Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Sites
2.2. Collection and Analysis of Plant Material
2.3. Statistical Analysis
2.4. Weather Conditions
- p—the total monthly rainfall (mm),
- Σt—the monthly total of average daily air temperatures > 0 °C.
3. Results
3.1. Soil Conditions
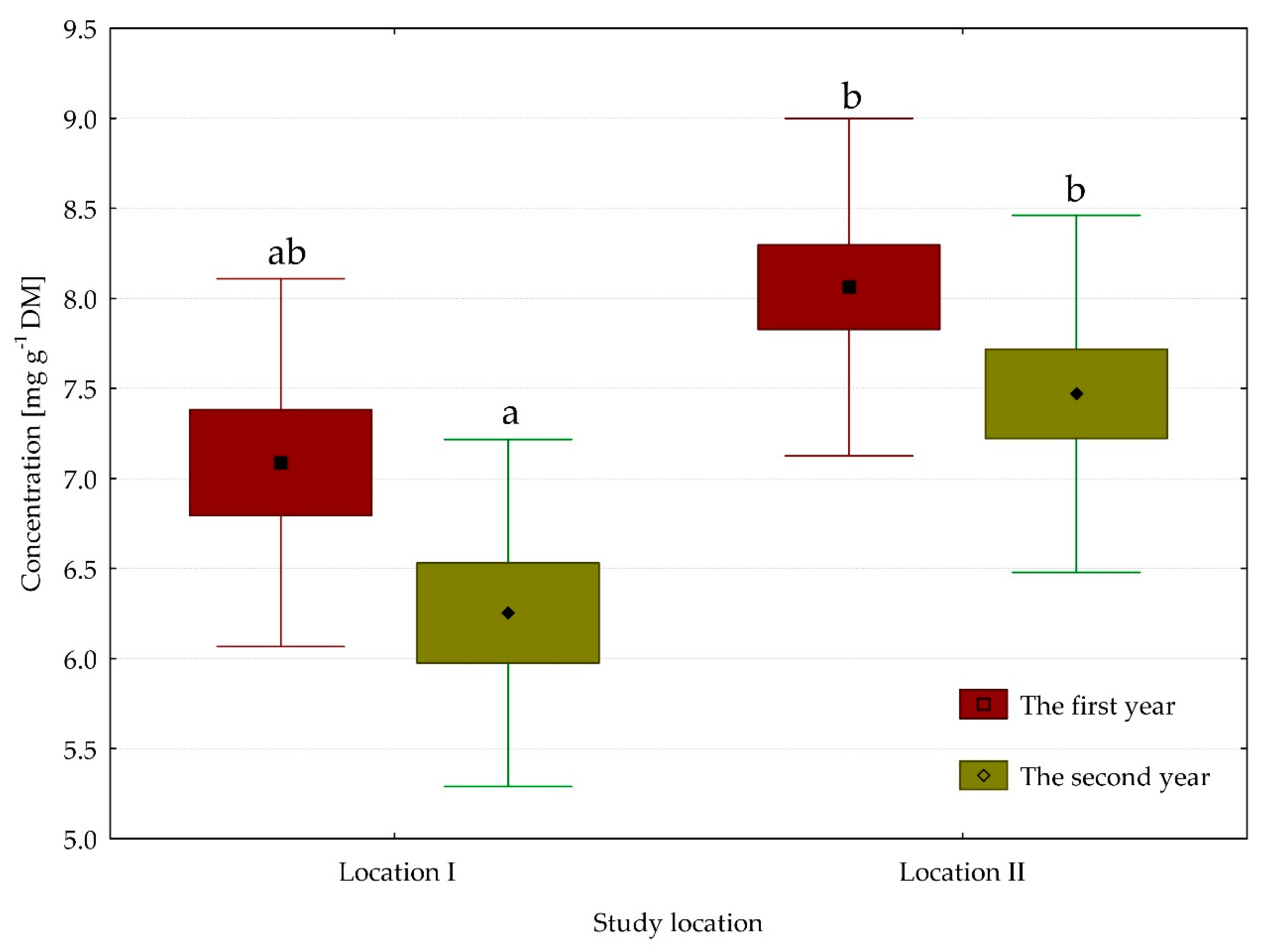
3.2. Content of Chlorophyll Pigments
3.3. Content of Carotene Pigments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frey, L. Trawy niezwyciężone (wybrane zagadnienia z historii, taksonomii i biologii Poaceae). Grassld. Sci. Pol. 2000, 3, 9–17. [Google Scholar]
- Kozłowski, S.; Goliński, P.; Golińska, B. Pozapaszowa funkcja traw. Grassld. Sci. Pol. 2000, 3, 79–94. [Google Scholar]
- Kozłowski, S.; Swędrzyński, A. Traw śródleśnych piękno. Mater. Ośrodka Kult. Leśnej 2009, 8, 47–58. [Google Scholar]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland ecosystem services: A systematic review of research advances and future directions. Landsc. Ecol. 2020, 35, 793–814. [Google Scholar] [CrossRef]
- Ferchmin, M. Szata roślinna wydm i bagien Puszczy Kampinowskiej. W: Z Mazowsza na Polesie i Wileńszczyzną. Zróżnicowanie i ochrona szaty roślinnej pogranicza Europy Środkowej i Północno-Wschodniej. Pod red. A. Obidzińskiego. In Monografia Sesji Terenowej LV Zjazdu Polskiego Towarzystwa Botanicznego Planta In Vivo, In Vitro et In Silico; PTB: Warszawa, Poland, 2010; pp. 57–66. [Google Scholar]
- Zając, A.; Zając, M. Atlas rozmieszczenia roślin naczyniowych w Polsce; Nakład Pracowni Chorologii Komputerowej Instytutu Botaniki UJ: Kraków, Poland, 2001. [Google Scholar]
- Tsvelev, N.N. Zlaki SSSR; Nauka: Leningrad, Russia, 1976. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europea; Cambridge University Press: Cambridge, UK, 1974; Volume 5. [Google Scholar]
- Dżugan, M. Czynniki wpływające na stabilność zielonych barwników roślin. Zesz. Nauk. PTG 2006, 7, 27–33. [Google Scholar]
- Rajalakshmi, K.; Banu, N. Extraction and estimation of chlorophyll from medicinal plants. Intern. J. Sci. Res. 2015, 4, 209–212. [Google Scholar]
- Falkowski, M.; Olszewska, L.; Kukułka, I.; Kozłowski, S. Reakcja odmian życicy trwałej (Lolium perenne L.) na azot i wodę. Biul. Oceny Odmian 1986, 16, 103–112. [Google Scholar]
- Zielewicz, W.; Kozłowski, S. Żywotność Sorghum saccharatum (L.) Pers. w aspekcie możliwości jego uprawy w Polsce. Fragm. Flor. Geobot. Polon. 2007, 9, 173–181. [Google Scholar]
- Gregorczyk, A.; Raczyńska, A. Badania korelacji między metodą Arnona a pomiarami zawartości chlorofilu za pomocą chlorofilometru. Zesz. Nauk. AR Szczec. Rol. 1997, 68, 119–123. [Google Scholar]
