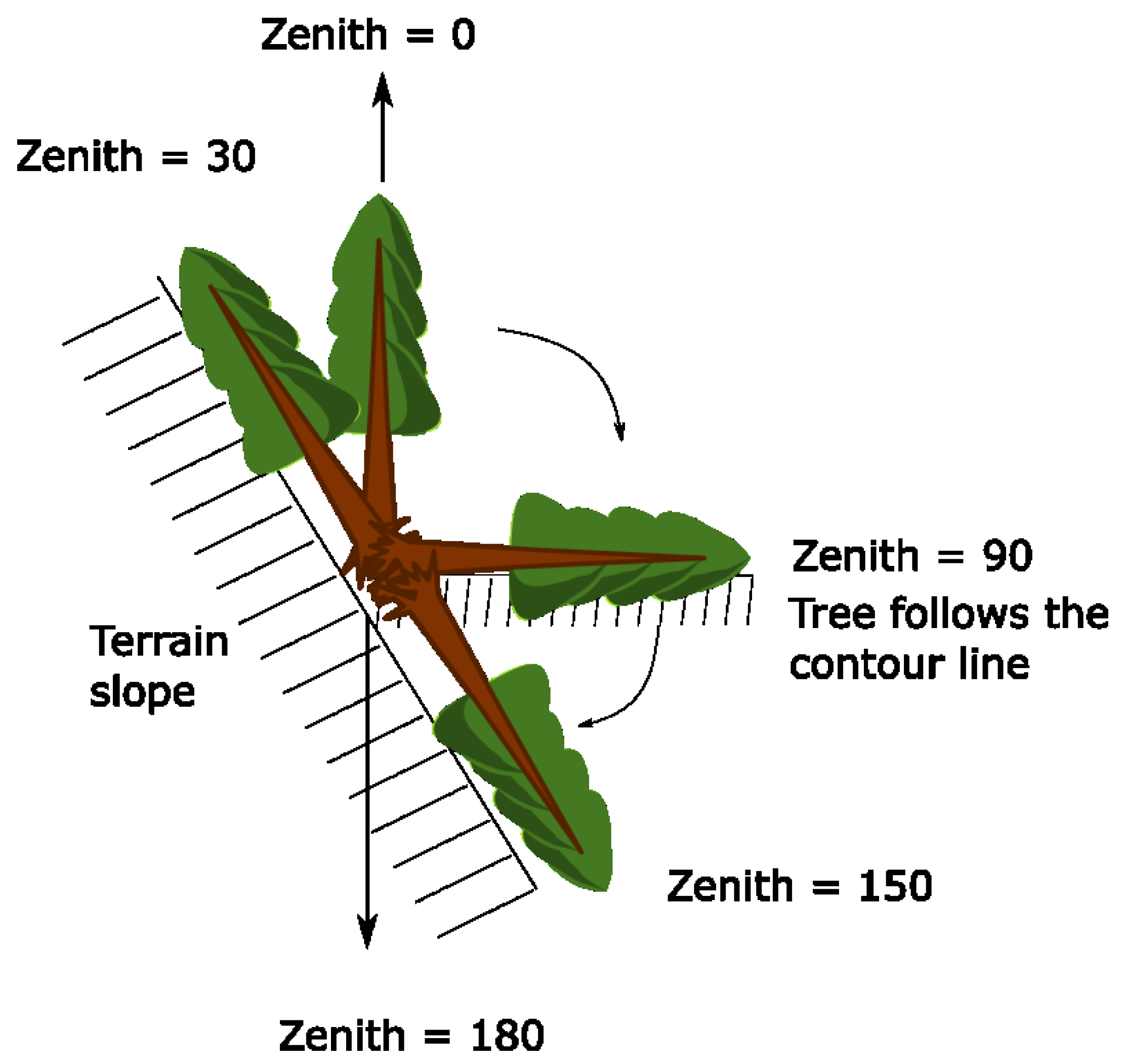
2.2. Data Collection
2.3. Statistical Analyses
3. Results
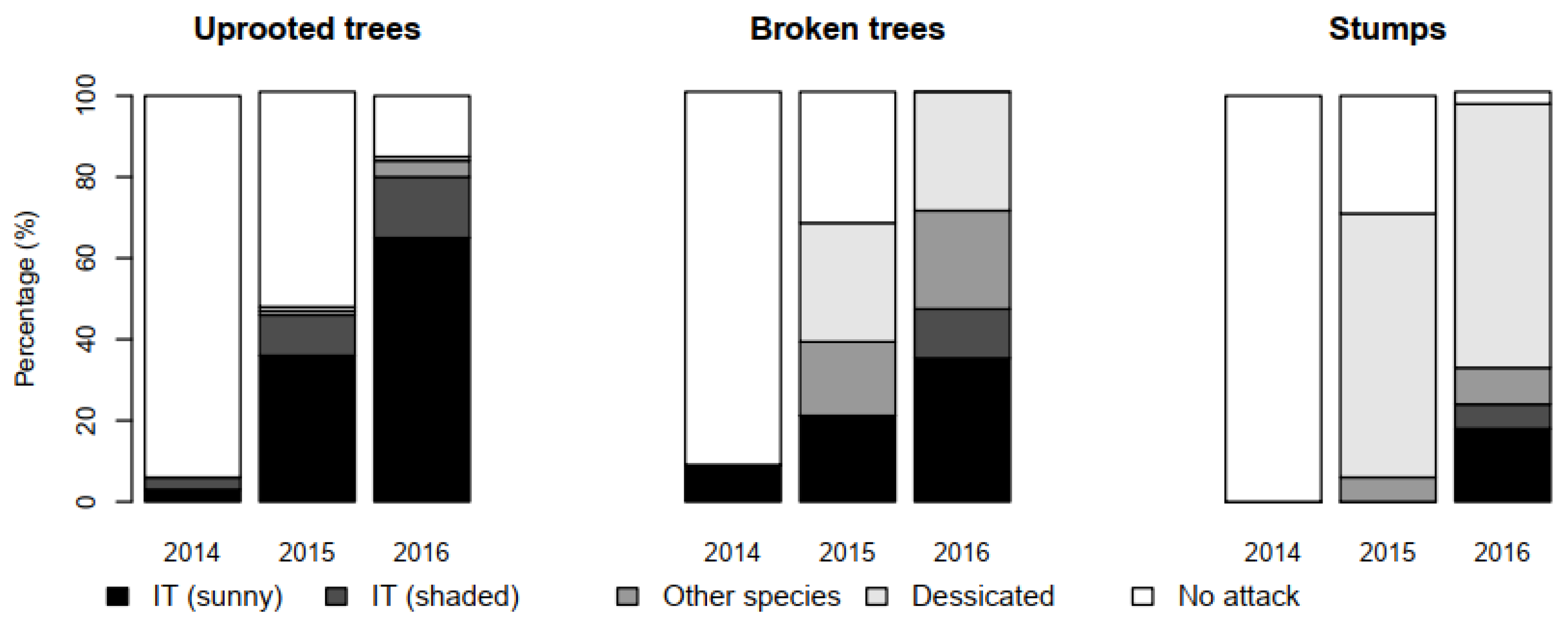
3.1. Infestation Dynamics by Damage Type
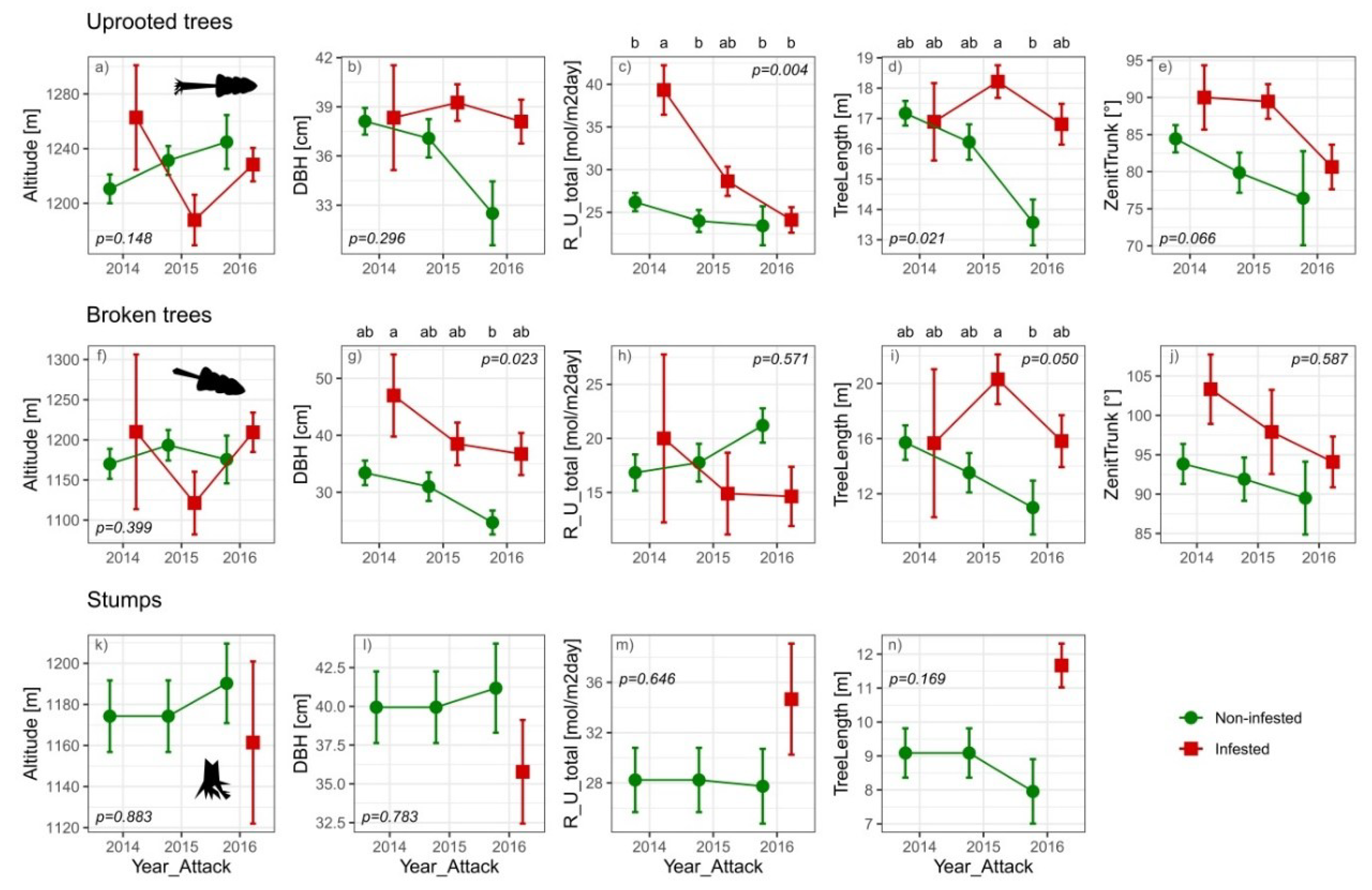
3.2. Environmental and Morphological Parameters of Downed Trees
3.3. Morphological and Environmental Effects on Beetle Infestation
4. Discussion
4.1. General I. typographus Infestation Patterns
4.2. Drivers of I. typographus Infestation Patterns
4.3. Management Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Després, T.; VaItkov, L.; Bace, R.; Cada, V.; Janda, P.; Mikolas, M.; Schurman, J.S.; Trotsiuk, V.; Svoboda, M. Past disturbances and intraspecific competition as drivers of spatial pattern in primary spruce forests. Ecosphere 2017, 8, e02037. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; Mcdowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, P.H.W.; Müller, J.; Grégoire, J.C.; Gruppe, A.; Hagge, J.; Hammerbacher, A.; Hofstetter, R.W.; Kandasamy, D.; Kolarik, M.; Kostovcik, M.; et al. Bark Beetle Population Dynamics in the Anthropocene: Challenges and Solutions. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Roussi, A. Why gigantic locust swarms are challenging governments and researchers. Nature 2020, 579, 330. [Google Scholar] [CrossRef]
- Bartík, M.; Jančo, M.; Střelcová, K.; Škvareninová, J.; Škvarenina, J.; Mikloš, M.; Vido, J.; Waldhauserová, P.D. Rainfall interception in a disturbed montane spruce (Picea abies) stand in the West Tatra Mountains. Biologia 2016, 71, 1002–1008. [Google Scholar] [CrossRef]
- Six, D.; Biber, E.; Long, E. Management for Mountain Pine Beetle Outbreak Suppression: Does Relevant Science Support Current Policy? Forests 2014, 5, 103–133. [Google Scholar] [CrossRef]
