Seed Shadows of Northern Pigtailed Macaques within a Degraded Forest Fragment, Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Troop
2.3. Data Collection
2.4. Fruit Availability
2.5. Ranging and Movement Patterns
2.6. In Situ Retention Times and Dispersal Distances
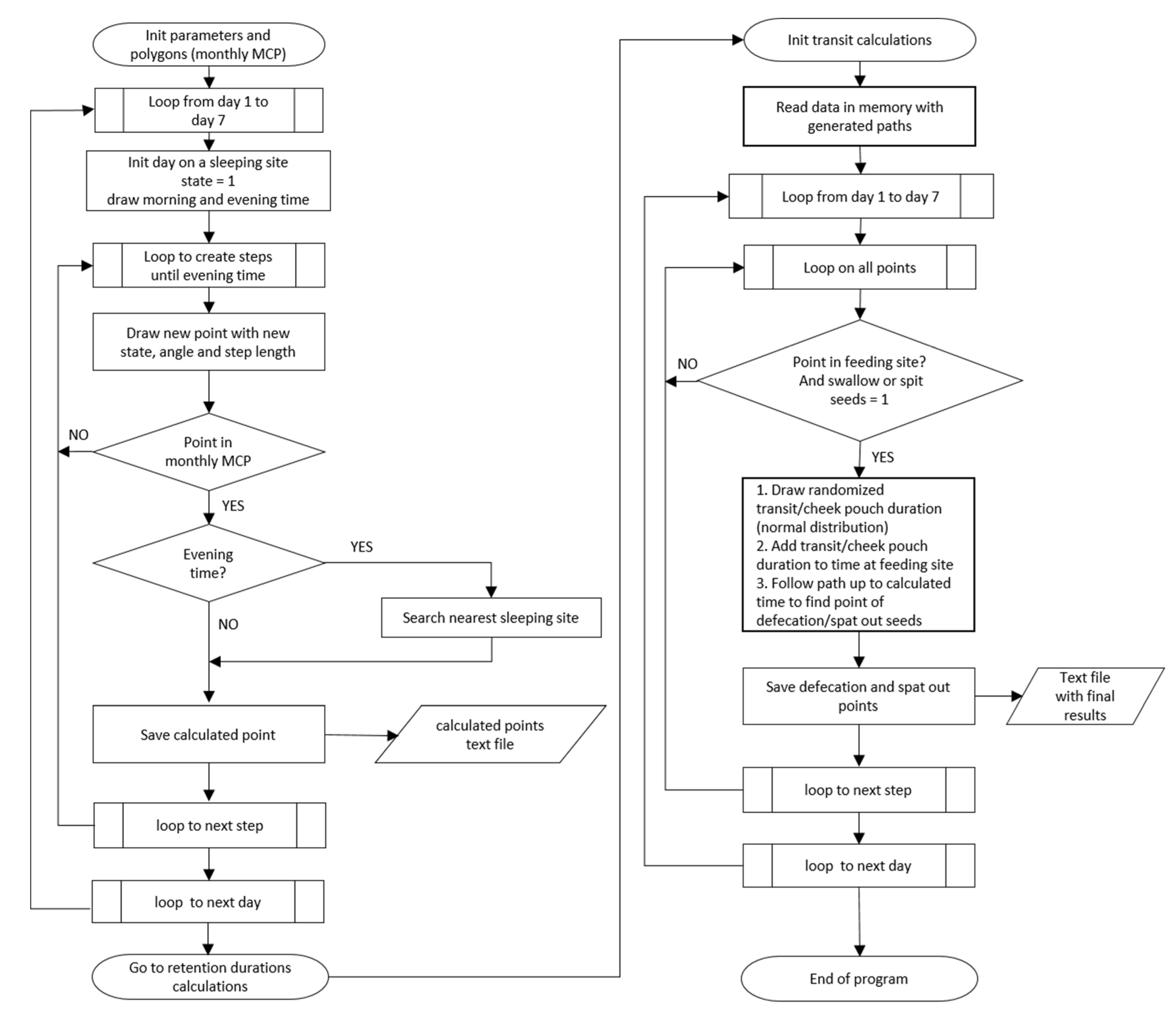
2.7. Mechanistic Model of Seed Transfer (MOST) and Data Analysis
3. Results
3.1. Ranging, Movement Patterns and Seasonality
3.2. Feeding Sites and Seed Processing
3.3. In Situ Retention Times and Dispersal Distances
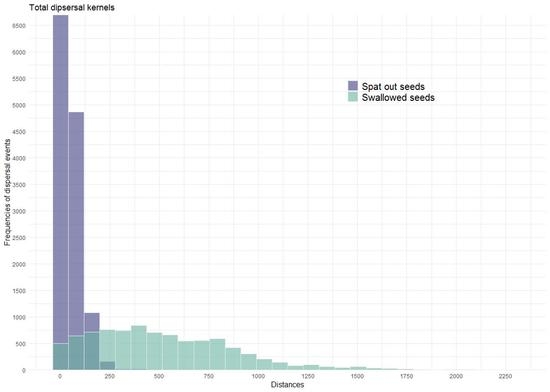
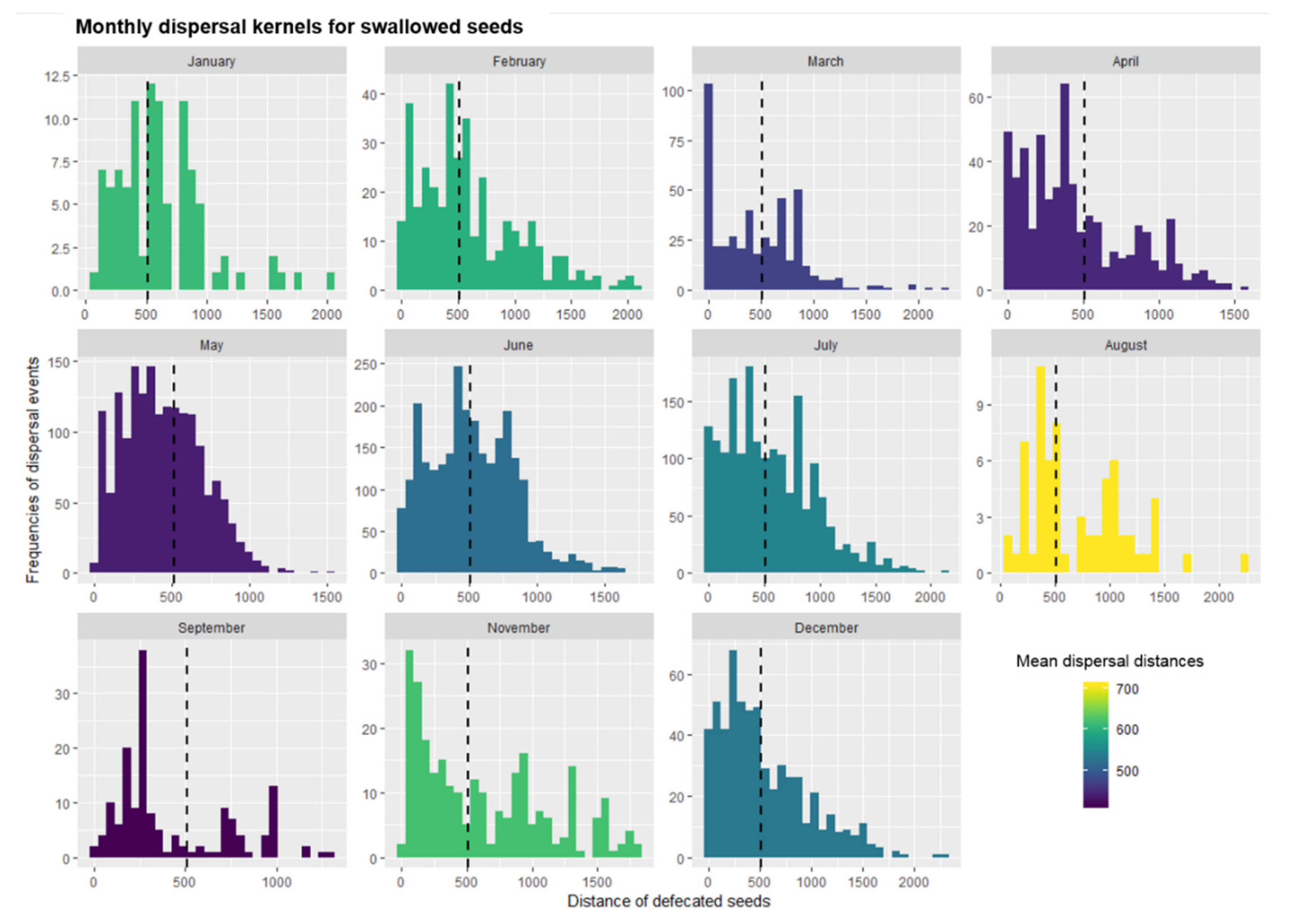
3.4. Simulation of Monthly Seed Shadows
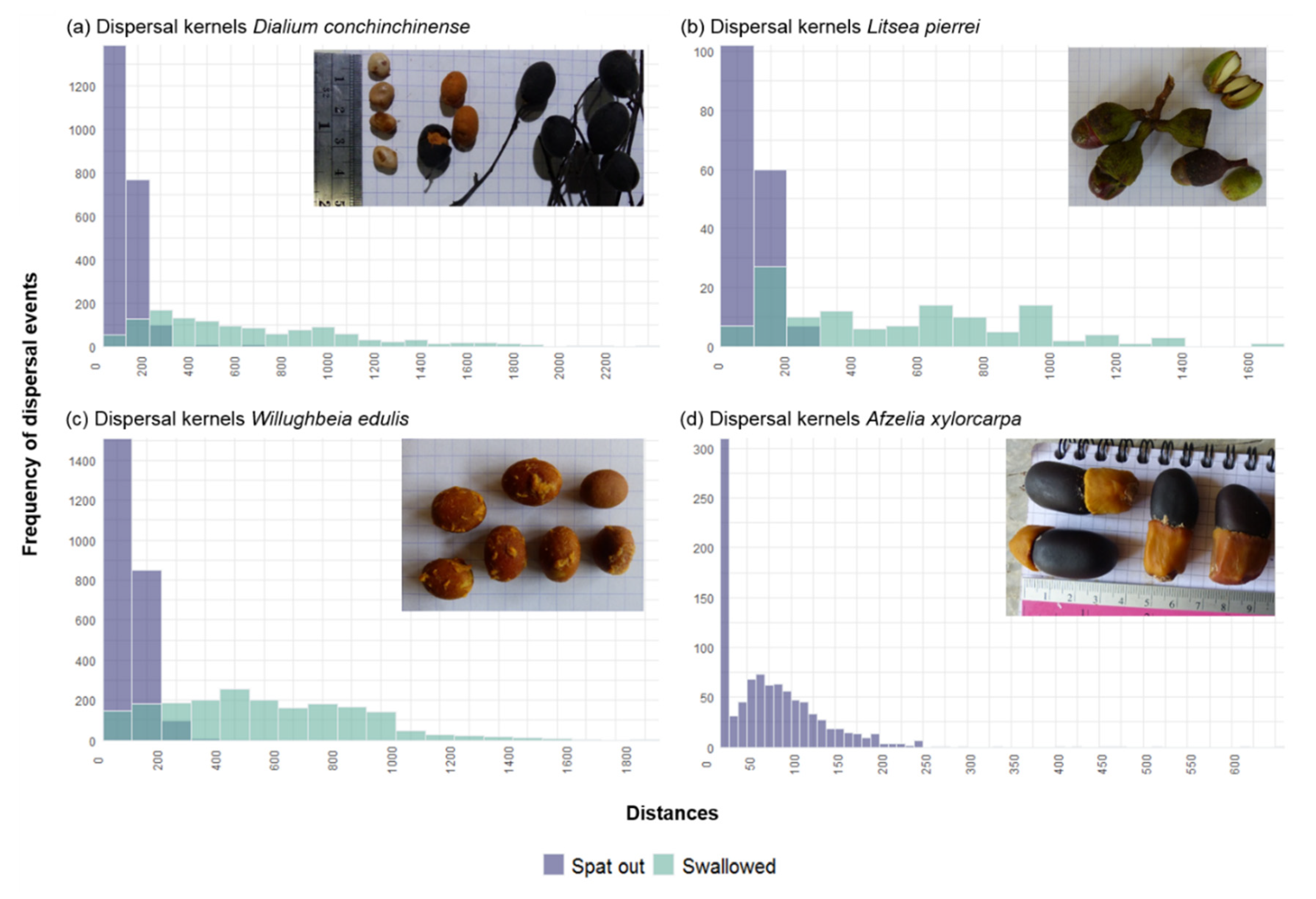
3.5. Simulation of a Species Seed Shadow
4. Discussion
4.1. Seed Dispersal Effectiveness in Degraded Habitats
4.1.1. Seed Category and Treatment
4.1.2. Seed Shadow Simulations
4.1.3. Macaques as Effective Seed Dispersers in Degraded Habitats?
4.2. Conservation Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Months | Entry Time | Exit Time |
|---|---|---|
| January 2018 | 18 h 09 min ± SD 8 | 06 h 44 min ± SD 8 |
| February 2018 | 18 h 22 min ± SD 5 | 06 h 39 min ± SD 3 |
| March 2018 | 18 h 25 min ± SD 7 | 06 h 36 min ± SD 9 |
| April 2017 | 18 h 17 min ± SD 9 | 06 h 14 min ± SD 9 |
| May 2017 | 18 h 36 min ± SD 5 | 06 h 20 min ± SD 16 |
| June 2017 | 18 h 28 min ± SD 11 | 05 h 58 min ± SD 10 |
| July 2017 | 18 h 47 min ± SD 10 | 06 h 02 min ± SD 7 |
| August 2018 | 18 h 23 min ± SD 10 | 06 h 17 min ± SD 10 |
| September 2018 | 18 h 08 min ± SD 9 | 06 h 09 min ± SD 11 |
| October 2018 | 17 h 55 min ± SD 7 | 06 h 09 min ± SD 03 |
| November 2017 | 17 h 39 min ± SD 7 | 06 h 25 min ± SD 12 |
| December 2017 | 17 h 52 min ± SD 2 | 06 h 17 min ± SD 3 |

| Species | Stat | Cov. | Pref. | Forest Types | Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul. | Aug. | Sept. | Oct. | Nov. | Dec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afzelia xylocarpa | EN | 0.25 | 4.47 | DEF (622) PF (105) | S (37) | - | - | - | - | - | S (462) | S (42) | S (81) | - | S (51) | S (54) |
| Aglaia sp. | - | 0.7 | 4.07 | DEF (2099) PF (59) | - | - | - | - | W (76) S (102) | W (478) S (660) | W (352) S (490) | - | - | - | - | - |
| Artocarpus lacucha | NE | 0.2 | 0.36 | DEF (0) PF (1) | - | - | S (1) | - | - | - | - | - | - | - | - | - |
| Baccaurea ramiflora | LC | - | - | DEF (20) PF (1) | - | - | W (10) S (11) | - | - | - | - | - | - | - | - | - |
| Canarium subulatum guillaumin | NE | 0.05 | 7.64 | DEF (7) PF (1) | - | - | - | W (2) S (6) | - | - | - | - | - | - | - | - |
| Dehaasia candolleana | NE | 0.1 | 0.85 | DEF (117) PF (12) | - | - | S (129) | - | - | - | - | - | - | - | - | - |
| Dialium conchinchinense | NT | 0.65 | 22.92 | DEF (2040) PF (244) | W (100) S (102) | W (35) S (44) | W (23) S (32) | - | - | - | W (114) S (149) | W (21) S (23) | W (54) S (54) | S (591) | W (217) S (309) | W (542) S (728) |
| Diospyros sp. | - | 0.6 | 0.17 | DEF (148) PF (26) | - | - | - | - | - | - | W (62) S (87) | W (10) S (15) | - | - | - | - |