- Falkowski, M.; Kukułka, I. Zawartość chlorofilu jako wskaźnika biologicznych właściwości roślin łąkowych. Roczn. Nauk. Rol. Seria F 1977, 79, 87–104. [Google Scholar]
- Kozłowski, S.; Goliński, P.; Golińska, B. Barwniki chlorofilowe jako wskaźniki wartości użytkowej gatunków i odmian traw. Zesz. Probl. Post. Nauk. Rol. 2001, 474, 215–223. [Google Scholar]
- Goliński, P. Efektywność nawożenia azotem w produkcji nasion Lolium perenne L. Rozpr. Nauk. Akad. Rol. Pozn. 2001, 321, 20–29. [Google Scholar]
- Selzer, L.J.; Busso, C.A. Pigments and photosynthesis of understory grasses: Light irradiance and soil moisture effects. Russ. J. Plant Phys. 2016, 63, 224–234. [Google Scholar] [CrossRef]
- Golińska, B. Chlorofil jako wskaźnik azotowej kondycji Poa pratensis (Poaceae) w warunkach wielokrotnej defoliacji jej runi. Fragm. Flor. Geobot. Pol. 2007, 9, 137–145. [Google Scholar]
- Gáborčik, N. Relationship between contents of chlorophyll (a+b) (SPAD values) and nitrogen of some temperate grasses. Photosynthetica 2003, 41, 285–287. [Google Scholar] [CrossRef]
- Gáborčik, N. Koncentrácia minerálnych živin chlorofylu a+b (SPAD hodnoty) v listoch tokajskỳch odrôd viniča. Vinič Vino 2006, 3, 2–4. [Google Scholar]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Bioch. Bioph. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, M.; Kukułka, I. Zawartość karotenu jako cecha charakterystyczna roślin łąkowych. Roczn. Nauk. Roln. Seria F 1977, 79, 105–112. [Google Scholar]
- Kozłowski, S.; Kukułka, I. Zróżnicowanie polskich odmian hodowlanych Lolium perenne pod względem barwników. Pr. Zakr. Nauk. Rol. PTPN 1996, 81, 103–111. [Google Scholar]
- Olszewska, M. Wpływ stresu wodnego na intensywność fotosyntezy, zawartość chlorofilu i plonowanie Lolium perenne. Grassld. Sci. Pol. 2002, 5, 163–172. [Google Scholar]
- Smith, J.H.C.; Benitez, A. Chlorophylls: Analysis in plant materials. In Moderne Methoden der Pflanzenanalyse, Band 4; Peach, K., Tracey, M.V., Eds.; Springer: Berlin/Heidelberg, Germany, 1955; pp. 142–196. [Google Scholar]
- Berger, S. Metoda ilościowego oznaczania beta karotenu (Prowitamina A) i sumy karotenoidów w niektórych produktach roślinnych. Rocz. Państwowego Zakładu Hig. 1953, 4, 473–479. [Google Scholar]
- Selyaninov, G.T. Methods of climate description to agricultural purposes. In World Climate and Agriculture Handbook; Selyaninov, G.T., Ed.; Gidrometeoizdat: Leningrad, Russia, 1937; pp. 5–27. [Google Scholar]