- Synek, M.; Janda, P.; Mikoláš, M.; Nagel, T.A.; Schurman, J.S.; Pettit, J.L.; Trotsiuk, V.; Morrissey, R.C.; Bače, R.; Čada, V.; et al. Contrasting patterns of natural mortality in primary Picea forests of the Carpathian Mountains. For. Ecol. Manag. 2020, 457. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Boratynski, A.; Bugala, W. Biology and Ecology of Norway Spruce; Springer: Dordrecht, The Netherlands, 2007; ISBN 9781402048401. [Google Scholar]
- Holeksa, J.; Jaloviar, P.; Kucbel, S.; Saniga, M.; Svoboda, M.; Szewczyk, J.; Szwagrzyk, J.; Zielonka, T.; Żywiec, M. Models of disturbance driven dynamics in the West Carpathian spruce forests. For. Ecol. Manag. 2017, 388, 79–89. [Google Scholar] [CrossRef]
- Janda, P.; Trotsiuk, V.; Mikoláš, M.; Bače, R.; Nagel, T.A.; Seidl, R.; Seedre, M.; Morrissey, R.C.; Kucbel, S.; Jaloviar, P.; et al. The historical disturbance regime of mountain Norway spruce forests in the Western Carpathians and its influence on current forest structure and composition. For. Ecol. Manag. 2017, 388, 67–78. [Google Scholar] [CrossRef]
- Yang, L.H.; Bastow, J.L.; Spence, K.O.; Wright, A.N. What can we learn from resource pulses? Ecology 2008, 89, 621–634. [Google Scholar] [CrossRef]
- Modlinger, R.; Novotný, P. Quantification of time delay between damages caused by windstorms and by Ips typographus. For. J. 2015, 61, 221–231. [Google Scholar] [CrossRef]
- Økland, B.; Nikolov, C.; Krokene, P.; Vakula, J. Transition from windfall- to patch-driven outbreak dynamics of the spruce bark beetle Ips typographus. For. Ecol. Manag. 2016, 363, 63–73. [Google Scholar] [CrossRef]