| Garaga pinnata | NE | - | - | DEF (30) PF (4) | - | - | - | - | - | - | - | W (11) S (23) | - | - | - | - |
| Knema globularia | LC | 0.25 | 0.14 | DEF (92) PF (2) | - | - | - | W (11) S (13) | - | W (34) S (36) | - | - | - | - | - | - |
| Litsea pierrei | LC | 0.1 | 2.95 | DEF (199) PF (5) | - | - | W (40) S (47) | W (44) S (73) | - | - | - | - | - | - | - | - |
| Memecylon ovatum | LC | 0.55 | 0.22 | DEF (440) PF (6) | - | - | - | - | - | W (5) S (7) | W (183) S (241) | W (5) S (5) | - | - | - | - |
| Memecylon scutellatum | NE | 0.25 | 0.66 | DEF (86) PF(2) | - | - | - | W (39) S (49) | - | - | - | - | - | - | - | - |
| Microcos tomentosa | LC | 0.65 | 0.07 | DEF (11) PF (39) | - | - | - | - | - | - | - | - | - | - | W (22) S (28) | - |
| Salacia sp. | - | - | - | DEF (171) PF (2) | - | - | - | - | W (22) S (34) | W (53) S (64) | - | - | - | - | - | |
| Sapotaceae sp. | - | 0.55 | 0.95 | DEF (150) PF (12) | - | - | - | - | - | - | - | - | - | - | W (22) S (51) | W (35) S (54) |
| Species no. 3 | - | - | - | DEF (1568) PF (80) | - | W (341) S (458) | W (386) S (442) | - | - | - | - | - | - | - | - | W (9) S (12) |
| Species no. 11 | - | - | - | DEF (11) PF(0) | - | - | - | - | - | - | W (11) | - | - | - | - | - |
| Species no. 12 | - | - | - | DEF (507) PF (24) | - | - | - | - | - | - | W (227) S (304) | - | - | - | - | - |
| Spondias pinnata | NE | 0.15 | 0.21 | DEF (15) PF (104) | S (76) | - | - | - | - | - | - | S (7) | - | - | S (36) | - |
| Syzygium sp. | – | 0.4 | 0.4 | DEF (664) PF (17) | - | - | - | W (35) S (41) | W (58) S (73) | W (198) S (276) | - | - | - | - | - | - |
| Vitex quinata | LC | 0.15 | 2.55 | DEF (250) PF (52) | - | - | - | - | - | - | - | W (20) S (49) | W (102) S (131) | - | - | - |
| Willughbeia edulis | NE | - | - | DEF (2919) PF (182) | - | - | - | - | W (394)S (508) | W (719) S (895) | W (280) S (305) | - | - | - | - | - |
| Xerospermum noronhianum | NE | 0.15 | 4.79 | DEF (6092) PF (185) | - | - | - | W (428) S (534) | W (1220) S (1575) | W (1160) S (1360) | - | - | - | - | - | - |
References
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Sodhi, N.S. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F. Have we overstated the tropical biodiversity crisis? Trends Ecol. Evol. 2007, 22, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Muller-Landau, H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef]
- Russo, S.E.; Portnoy, S.; Augspurger, C.K. Incorporating animal behavior into seed dispersal models: Implications for seed shadows. Ecology 2006, 87, 3160–3174. [Google Scholar] [CrossRef] [Green Version]
- Trolliet, F.; Forget, P.M.; Doucet, J.L.; Gillet, J.F.; Hambuckers, A. Frugivorous birds influence the spatial organization of tropical forests through the generation of seedling recruitment foci under zoochoric trees. Acta Oecologica 2017, 85, 69–76. [Google Scholar] [CrossRef]