- Skowera, B.; Puła, J. Skrajne warunki pluwiotermiczne w okresie wiosennym na obszarze Polski w latach 1971–2000. Acta Agroph. 2004, 3, 171–177. [Google Scholar]
- Zielewicz, W.; Kozłowski, S. Występowanie barwników chlorofilowych i karotenowych w trawach leśnych. Grassld. Sci. Pol. 2011, 14, 161–170. [Google Scholar]
- Kozłowski, S.; Swędrzyński, A. Zmienność występowania barwników chlorofilowych i karotenoidowych w odmianach hodowlanych Lolium perenne (Poaceae). Fragm. Flor. Geobot. Pol. 2007, 9, 163–171. [Google Scholar]
- Noziere, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Strusińska, D.; Antoszkiewicz, Z.; Kaliniewicz, J. The concentrations of β-carotene, vitamin A and vitamin E in bovine milk in regard to the feeding season and the share of concentrate in the feed ration. Rocz. Nauk. Pol. Tow. Zoot. 2010, 6, 213–220. [Google Scholar]
- Noziere, P.; Groiler, P.; Durand, D.; Ferlay, A.; Pradel, P.; Martin, B. Variations in carotenoids, fatsoluble micronutrients and color in cows plasma and milk following changes in forage and feeding level. J. Dairy Sci. 2006, 89, 2634–2648. [Google Scholar] [CrossRef] [Green Version]
- Chauveau-Duriot, B.; Thomas, D.; Portelli, J.; Doreau, M. Carotenoids content in forages: Variation during conservation. Renc. Rech. Rumin. 2005, 12, 117. [Google Scholar]
- Prache, S.; Priolo, A.; Groiler, P. Persistence of carotenoid pigments in the blood of concentrate-finished grazing sheep: Its significance for traceability of grass-feeding. J. Anim. Sci. 2003, 81, 360–367. [Google Scholar] [CrossRef]
- Reynoso, C.R.; Mora, O.; Nieves, V.; Shimada, A.; De Mejia, E.G. Beta-carotene and lutein in forage and bovine adipose tissue in two tropical regions of Mexico. Anim. Feed Sci. Technol. 2004, 113, 183–190. [Google Scholar] [CrossRef]
- Williams, P.E.V.; Ballet, N.; Robert, J.C. A review of the provision of vitamins for ruminants. In Proceedings of the Preconference Symp. of the Cornell Nutrition Conference 1998. Provision of Vitamins and Amino Acids for Ruminants; Rhone Poulen Animal Nutrition: Anthony, France, 1998; pp. 7–37. [Google Scholar]
- Kalač, P. The effects of silage feeding on some sensory and heath attributes of cow’s milk. Rev. Food Chem. 2011, 125, 307–317. [Google Scholar] [CrossRef]
- Krzyżewski, J.; Strzałkowska, N.; Bagnicka, E.; Jóźwik, A.; Horbańczuk, J.O. Wpływ antyoksydantów zawartych w tłuszczu pasz objętościowych na jakość mleka krów. Żywność Nauka Technol. Jakość 2012, 3, 35–45. [Google Scholar]