- Mezei, P.; Grodzki, W.; Blaženec, M.; Jakuš, R. Factors influencing the wind–bark beetles’ disturbance system in the course of an Ips typographus outbreak in the Tatra Mountains. For. Ecol. Manag. 2014, 312, 67–77. [Google Scholar] [CrossRef]
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405. [Google Scholar] [CrossRef]
- Zielonka, T. When does dead wood turn into a substrate for spruce replacement? J. Veg. Sci. 2006, 17, 739. [Google Scholar] [CrossRef]
- Bače, R.; Svoboda, M.; Pouska, V.; Janda, P.; Červenka, J. Natural regeneration in Central-European subalpine spruce forests: Which logs are suitable for seedling recruitment? For. Ecol. Manag. 2012, 266, 254–262. [Google Scholar] [CrossRef]
- Bujoczek, L.; Bujoczek, M.; Banaś, J.; Zięba, S. Spruce regeneration on woody microsites in a subalpine forest in the western Carpathians: Density and occurrence probability. Silva Fenn. 2015, 49, 1337. [Google Scholar] [CrossRef]
- Netherer, S.; Ehn, M.; Blackwell, E.; Kirisits, T. Defence reactions of mature Norway spruce (Picea abies) before and after inoculation of the blue-stain fungus Endoconidiophora polonica in a drought stress experiment. For. J. 2016, 62, 169–177. [Google Scholar] [CrossRef]
- Louis, M.; Dohet, L.; Grégoire, J.C. Fallen trees’ last stand against bark beetles. For. Ecol. Manag. 2016, 359, 44–50. [Google Scholar] [CrossRef]
- Louis, M.; Grégoire, J.-C.; Pélisson, P.-F. Exploiting fugitive resources: How long-lived is “fugitive”? Fallen trees are a long-lasting reward for Ips typographus (Coleoptera, Curculionidae, Scolytinae). For. Ecol. Manag. 2014, 331, 129–134. [Google Scholar] [CrossRef]