- Caughlin, T.T.; Ferguson, J.M.; Lichstein, J.W.; Zuidema, P.A.; Bunyavejchewin, S.; Levey, D.J. Loss of animal seed dispersal increases extinction risk in a tropical tree species due to pervasive negative density dependence across life stages. Proc. R. Soc. B 2014, 282, e20142095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corlett, R.T. The Ecology of Tropical East Asia, 2nd ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed dispersal effectiveness revisited: A conceptual review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef] [Green Version]
- Jordano, P. What is long-distance dispersal? And a taxonomy of dispersal events. J. Ecol. 2017, 105, 75–84. [Google Scholar] [CrossRef]
- Culot, L.; Huynen, M.C.; Heymann, E.W. Partitioning the relative contribution of one-phase and two-phase seed dispersal when evaluating seed dispersal effectiveness. Methods Ecol. Evol. 2015, 6, 178–186. [Google Scholar] [CrossRef]
- Reid, J.L.; Holl, K.D.; Zahawi, R.A. Seed dispersal limitations shift over time in tropical forest restoration. Ecol. Appl. 2015, 25, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Westcott, D.A.; Bentrupperbäumer, J.; Bradford, M.G.; Mckeown, A. Incorporating patterns of disperser behavior into models of seed dispersal and its effects on estimated dispersal. Oecologia 2005, 146, 57–67. [Google Scholar] [CrossRef] [PubMed]
- McConkey, K.R.; Prasad, S.; Corlett, R.T.; Campos-Arceiz, A.; Brodie, J.F.; Rogers, H.; Santamaria, L. Seed dispersal in changing landscapes. Biol. Conserv. 2012, 146, 1–13. [Google Scholar] [CrossRef]
- Pessoa, M.S.; Rocha-Santos, L.; Talora, D.C.; Faria, D.; Mariano-Neto, E.; Hambuckers, A.; Cazetta, E. Fruit biomass availability along a forest cover gradient. Biotropica 2016, 49, 45–55. [Google Scholar] [CrossRef]
- Pessoa, M.S.; Hambuckers, A.; Benchimol, M.; Rocha-Santos, L.; Bomfim, J.A.; Faria, D.; Cazetta, E. Deforestation drives functional diversity and fruit quality changes in a tropical tree assemblage. Perspect. Plant Ecol. Evol. Syst. 2017, 28, 78–86. [Google Scholar] [CrossRef]
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernandez-Duque, E.; Di Fiore, A.; Nekaris, K.A.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef] [Green Version]
- Chaves, O.M.; Bicca-Marques, J.C.; Chapman, C.A. Quantity and quality of seed dispersal by a large arboreal frugivore in small and large Atlantic forest fragments. PLoS ONE 2018, 13, e0193660. [Google Scholar] [CrossRef] [Green Version]
- Corlett, R.T.; Hau, B.C. Seed dispersal and forest restoration. In Forest Restoration for Wildlife Conservation; Elliot, S., Kerby, J., Blakesley, D., Hardwick, K., Woods, K., Anusarnsunthorn, V., Eds.; International Tropical Timber Organization and The Forest Restoration Research Unit: Chiang Mai, Thailand, 2000; pp. 317–325. [Google Scholar]
- Corlett, R.T. Frugivory and seed dispersal by vertebrates in tropical and subtropical Asia: An update. Glob. Ecol. Conserv. 2017, 11, 1–22. [Google Scholar] [CrossRef]
- Kitamura, S.; Yumoto, T.; Poonswad, P.; Chuailua, P.; Plongmai, K.; Maruhashi, T.; Noma, N. Interactions between fleshy fruits and frugivores in a tropical seasonal forest in Thailand. Oecologia 2002, 133, 559–572. [Google Scholar] [CrossRef]