- Grzebisz, W.; Przygocka-Cyna, K.; Szczepaniak, W.; Diatta, J.B.; Potarzycki, J. Magnesium as a nutritional tool of nitrogen efficient management—plant production and environment. J. Elementol. 2010, 15, 771–788. [Google Scholar] [CrossRef] [Green Version]
- Mazur, T.; Rogalski, L. Wpływ nawożenia mineralnego na cechy morfologiczne łodyg i zawartość barwników w liściach ziemniaków. Acta Agrobot. 1977, 30, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewski, B.; Majtkowska, G.; Majtkowski, W. Zawartość chlorofilu u wybranych ekotypów i odmian prosa rózgowatego (Panicum virgatum L.) w warunkach zróżnicowanego nawożenia. Biul. Inst. Hod. Akl. Roś. 2016, 280, 71–77. [Google Scholar]
- Higgins, S.; Morrison, S.; Watson, C.J. Effect of annual applications of pelletised dolomitic lime on soil chemical properties and grass productivity. Soil Use Manag. 2012, 28, 62–69. [Google Scholar] [CrossRef]
- Suttle, N.F. Mineral nutrition of Livestock. 2010. Available online: www.carbo.org (accessed on 4 June 2020).
- Kavanová, M.; Lattanzini, F.A.; Grimoldi, A.A.; Schnyder, H. Phosphorus deficiency decreases cell division and elongation in grass leaves. Plant Phys. 2006, 41, 766–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duru, M.; Ducrocq, H. A nitrogen and phosphorus herbage nutrient index as a tool for assessing the effect of N and P supply on the dry matter yield of permanent pastures. Nutr. Cycl. Agroecosyst. 1997, 47, 59–69. [Google Scholar] [CrossRef]
- Shipley, B.; Almeida-Cortez, J. Interspecific consistency and intraspecific variability of specific leaf area with respect to irradiance and nutrient availability. Ecoscience 2003, 10, 74–79. [Google Scholar] [CrossRef]
- Gommers, C.M.M.; Visser, E.J.W.; Onge, K.R.S.; Voesenek, L.A.C.J.; Ronald, P. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 1360–1385. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Dias-Filho, M.B. Photosynthetic light response of the C4 grasses Brachiaria brizantha and B. humidicola under shade. Sci. Agric. 2002, 59, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.Y.; Azmi, A.R.; Khan, A.H.; Ala, S.A. Effect of water stress on total phenols, peroxidase activity and chlorophyll content in wheat. Acta Physiol. Plant. 1994, 16, 185–191. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Sourour, A.; Afef, O.; Mounir, R.; Mongi, B.Y.A. Morphological, physiological, biochemical and molecular plant responses to water deficit stress. Intern. J. Eng. Sci. 2017, 6, 1–4. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Żebrowski, M. Intensywność fotosyntezy jedno- i dwuliściennych roślin C3 i C4 w różnych warunkach środowiska. Zesz. Probl. Post. Nauk. Rol. 2004, 496, 133–142. [Google Scholar]