- Jakuš Patch level variation on bark beetle attack (Col., Scolytidae) on snapped and uprooted trees in Norway spruce primeval natural forest in endemic condition: Effects of host and insolation. J. Appl. Entomol. 1998, 122, 409–421. [CrossRef]
- Potterf, M.; Nikolov, C.; Kočická, E.; Ferenčík, J.; Mezei, P.; Jakuš, R. Landscape-level spread of beetle infestations from windthrown- and beetle-killed trees in the non-intervention zone of the Tatra National Park, Slovakia (Central Europe). For. Ecol. Manag. 2019, 432, 489–500. [Google Scholar] [CrossRef]
- Jakuš, R. Bark beetle (Col., Scolytidae) communities and host and site factors on tree level in Norway spruce primeval natural forest. J. Appl. Entomol. 1995, 119, 643–651. [Google Scholar] [CrossRef]
- Hinze, J.; John, R. Effects of heat on the dispersal performance of Ips typographus. J. Appl. Entomol. 2020, 144, 144–151. [Google Scholar] [CrossRef]
- Baier, P.; Pennerstorfer, J.; Schopf, A. PHENIPS—A comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. For. Ecol. Manag. 2007, 249, 171–186. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Schroeder, L.M.; Lagergren, F.; Anderbrant, O.; Smith, B. Guess the impact of Ips typographus—An ecosystem modelling approach for simulating spruce bark beetle outbreaks. Agric. For. Meteorol. 2012, 166–167, 188–200. [Google Scholar] [CrossRef]
- Jakoby, O.; Lischke, H.; Wermelinger, B. Climate change alters elevational phenology patterns of the European spruce bark beetle (Ips typographus). Glob. Chang. Biol. 2019, 25, 4048–4063. [Google Scholar] [CrossRef]