- Chanthorn, W.; Wiegand, T.; Getzin, S.; Brockelman, W.Y.; Nathalang, A. Spatial patterns of local species richness reveal importance of frugivores for tropical forest diversity. J. Ecol. 2017, 106, 925–935. [Google Scholar] [CrossRef]
- Chapman, C.A.; Russo, S.E. Primate seed dispersal: Linking behavioral ecology with forest community structure. In Primates in Perspective; Campbell, C.J., Fuentes, A.F., MacKinnon, K.C., Panger, M., Bearder, S., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 510–525. [Google Scholar]
- McConkey, K.R. Seed dispersal by primates in Asian habitats: From species, to communities, to conservation. Int. J. Primatol. 2018, 39, 466–492. [Google Scholar] [CrossRef]
- Albert, A.; McConkey, K.; Savini, T.; Huynen, M.C. The value of disturbance-tolerant cercopithecine monkeys as seed dispersers in degraded habitats. Biol. Conserv. 2014, 170, 300–310. [Google Scholar] [CrossRef] [Green Version]
- McLennan, M.R.; Spagnoletti, N.; Hockings, K.J. The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. Int. J. Primatol. 2017, 38, 105–121. [Google Scholar] [CrossRef]
- Tsuji, Y.; Su, H.H. Macaques as seed dispersal agents in Asian forests: A review. Int. J. Primatol. 2018, 39, 356–376. [Google Scholar] [CrossRef]
- Sengupta, A.; McConkey, K.R.; Radhakrishna, S. Seed dispersal by rhesus macaques Macaca mulatta in northern India. Am. J. Primatol. 2014, 76, 1175–1184. [Google Scholar] [CrossRef]
- Albert, A.; Hambuckers, A.; Culot, L.; Savini, T.; Huynen, M.C. Frugivory and seed dispersal by northern pigtailed macaques (Macaca leonina), in Thailand. Int. J. Primatol. 2013, 34, 170–193. [Google Scholar] [CrossRef]
- Fuzessy, L.F.; Janson, C.H.; Silveira, F.A. How far do Neotropical primates disperse seeds? Am. J. Primatol. 2017, 79, e22659. [Google Scholar] [CrossRef]
- Phiphatsuwannachai, S.; Westcott, D.A.; McKeown, A.; Savini, T. Inter-group variability in seed dispersal by white-handed gibbons in mosaic forest. Biotropica 2018, 50, 106–115. [Google Scholar] [CrossRef]
- Razafindratsima, O.H.; Jones, T.A.; Dunham, A.E. Patterns of movement and seed dispersal by three lemur species. Am. J. Primatol. 2014, 76, 84–96. [Google Scholar] [CrossRef]
- Gazagne, E.; José-Domínguez, J.M.; Huynen, M.C.; Hambuckers, A.; Poncin, P.; Savini, T.; Brotcorne, F. Northern pigtailed macaques rely on old growth plantations to offset low fruit availability in a degraded forest fragment. Am. J. Primatol. 2020, e23117. [Google Scholar] [CrossRef]
- Raghunathan, N. Climate Change Impacts on the Distribution of Key Tree Species Used by Lion Tamarins in the Brazilian Atlantic Forest: Applications to Conservation. Ph.D. Thesis, Université de Liège, Liège, Belgium, 2019. Available online: http://hdl.handle.net/2268/236209 (accessed on 14 May 2019).
- Thai Institute of Scientific and Technological Research. Meteorological Observations. Available online: http://www.tistr.or.th/sakaerat (accessed on 13 February 2019).