- Olszewska, M.; Grzegorczyk, S.; Olszewski, J.; Bałuch-Małecka, A. Porównanie reakcji wybranych gatunków traw na stres wodny. Grassld. Sci. Pol. 2010, 13, 127–136. [Google Scholar]
- Falkowski, M. Trawy Polskie; Praca zbiorowa pod redakcją M. Falkowskiego; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 1982. [Google Scholar]
- Falkowski, M.; Kukułka, I.; Kozłowski, S. Właściwości chemiczne roślin łąkowych; Wydawnictwo Akademii Rolniczej im. Augusta Cieszkowskiego w Poznaniu: Poznań, Poland, 2000; pp. 59–84. [Google Scholar]





| Month | Average Air Temperature (°C) | Total Rainfall (mm) | Selyaninov’s Coeficient K | |||
|---|---|---|---|---|---|---|
| 2007 | 2008 | 2007 | 2008 | 2007 | 2008 | |
| April | 12.7 | 10.0 | 7.4 | 77.5 | 0.19 | 2.58 |
| May | 16.8 | 16.2 | 73.1 | 9.5 | 1.40 | 0.19 |
| June | 20.6 | 20.6 | 44.3 | 8.4 | 0.72 | 0.14 |
| July | 19.9 | 22.2 | 72.2 | 46.6 | 1.17 | 0.68 |
| August | 20.6 | 19.7 | 65.7 | 88.6 | 1.03 | 1.45 |
| September | 14.6 | 14.4 | 32.6 | 16.8 | 0.74 | 0.39 |
| October | 9.0 | 9.9 | 20.3 | 9.4 | 0.73 | 0.31 |
| Mean/Sum | 16.3 | 16.1 | 315.6 | 256.8 | 0.85 | 0.82 |
| Minerals and Soil Reaction | Mean | Minimum | Maximum | Standard Deviation | Variation Coefficient (%) |
|---|---|---|---|---|---|
| Year 2007 (n = 3) | |||||
| pH | 3.56 | 3.32 | 3.76 | 0.22 | 6.2 |
| P2O5 | 7.54 | 6.64 | 8.68 | 1.04 | 13.8 |
| K2O | 6.09 | 5.18 | 6.78 | 0.82 | 13.4 |
| MgO | 5.40 | 5.07 | 5.67 | 0.30 | 5.5 |
| Year 2008 (n = 3) | |||||
| pH | 3.55 | 3.43 | 3.66 | 0.11 | 3.2 |
| P2O5 | 6.79 | 6.42 | 7.12 | 0.35 | 5.1 |
| K2O | 6.12 | 5.43 | 6.44 | 0.62 | 10.2 |
| MgO | 5.34 | 5.13 | 5.57 | 0.22 | 4.1 |
| Average over the years (n = 6) | |||||
| pH | 3.55 | 3.32 | 3.76 | 0.16 | 4.7 |
| P2O5 | 7.16 | 6.42 | 8.68 | 0.69 | 9.5 |
| K2O | 6.10 | 5.18 | 6.78 | 0.72 | 11.8 |
| MgO | 5.37 | 5.07 | 5.67 | 0.26 | 4.8 |
| Minerals and Soil Reaction | Mean | Minimum | Maximum | Standard Deviation | Variation Coefficient (%) |
|---|---|---|---|---|---|
| Year 2007 (n = 3) | |||||
| pH | 3.37 | 3.18 | 3.62 | 0.22 | 6.7 |
| P2O5 | 8.75 | 8.29 | 9.42 | 0.59 | 6.7 |
| K2O | 6.89 | 6.32 | 7.46 | 0.57 | 8.2 |
| MgO | 6.70 | 5.12 | 8.66 | 1.79 | 26.8 |
| Year 2008 (n = 3) | |||||
| pH | 3.30 | 3.18 | 3.43 | 0.12 | 3.7 |
| P2O5 | 8.03 | 7.42 | 8.66 | 0.57 | 7.1 |
| K2O | 6.74 | 5.77 | 7.78 | 1.00 | 14.9 |
| MgO | 6.28 | 5.57 | 6.88 | 0.66 | 10.5 |