- Bentz, B.J.; Jönsson, A.M.; Schroeder, M.; Weed, A.; Wilcke, R.A.I.; Larsson, K. Ips typographus and Dendroctonus ponderosae Models Project Thermal Suitability for Intra- and Inter-Continental Establishment in a Changing Climate. Front. For. Glob. Chang. 2019, 2. [Google Scholar] [CrossRef]
- Sproull, G.J.; Adamus, M.; Bukowski, M.; Krzyżanowski, T.; Szewczyk, J.; Statwick, J.; Szwagrzyk, J. Tree and stand-level patterns and predictors of Norway spruce mortality caused by bark beetle infestation in the Tatra Mountains. For. Ecol. Manag. 2015, 354, 261–271. [Google Scholar] [CrossRef]
- Netherer, S.; Nopp-Mayr, U. Predisposition assessment systems (PAS) as supportive tools in forest management—Rating of site and stand-related hazards of bark beetle infestation in the High Tatra Mountains as an example for system application and verification. For. Ecol. Manag. 2005, 207, 99–107. [Google Scholar] [CrossRef]
- Hanson, J.J.; Lorimer, C.G. Forest structure and light regimes following moderate wind storms: Implications for multi-cohort management. Ecol. Appl. 2007, 17, 1325–1340. [Google Scholar] [CrossRef]
- Emmel, C.; Paul-Limoges, E.; Black, T.A.; Christen, A. Vertical Distribution of Radiation and Energy Balance Partitioning Within and Above a Lodgepole Pine Stand Recovering from a Recent Insect Attack. Bound. Layer Meteorol. 2013, 149, 133–163. [Google Scholar] [CrossRef]
- Vanderhoof, M.; Williams, C.A.; Shuai, Y.; Jarvis, D.; Kulakowski, D.; Masek, J. Albedo-induced radiative forcing from mountain pine beetle outbreaks in forests, south-central Rocky Mountains: Magnitude, persistence, and relation to outbreak severity. Biogeosciences 2014, 11, 563–575. [Google Scholar] [CrossRef]