- Oliver, K.; Ngoprasert, D.; Savini, T. Slow loris density in a fragmented, disturbed dry forest, north-east Thailand. Am. J. Primatol. 2019, 81, e22957. [Google Scholar] [CrossRef]
- Suwanrat, J.; Artchawakom, T.; Suwanwaree, P. Mammal diversity study using camera trap at Sakaerat Environmental Research Station. In Proceedings of the 32th Thailand Wildlife Seminar, Kasetsart University, Bangkok, Thailand, December 2011. [Google Scholar]
- Khamcha, D.; Corlett, R.T.; Powell, L.A.; Savini, T.; Lynam, A.J.; Gale, G.A. Road induced edge effects on a forest bird community in tropical Asia. Avian Res. 2018, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Petersen, W.J.; Savini, T.; Steinmetz, R.; Ngoprasert, D. Periodic resource scarcity and potential for interspecific competition influences distribution of small carnivores in a seasonally dry tropical forest fragment. Mamm. Biol. 2019, 95, 112–122. [Google Scholar] [CrossRef]
- Gazagne, E.; Savini, T.; Ngoprasert, D.; Poncin, P.; Huynen, M.C.; Brotcorne, F. When northern pigtailed macaques (Macaca leonina) cannot select for ideal sleeping sites in a degraded habitat. Int. J. Primatol. 2020, 41, 614–633. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelot, T.; Langrock, R.; Patterson, T.A.; McInerny, G. moveHMM: An R package for the statistical modelling of animal movement data using hidden Markov models. Methods Ecol. Evol. 2016, 7, 1308–1315. [Google Scholar] [CrossRef] [Green Version]
- Langrock, R.; Kneib, T.; Glennie, R.; Michelot, T. Markov-switching generalized additive models. Stat. Comput. 2017, 27, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bolker, B.M. Incorporating periodic variability in hidden Markov models for animal movement. Mov. Ecol. 2017, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Whoriskey, K.; Auger-Méthé, M.; Albertsen, C.M.; Whoriskey, F.G.; Binder, T.R.; Krueger, C.C.; Mills Flemming, J. A hidden Markov movement model for rapidly identifying behavioral states from animal tracks. Ecol. Evol. 2017, 7, 2112–2121. [Google Scholar] [CrossRef] [Green Version]
- Dennis, A.J.; Westcott, D.A. Estimating dispersal kernels produced by a diverse community of vertebrates. In Seed Dispersal: Theory and Its Application in a Changing World; Dennis, A.J., Green, R.J., Schupp, E.W., Westcott, D.A., Eds.; CAB International: Wallingford, UK, 2007; pp. 201–228. [Google Scholar]
- McConkey, K.R. Primary seed shadow generated by gibbons in the rainforests of Barito Ulu, Central Borneo. Am. J. Primatol. 2000, 52, 13–29. [Google Scholar] [CrossRef]
- Heymann, E.W.; Lüttmann, K.; Michalczyk, I.M.; Saboya, P.P.P.; Ziegenhagen, B.; Bialozyt, R. DNA fingerprinting validates seed dispersal curves from observational studies in the Neotropical legume Parkia. PLoS ONE 2012, 7, e35480. [Google Scholar] [CrossRef] [Green Version]
- Burkkardt, J. CDFLIB: Fortran Library for Cumulative Density Functions. 2010. Available online: http://people.sc.fsu.edu/~jburkardt/f_src/cdflib/cdflib.html (accessed on 11 March 2020).
- Sunday, D. 2001. Available online: http://geomalgorithms.com/a03-_inclusion.html (accessed on 11 March 2020).
- Sengupta, A.; Gazagne, E.; Albert-Daviaud, A.; Tsuji, Y.; Radhakrishna, S. Reliability of macaques as seed dispersers. Am. J. Primatol. 2020, 82, e23115. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A. Introduction to Categorical Data Analysis; Wiley: Hoboken, NJ, USA, 2007; p. 372. [Google Scholar]
- World Conservation Monitoring Centre. Dialium cochinchinense. In IUCN Red List; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 1998. [Google Scholar] [CrossRef]
- De Kok, R. Litsea pierrei. In IUCN Red List Threat. Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2020. [Google Scholar] [CrossRef]
- Nghia, N.H. Afzelia xylocarpa. In IUCN Red List Threat. Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 1998. [Google Scholar] [CrossRef]
- Clark, C.J.; Poulsen, J.R.; Bolker, B.M.; Connor, E.F.; Parker, V.T. Comparative seed shadows of bird-, monkey-, and wind-dispersed trees. Ecology 2005, 86, 2684–2694. [Google Scholar] [CrossRef] [Green Version]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A. Mechanisms of long-distance seed dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.S.; Chapman, C.A. Limitations of animal seed dispersal for enhancing forest succession on degraded lands. In Seed-Dispersal and Frugivory: Ecology, Evolution and Conservation; Levey, D.J., Silva, W.R., Galetti, M., Eds.; CAB International: New York, NY, USA, 2002; pp. 437–450. [Google Scholar]
- Costa, J.B.; Melo, F.P.; Santos, B.A.; Tabarelli, M. Reduced availability of large seeds constrains Atlantic forest regeneration. Acta Oecologica 2012, 39, 61–66. [Google Scholar] [CrossRef]
- Lambert, J.E.; Rothman, J.M. Fallback foods, optimal diets, and nutritional targets: Primate responses to varying food availability and quality. Annu. Rev. Anthropol. 2015, 44, 493–512. [Google Scholar] [CrossRef]
- Rebout, N.; Desportes, C.; Thierry, B. Resource partitioning in tolerant and intolerant macaques. Aggress. Behav. 2017, 43, 513–520. [Google Scholar] [CrossRef]