| Average over the years (n = 6) | |||||
| pH | 3.33 | 3.18 | 3.62 | 0.17 | 5.2 |
| P2O5 | 8.39 | 7.42 | 9.42 | 0.58 | 6.9 |
| K2O | 6.81 | 5.77 | 7.78 | 0.78 | 11.5 |
| MgO | 6.49 | 5.12 | 8.66 | 1.22 | 18.6 |
| Plant Pigments | Mean | Minimum | Maximum | Standard Deviation | Variation Coefficient (%) |
|---|---|---|---|---|---|
| Location I (n = 12) | |||||
| Chlorophyll a | 6.67 | 5.09 | 8.77 | 0.99 | 14.87 |
| Chlorophyll b | 2.44 | 1.79 | 3.63 | 0.39 | 16.36 |
| Chlorophyll (a + b) | 9.11 | 6.92 | 11.69 | 1.28 | 14.16 |
| β-carotene | 0.61 | 0.42 | 0.92 | 0.11 | 17.32 |
| Carotene sum | 1.60 | 1.21 | 1.94 | 0.21 | 13.10 |
| Location II (n = 16) | |||||
| Chlorophyll a | 7.76 | 6.04 | 9.29 | 0.96 | 12.39 |
| Chlorophyll b | 2.36 | 1.61 | 2.96 | 0.26 | 11.57 |
| Chlorophyll (a + b) | 10.13 | 7.65 | 12.14 | 1.18 | 11.73 |
| β-carotene | 0.62 | 0.46 | 0.83 | 0.07 | 11.35 |
| Carotene sum | 1.40 | 0.93 | 1.79 | 0.19 | 14.06 |
| Plant Pigments | Forest Habitat | Year of Study | Significance | ||||
|---|---|---|---|---|---|---|---|
| Location I | Location II | Year 1 | Year 2 | Location | Year | Interaction | |
| Chlorophyll a | 6.67 | 7.77 | 7.64 | 6.95 | ** | * | * |
| Chlorophyll b | 2.44 | 2.37 | 2.66 | 2.14 | ns | ** | ** |
| Chlorophyll (a + b) | 9.12 | 10.14 | 10.30 | 9.09 | ** | ** | * |
| β-carotene | 0.61 | 0.62 | 0.66 | 0.58 | ns | ** | * |
| Total carotenoids | 1.60 | 1.41 | 1.53 | 1.46 | ** | ns | * |
| Plant Pigments | Soil Parameters | |||
|---|---|---|---|---|
| pH | P2O5 | K2O | MgO | |
| Chlorophyll a | −0.25 | 0.55 ** | 0.44 ** | 0.55 ** |
| Chlorophyll b | 0.38 ** | 0.22 | −0.01 | 0.22 |
| Chlorophyll (a + b) | −0.08 | 0.48 ** | 0.32 * | 0.48 ** |
| β-carotene | 0.08 | 0.29 * | 0.11 | 0.29 * |
| Carotene sum | 0.41 ** | −0.28 * | −0.23 | −0.28 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielewicz, W.; Wróbel, B.; Niedbała, G. Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables. Forests 2020, 11, 1197. https://doi.org/10.3390/f11111197
Zielewicz W, Wróbel B, Niedbała G. Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables. Forests. 2020; 11(11):1197. https://doi.org/10.3390/f11111197
Chicago/Turabian StyleZielewicz, Waldemar, Barbara Wróbel, and Gniewko Niedbała. 2020. "Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables" Forests 11, no. 11: 1197. https://doi.org/10.3390/f11111197
APA StyleZielewicz, W., Wróbel, B., & Niedbała, G. (2020). Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables. Forests, 11(11), 1197. https://doi.org/10.3390/f11111197