- Grodzki, W.; Jakuš, R.; Lajzová, E.; Sitková, Z.; Maczka, T.; Škvarenina, J. Effects of intensive versus no management strategies during an outbreak of the bark beetle Ips typographus (L.) (Col.: Curculionidae, Scolytinae) in the Tatra Mts. in Poland and Slovakia. Ann. For. Sci. 2006, 63, 55–61. [Google Scholar] [CrossRef]
- Thom, D.; Seidl, R.; Steyrer, G.; Krehan, H.; Formayer, H. Slow and fast drivers of the natural disturbance regime in Central European forest ecosystems. For. Ecol. Manag. 2013, 307, 293–302. [Google Scholar] [CrossRef]
- Mezei, P.; Jakuš, R.; Pennerstorfer, J.; Havašová, M.; Škvarenina, J.; Ferenčík, J.; Slivinský, J.; Bičárová, S.; Bilčík, D.; Blaženec, M.; et al. Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—An infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agric. For. Meteorol. 2017, 242, 85–95. [Google Scholar] [CrossRef]
- Školek, J. Flora and vegetation of the National Nature Reserve Suchá dolina in the West Tatra Mts. In Štúdie o Tatranskom Národnom Parku; Popradské noviny: Poprad, Slovakia, 2004; pp. 109–187. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 027–046. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An introduction with R.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006; ISBN 1584884746. [Google Scholar]
- Mellor, A.F.P.; Cey, E.E. Using generalized additive mixed models to assess spatial, temporal, and hydrologic controls on bacteria and nitrate in a vulnerable agricultural aquifer. J. Contam. Hydrol. 2015, 182, 104–116. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; Volume 36, ISBN 978-0-387-87458-6. [Google Scholar]
- R Development Core Team. The R Project for Statistical Computing. Available online: http://r-project.org (accessed on 24 October 2020).