- McConkey, K.R.; Brockelman, W.Y. Non-redundancy in the dispersal network of a generalist tropical forest tree. Ecology 2011, 92, 1492–1502. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Ahumada, J.A.; Condit, R.; Foster, R.B. Local neighborhood effects on long-term survival of individual trees in a neotropical forest. Ecol. Res. 2001, 16, 859–875. [Google Scholar] [CrossRef]
- Uriarte, M.; Hubbell, S.P.; John, R.; Condit, R.; Canham, C.D. Neighborhood effects on sapling growth and survival in a neotropical forest and the ecological equivalence hypothesis. In Biotic Interactions in the Tropics: Their Role in the Maintenance of Species Diversity; Hartley, S., Burslem, D.F.R.P., Pinard, M., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 89–106. [Google Scholar] [CrossRef] [Green Version]
- Nathan, R. Long-distance dispersal of plants. Science 2006, 313, 786–788. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Rodríguez, V.; Aguilar-Barajas, E.; González-Zamora, A.; Rocha-Ramirez, V.; González-Rodríguez, A.; Oyama, K. Parent-parent and parent-offspring distances in Spondias radlkoferi seeds suggest long-distance pollen and seed dispersal: Evidence from latrines of the spider monkey. J. Trop. Ecol. 2017, 33, 95–106. [Google Scholar] [CrossRef]
- Cain, M.L.; Milligan, B.G.; Strand, A.E. Long-distance seed dispersal in plant populations. Am. J. Bot. 2000, 87, 1217–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, Y.; Morimoto, M. Endozoochorous seed dispersal by Japanese macaques (Macaca fuscata): Effects of temporal variation in ranging and seed characteristics on seed shadows. Am. J. Primatol. 2016, 78, 185–191. [Google Scholar] [CrossRef] [PubMed]
- José-Domínguez, J.M.; Huynen, M.C.; Garcia, C.J.; Albert-Daviaud, A.; Savini, T.; Asensio, N. Non-territorial macaques can range like territorial gibbons when partially provisioned with food. Biotropica 2015, 47, 733–744. [Google Scholar] [CrossRef]
- Hooper, E.; Legendre, P.; Condit, R. Barriers to forest regeneration of deforested and abandoned land in Panama. J. Appl. Ecol. 2005, 42, 1165–1174. [Google Scholar] [CrossRef]
- Cords, M. The behavior, ecology, and social evolution of cercopithecine monkeys. In The Evolution of Primate Societies; Mitani, J.C., Call, J., Kappeler, P.M., Palombit, R.A., Silk, J.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 91–112. [Google Scholar]
- Schülke, O.; Ostner, J. Ecological and social influences on sociality. In The Evolution of Primate Societies; Mitani, J.C., Call, J., Kappeler, P.M., Palombit, R.A., Silk, J.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 195–219. [Google Scholar]
- So, T.; Theilade, I.; Dell, B. Conservation and utilization of threatened hardwood species through reforestation—An example of Afzelia xylocarpa (Kruz.) Craib and Dalbergia cochinchinensis Pierre in Cambodia. Pac. Conserv. Biol. 2010, 16, 101–116. [Google Scholar] [CrossRef]
- Boonratana, R.; Chetry, D.; Long, Y.; Jiang, X.L.; Htun, S.; Timmins, R.J. Macaca leonina. In IUCN Red List Threat. Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2020. [Google Scholar] [CrossRef]
- Andresen, E.; Arroyo-Rodríguez, V.; Ramos-Robles, M. Primate seed dispersal: Old and new challenges. Int. J. Primatol. 2018, 39, 443–465. [Google Scholar] [CrossRef]


| Swallow Seeds | Spit Seeds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | HR Size (ha) | PF Cover. (%) | DPL (m) | FAI DEF | Frug. (%) | Freq. | Mean dist. (m) | Disp. in PF (%) | Freq. | Mean dist. (m) | Disp. in PF (%) |
| January 2018 | 441.6 | 22.9 | 2055.8 | 3.159 ** | 76.4 | 100 | 613.4 | 7 | 215 | 60.4 | 34.9 |
| February 2018 | 446.7 | 13.9 | 2171.3 | 1.49 * | 74.1 | 376 | 601 | 12 | 502 | 46 | 5.4 |
| March 2018 | 513.1 | 38.7 | 2201.5 | 0.796 * | 84.6 | 459 | 474.7 | 2.6 | 662 | 57.7 | 2.1 |
| April 2017 | 256.8 | 13.3 | 2222.1 | 5.62 *** | 65.8 | 559 | 450.2 | 3.2 | 716 | 42.9 | 0.1 |
| May 2017 | 140.7 | 7.4 | 2240.4 | 5.293 *** | 86.6 | 1748 | 440.7 | 3.6 | 2258 | 54 | 0.3 |
| June 2017 | 179.0 | 5.5 | 2227.2 | 3.922 *** | 73.8 | 2616 | 521.1 | 8.7 | 3268 | 47.1 | 3.6 |
| July 2017 | 290.6 | 6.7 | 2296.1 | 2.943 ** | 79.7 | 1849 | 547.1 | 3.2 | 2713 | 45.6 | 1.3 |
| August 2018 | 878.8 | 30.5 | 2269.3 | 2.066 * | 65.8 | 67 | 706.7 | 38.8 | 164 | 74.9 | 47.6 |
| September 2018 | 145.6 | 7.9 | 2089.3 | 2.098 * | 84.2 | 156 | 417.2 | 1.3 | 213 | 44.4 | 0 |
| October 2018 | 139.8 | 7.6 | 2080.5 | 1.949 * | 77.1 | 0 | NA | NA | 591 | 32.6 | 0 |
| November 2017 | 507.7 | 19.1 | 2056.8 | 3.215 ** | 81.2 | 261 | 621.2 | 18 | 700 | 45.1 | 23.6 |
| December 2017 | 441.2 | 11.7 | 21101 | 3.326 ** | 68.9 | 586 | 532.1 | 8.7 | 848 | 55.4 | 14.4 |
| Species | Family | Life Form | Fruit Type | Seed Category | M-/S-Seeded Fruit | Part Consumed | Process | Feeding Obs. |
|---|---|---|---|---|---|---|---|---|
| Acacia auricularis | Mimosaceae | MT | D | Small | M | Seed | C | 226 |
| Acacia mangium | Mimosaceae | MT | D | Small | M | Seed | C | 410 |
| Afzelia xylocarpa | Fabaceae | TT | D | Large | M | Mes, Seed | S, C, D | 350 |
| Aglaia sp. | Meliaceae | MT | FP | Large | S | FR | W, S | 179 |
| Antiaris toxicaria | Moraceae | TT | FP | Small | M | FR | W, C | 4 |
| Artocarpus lacucha | Moraceae | MT | FU | Large | M | FR | S, C | 16 |