- Vakula, J.; Zúbrik, M.; Brutovský, D.; Gubka, A.; Ferenčík, J.; Kaštier, P.; Kunca, A.; Leontovyč, R.; Longauerová, V.; Nikolov, C.; et al. Projekt ochrany lesa na území TANAP-u po vetrovej kalamite zo dňa 19.11.2004 pre štátne a neštátne subjekty—Realizačný projekt pre rok 2006. Zvolen Lesnícky Výskumný Ústav 2007, 140, 1–80. [Google Scholar]
- Kautz, M.; Schopf, R.; Imron, M.A. Individual traits as drivers of spatial dispersal and infestation patterns in a host-bark beetle system. Ecol. Modell. 2014, 273, 264–276. [Google Scholar] [CrossRef]
- Mezei, P.; Potterf, M.; Škvarenina, J.; Rasmussen, J.G.; Jakuš, R. Potential Solar Radiation as a Driver for Bark Beetle Infestation on a Landscape Scale. Forests 2019, 10, 604. [Google Scholar] [CrossRef]
- Zumr, V. Biologie a Ekologie Lýkožrouta Smrkového (Ips typographus) a Ochrana Proti Němu; ACADEMIA, nakladatelství ČSAV: SPraha, Czech Republic, 1985. [Google Scholar]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017. in print. [Google Scholar] [CrossRef]
- Matthews, B.; Netherer, S.; Katzensteiner, K.; Pennerstorfer, J.; Blackwell, E.; Henschke, P.; Hietz, P.; Rosner, S.; Jansson, P.E.; Schume, H.; et al. Transpiration deficits increase host susceptibility to bark beetle attack: Experimental observations and practical outcomes for Ips typographus hazard assessment. Agric. For. Meteorol. 2018, 263, 69–89. [Google Scholar] [CrossRef]
- Blomqvist, M.; Kosunen, M.; Starr, M.; Kantola, T.; Holopainen, M.; Lyytikäinen-Saarenmaa, P. Modelling the predisposition of Norway spruce to Ips typographus L. infestation by means of environmental factors in southern Finland. Eur. J. For. Res. 2018, 137, 675–691. [Google Scholar] [CrossRef]
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Pennerstorfer, J.; Rosner, S.; Kikuta, S.; Schume, H.; et al. Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Rouault, G.; Candau, J.; Lieutier, F.; Nageleisen, L.; Martin, J.; Warzee, N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann. For. Sci. 2006, 63, 613–624. [Google Scholar] [CrossRef]
- White, T.C.R. Are outbreaks of cambium-feeding beetles generated by nutritionally enhanced phloem of drought-stressed trees? J. Appl. Entomol. 2015, 139, 567–578. [Google Scholar] [CrossRef]
- White, T.C.R. An alternative hypothesis explains outbreaks of conifer-feeding budworms of the genus Choristoneura (Lepidoptera: Tortricidae) in Canada. J. Appl. Entomol. 2018, 142, 725–730. [Google Scholar] [CrossRef]
- Mezei, P.; Grodzki, W.; Blaženec, M.; Škvarenina, J.; Brandýsová, V.; Jakuš, R. Host and site factors affecting tree mortality caused by the spruce bark beetle (Ips typographus) in mountainous conditions. For. Ecol. Manag. 2014, 331, 196–207. [Google Scholar] [CrossRef]
- Jurc, M.; Perko, M.; Džeroski, S.; Demšar, D.; Hrašovec, B. Spruce bark beetles (Ips typographus, Pityogenes chalcographus, Col.: Scolytidae) in the Dinaric mountain forests of Slovenia: Monitoring and modeling. Ecol. Modell. 2006, 194, 219–226. [Google Scholar] [CrossRef]
- Akkuzu, E.; Sariyildiz, T.; Kucuk, M.; Duman, A. Ips typographus (L.) and Thanasimus formicarius (L.) populations influenced by aspect and slope position in Artvin-Hatila valley national park, Turkey. Afr. J. Biotechnol. 2009, 8, 877–882. [Google Scholar]
- Mezei, P.; Jakuš, R.; Blaženec, M.; Belánová, S.; Šmídt, J. The relationship between potential solar radiation and spruce bark beetle catches in pheromone traps. Ann. For. Res. 2012, 55, 243–252. [Google Scholar]
- Fahse, L.; Heurich, M. Simulation and analysis of outbreaks of bark beetle infestations and their management at the stand level. Ecol. Modell. 2011, 222, 1833–1846. [Google Scholar] [CrossRef]


| Parameter | Description | Units |
|---|---|---|
| IT | Presence/absence of I. typographus attack | Presence/absence |
| Position | Attack location on trunk: on sun exposed; ground facing side; or dried bark (not suitable for beetle infestation) | IT_sunny, IT_shaded, desiccated |
| Species | Bark beetle species other than I. typographus | Species name |
| Year_of_attack | Year of attack | 2014, 2015, 2016 |
| Acronym | Description | Units | Type | Min | Max | Mean |
|---|---|---|---|---|---|---|
| Altitude | Tree altitude | [m] | Site | 971 | 1400 | 1201 |
| Aspect_terrain | Terrain aspect | [°] | Site | 5 | 360 | 280 |
| Slope_terrain | Terrain slope | [%] | Site | 0 | 55 | 28 |
| Openess | Canopy openness | [-] | Site | 6 | 95 | 46 |
| R_O_total | Total solar radiation above canopy | [mol.m−2day−1] | Site | 12 | 59 | 44 |
| R_U_total | Total solar radiation under canopy | [mol.m−2day−1] | Site | 2 | 53 | 27 |
| Aspct_trunk | Trunk zenith | [°] | Tree | 0 | 350 | 151 |
| Connection_roots | Degree of root-soil connectivity | [%] | Tree | 0 | 100 | 40 |
| Crown_length | Tree crown length | [m] | Tree | 0 | 22 | 7 |
| Crown_Percentage | Crown ratio as a percentage of tree length | [%] | Tree | 0 | 100 | 30 |
| DBH | Diameter at breast height | [cm] | Tree | 13 | 66 | 38 |
| Tree_length | Length of tree | [m] | Tree | 1 | 32 | 16 |
| Slope_trunk | Slope of tree | [°] | Tree | −40 | 90 | 17 |
| Zenith_Trunk | Zenith angle of tree | [°] | Tree | 0 | 130 | 73 |
| Parameter | Estimate | Std. Error | t-Value | p-Value |
|---|---|---|---|---|
| Crown length | −0.061523 | 0.100652 | −0.611 | 0.5412 |
| Year_of_attack | 2.354738 | 0.150526 | 15.643 | <0.01 * |
| Crown_Percentage | 0.002991 | 0.015845 | 0.189 | 0.8503 |
| DBH | 0.023468 | 0.015943 | 1.472 | 0.1414 |
| Connection_roots | 0.013493 | 0.009059 | 1.489 | 0.1367 |
| Tree_length | 0.178461 | 0.060368 | 2.956 | 0.0032 * |
| Approximate significance of smooth terms (nonlinear variables) | ||||
| Parameter | edf | F | p-value | |
| R_U_total | 2.720 | 5.914 | <0.01 * | |
| Zenith_Trunk | 2.436 | 10.498 | <0.01 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hroššo, B.; Mezei, P.; Potterf, M.; Majdák, A.; Blaženec, M.; Korolyova, N.; Jakuš, R. Drivers of Spruce Bark Beetle (Ips typographus) Infestations on Downed Trees after Severe Windthrow. Forests 2020, 11, 1290. https://doi.org/10.3390/f11121290
Hroššo B, Mezei P, Potterf M, Majdák A, Blaženec M, Korolyova N, Jakuš R. Drivers of Spruce Bark Beetle (Ips typographus) Infestations on Downed Trees after Severe Windthrow. Forests. 2020; 11(12):1290. https://doi.org/10.3390/f11121290
Chicago/Turabian StyleHroššo, Branislav, Pavel Mezei, Mária Potterf, Andrej Majdák, Miroslav Blaženec, Nataliya Korolyova, and Rastislav Jakuš. 2020. "Drivers of Spruce Bark Beetle (Ips typographus) Infestations on Downed Trees after Severe Windthrow" Forests 11, no. 12: 1290. https://doi.org/10.3390/f11121290
APA StyleHroššo, B., Mezei, P., Potterf, M., Majdák, A., Blaženec, M., Korolyova, N., & Jakuš, R. (2020). Drivers of Spruce Bark Beetle (Ips typographus) Infestations on Downed Trees after Severe Windthrow. Forests, 11(12), 1290. https://doi.org/10.3390/f11121290