| Artocarpus sp. | Moraceae | MT | FU | Small | M | FR | W, C | 1 |
| Baccaurea ramiflora | Euphorbiaceae | ST | FP | Large | M | FR | W, S, D | 2 |
| Beilschmiedia sp. | Lauraceae | MT | FU | Large | S | FR | S, D | 2 |
| Calamus siamensis becc. | Arecaceae | HE | FP | Medium | S | FR | W, S, C | 21 |
| Canarium subulatum guillaumin | Burseraceae | MT | FU | Large | S | FR | S, D | 24 |
| Caryota bacsonensis Magalon | Arecaceae | SH | FU | Medium | S | FR | C | 5 |
| Cinnamomum porrectum | Lauraceae | MT | FU | Medium | S | FR | - | 3 |
| Cissus javana | Vitaceae | SH | FP | Small | S | FR | W | 2 |
| Dehaasia cand. | Lauraceae | MT | FU | Large | S | FR | S, D | 16 |
| Dialium conchinchinense | Fabaceae | TT | FU | Medium | S | FR | W, S, C | 2137 |
| Diospyros castanea | Ebenaceae | MT | FU | Large | M | FR | S, C | - |
| Diospyros rhodocalyx | Ebenaceae | MT | FU | Large | M | FR | W, S, C | 2 |
| Diospyros sp. 1 | Ebenaceae | MT | FU | Large | M | FR | W, S, C | 6 |
| Diospyros sp. 2 | Ebenaceae | MT | FU | Large | M | FR | W, S, C | 4 |
| Dysoxylum cyrtobotryum | Meliaceae | ST | FP | Large | M | FR | - | - |
| Ficus altissima | Moraceae | TT | FU | Small | M | FR | W | 2 |
| Ficus macrophylla | Moraceae | TT | FU | Small | M | FR | W | - |
| Ficus spp. | Moraceae | TT | FU | Small | M | FR | W | - |
| Garaga pinnata | Meliaceae | MT | FP | Medium | S | FR | W, S | 3 |
| Garcinia cowa | Clusiaceae | MT | FU | Large | M | FR | W, S | - |
| Garcinia speciose | Clusiaceae | MT | FU | Large | M | FR | W, S, C | 1 |
| Glycosmis pentaphylla | Rutaceae | ST | FU | Small | S | FR | W, S, C | 4 |
| Gonocaryium lobbianum | Cardiopteridaceae | ST | FU | Large | S | FR | D | - |
| Hydnocarpus ilicifolia | Achariaceae | ST | FP | Medium | M | Mes, Seed | C, D | 5 |
| Hypobathrum racemosum | Rubiaceae | ST | FU | Medium | S | FR | W, S | 10 |
| Irvingia malayana | Irvingiaceae | TT | FU | Large | S | FR | D | - |
| Knema globularia | Myristicaceae | ST | FP | Large | S | FR | W, S, D | 15 |
| Knema linifolia | Myristicaceae | MT | FP | Medium | S | FR | W, S, D | 20 |
| Leucaena leucocephala | Fabaceae | MT | D | Small | M | Mes, Seed | C | 145 |
| Litsea pierrei | Lauraceae | MT | FU | Large | S | FR | W, S, D | 37 |
| Mangifera sp. | Anarcardiaceae | MT | FU | Large | S | FR | D | 13 |
| Melia azedarach | Meliaceae | MT | FU | Large | S | FR | S, D | - |
| Melodorum fructicosum | Annonaceae | ST | FU | Medium | S | FR | W, S | 7 |
| Memecylon ovatum | Melastomataceae | ST | FU | Medium | S | FR | W, S | 38 |
| Memecylon scutellatum | Melastomataceae | ST | FU | Medium | S | FR | W, S | 29 |
| Memecylon sp. | Melastomataceae | ST | FU | Medium | S | FR | W, S | 3 |
| Microcos tomentosa | Malvaceae | MT | FU | Medium | S | FR | W, S | 17 |
| Micromelum minutum | Rutaceae | ST | FU | Small | S | FR | W, S | 12 |
| Miliuasa lineata | Annonaceae | MT | FP | Medium | M | FR | S | - |
| Nephelium hypoleucum | Sapindaceae | MT | FP | Large | S | FR | W, S, D | 9 |
| Parkia sumatrana | Fabaceae | TT | D | Large | M | Mes | S, D | 27 |
| Peltophorum pterocarpum | Fabaceae | MT | D | Medium | M | Mes, Seed | C | 11 |
| Polyathia cerasoides | Annonaceae | ST | FU | Small | S | FR | W, S | 3 |
| Prunus javanica | Rosaceae | TT | FU | Medium | S | FR | W, S | 4 |
| Rourea minor Leenh | Connaraceae | SH | FP | Large | S | FR | W, S, D | 8 |
| Rutaceae sp. | Rutaceae | ST | FP | Small | S | FR | W, S | 25 |
| Salacia chinensis | Celastraceae | LI | FP | Large | M | FR | W, S, D | 10 |
| Salacia verrucosa | Celastraceae | LI | FP | Large | M | FR | W, S, D | 10 |
| Sapotaceae sp. | Sapotaceae | TT | FU | Large | M | FR | W, S, D | 63 |
| Schleichera oleosa | Sapindaceae | MT | FU | Large | S | FR | S | 9 |
| Spondias pinnata | Anarcardiaceae | MT | FU | Large | S | FR | S, C, D | 2 |
| Streptocaulon juventas | Apocynaceae | LI | D | Medium | S | Seed | C | 28 |
| Syzygium cumini | Myrtaceae | MT | FU | Small | S | FR | W, S | 1 |
| Syzygium siamense | Myrtaceae | ST | FU | Large | M | FR | C, D | - |
| Syzygium sp. 1 | Myrtaceae | MT | FU | Small | S | FR | W | 19 |
| Syzygium sp. 2 | Myrtaceae | MT | FU | Large | S | FR | S, D | 18 |
| Syzygium sp. 3 | Myrtaceae | LT | FU | Large | S | FR | S, D | 7 |
| Suregada multiflora | Euphorbiaceae | MT | FU | Large | M | FR | S, D | 3 |
| Toddalia asiatica | Rutaceae | LI | FP | Small | M | FR | W | 7 |
| Uvaria cordata | Annonaceae | SH | FP | Large | M | FR | W, S | - |
| Vitex quinata | Lamiaceae | ST | FU | Medium | S | FR | W, S, D | 32 |
| Walsura trichostemon | Meliaceae | ST | FP | Medium | S | FR | W, S | 2 |
| Willughbeia edulis | Apocynaceae | SH | FP | Large | M | FR | W, S, D | 188 |
| Xerospermum noronhianum | Sapindaceae | MT | FP | Large | S | FR | W, S, D | 546 |
| Species no. 1 | Herbaceae | HE | FU | Small | S | FR | W | 5 |
| Species no. 2 | Apocynaceae | LI | D | Large | M | Seed | C | 2 |
| Species no. 3 | - | LI | FP | Large | M | FR | W, S, C, D | 159 |
| Species no. 4 | - | LI | FU | Medium | S | FR | S | 2 |
| Species no. 5 | - | SH | FU | Medium | S | FR | W | 1 |
| Species no. 6 | - | - | FU | Large | S | FR | S, D | 1 |
| Species no. 7 | - | T | FU | Medium | S | FR | W | 3 |
| Species no. 8 | - | T | FP | Medium | S | FR | S | 1 |
| Species no. 9 | Dipterocarpaceae | T | D | Medium | S | Seed | C | 1 |
| Species no. 10 | - | - | D | Medium | S | Seed | C | 2 |
| Species no. 11 | - | T | FP | Medium | M | FR | W, C | 9 |
| Species no. 12 | Sapindaceae | T | FP | Large | S | FR | W, S, D | 12 |
| Species no. 13 | Fabaceae | - | D | Large | M | Mes, Seed | C | 1 |
| Species no. 14 | Apocynaceae | LI | FP | Large | M | FR | W, S, C, D | 17 |
| Species no. 15 | - | TT | FU | Large | S | FR | W, S | 4 |
| Species no. 16 | - | LI | FP | Large | M | FR | W, S | 1 |
| Species no. 17 | - | ST | FU | Small | S | FR | W | 9 |
| Species no. 18 | - | - | FU | Large | S | FR | W, S | 5 |
| Species no. 19 | - | LI | FP | - | - | - | - | 4 |
| Species no. 20 | - | LI | FP | Medium | M | FR | W, S, C, D | 2 |
| Species no. 21 | - | LI | FU | Medium | M | FR | W, S | - |
| Species no. 22 | Annonaceae | MT | FP | Large | M | FR | W, S | 5 |
| Species no. 23 | - | LI | FU | Large | S | FR | W | - |
| Species no. 24 | - | - | FU | Medium | S | FR | S, D | 10 |
| 13 unknown species | - | - | - | - | - | FR | W, S, C, D | 42 |
| Species | Seed Length (mm) | Seed Width (mm) | Seed Size Category | Number of Seeds | Dispersal Distance (m) | Gut Retention Time (min) | Forest Types |
|---|---|---|---|---|---|---|---|
| Aglaia sp. | 14 ± 4 | 10 ± 2 | Large | 8.9 ± 8.7 | - | - | DEF |
| Dialium conchinchinense | 8.8 ± 0.7 | 6.7 ± 0.7 | Medium | 10.7 ± 7.1 | 1584 ± 394 | 1542 ± 234 | DEF; PF |
| Garaga pinnata | 8.5 ± 0.5 | 7 ± 0 | Medium | 1 ± 0 | - | - | DEF; PF |
| Knema globularia | 10.6 | 10.1 | Large | 2.3 ± 1.9 | - | - | DEF |
| Listea pierrei | 16 ± 0 | 8.7 ± 0.5 | Large | 3 | - | - | DEF |
| Melodorum siamensis | 7.8 ± 0.9 | 4.2 ± 0.4 | Medium | 3.3 ± 2.1 | - | - | PF |
| Microcos tomentosa | 6 ± 3 | 6 ± 2 | Medium | 24 ± 20.4 | - | - | DEF; PF |
| Nephelium hypoleucum | 17.5 ± 0.5 | 9.5 ± 0.5 | Large | 2 | - | - | DEF |
| Toddalia asiatica | 4.3 ± 0.5 | 2.9 ± 0.4 | Small | 55 ± 37 | 1582 ± 319 | 1518 ± 171.7 | DEF; PF |
| Uvaria cordata | 18 | 11 | Large | 1 | - | - | PF |
| Salacia sp. | 15 ± 0 | 10 ± 0 | Large | 2 | - | - | DEF |
| Sapotaceae sp. | 14.4 ± 0.6 | 8.6 ± 1 | Large | 2 | - | - | DEF |
| Vitex quinata | 10 | 6 | Large | 1 | - | - | DEF |
| Willughbeia edulis | 15 ± 1.6 | 11 ± 2 | Large | 6.1 ± 4.4 | - | - | DEF; PF |
| Xerospermum noronhianum | 21 ± 1 | 14 ± 0.8 | Large | 2.5 ± 0.5 | - | - | DEF |
| Species no. 3 | 13 ± 1.1 | 11 ± 1.3 | Large | 13 ± 10 | - | - | DEF; PF |
| Species no. 12 | 15 | 10 | Large | 3 | - | - | DEF |
| Species no. 14 | 12 ± 3 | 7 ± 2 | Large | 4 ± 1 | - | - | DEF |
| Species no. 15 | 27 | 19 | Large | 1 | - | - | DEF |
| Species no. 16 | 11 ± 0 | 6.7 ± 0.5 | Large | 3 | - | - | DEF |
| Species no. 18 | 16 ± 0.8 | 13 ± 4.5 | Large | 3 | - | - | PF |
| Species no. 22 | 10 ± 1 | 6.5 ± 0.5 | Large | 2 | - | - | DEF |
| Species no. 23 | 16 ± 0 | 8 ± 0 | Large | 2 | - | - | DEF |
| Species no. 25 | 13 ± 1.6 | 9.3 ± 0.6 | Large | 5 | - | - | DEF |
| Species no. 26 | 0.8 | 0.5 | Medium | 1 | - | - | DEF |
| Species no. 29 | 7 | 7 | Medium | 1 | - | - | PF |
| Species no. 30 | 9.3 ± 1.1 | 5.4 ± 0.8 | Medium | 3.7 ± 1.2 | - | - | DEF |
| Species | Seed Length (mm) | Seed Width (mm) | Seed Size Category | Number of Seeds | Dispersal Distance (m) | Cheek Pouch Retention Time (min) |
|---|---|---|---|---|---|---|
| Afzelia xylocarpa | 37 ± 3.2 | 18 ± 1.6 | Large | 3 | 87.3 ± 31.6 | 20.5 ± 12.5 |
| Aglaia sp. | 14 ± 4 | 10 ± 2 | Large | 8.9 ± 8.7 | 38 ± 5.9 | 12.3 ± 2.9 |
| Beilschmiedia sp. | 16 | 8 | Large | 1 | - | - |
| Artocarpus lacucha | 13 ± 1 | 10 ± 1 | Large | - | - | - |
| Beilschmiedia sp. | 14 ± 2 | 9 ± 1 | Large | - | - | - |
| Dehaasia candolleana | 21 ± 4 | 10 ± 1 | Large | - | - | - |
| Dialium conchinchinense | 8.3 ± 0.7 | 6.7 ± 0.5 | Medium | 11 | 81.1 ± 53 | 25 ± 7 |
| Listea pierrei | 20.3 ± 0.9 | 10.3 ± 0.9 | Large | 1.5 ± 0.5 | - | |
| Memecylon ovaton | 6 ± 0 | 6 ± 0 | Medium | 19 | 132 ± 11.5 | 30.5 ± 3.5 |
| Uvaria cordata | 17 ± 3 | 12 ± 4 | Large | 5 ± 0 | - | - |
| Spondias pinnata | 34 ± 3 | 26 ± 1 | Large | - | - | - |
| Syzygium sp. 3 | 19 ± 1.5 | 16 ± 1.9 | Large | - | 10 | |
| Vitex quinata | 8.5 ± 0.5 | 6.2 ± 0.7 | Medium | 6 | 16 ± 5 | - |
| Willughbeia edulis | 15 ± 1.6 | 13 ± 2 | Large | 6.5 ± 0.5 | - | - |
| Xerospermum noronhianum | 21 ± 1 | 14 ± 0.8 | Large | 26.2 ± 46.5 | - | - |
| Species no. 3 | 13 ± 1.1 | 11 ± 1.3 | Large | 13 ± 10 | - | - |
| Species no. 12 | 15 | 10 | Large | 3 | 159.7 ± 11.8 | - |
| Species no. 14 | 12 ± 3 | 7 ± 2 | Large | 4 ± 1 | - | - |
| Species no. 15 | 27 | 19 | Large | - | - | - |
| Species no. 16 | 11 ± 0 | 6.7 ± 0.5 | Large | 3 | - | - |
| Species no. 18 | 16 ± 0.8 | 13 ± 4.5 | Large | 3 | - | - |
| Species no. 22 | 10 ± 1 | 6.5 ± 0.5 | Medium | 2 | 28.5 ± 3.5 | - |
| Species no. 24 | 6.9 ± 0.7 | 5.4 ± 0.4 | Medium | - | - | - |
| Species no. 28 | 24 | 18 | Large | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazagne, E.; Pitance, J.-L.; Savini, T.; Huynen, M.-C.; Poncin, P.; Brotcorne, F.; Hambuckers, A. Seed Shadows of Northern Pigtailed Macaques within a Degraded Forest Fragment, Thailand. Forests 2020, 11, 1184. https://doi.org/10.3390/f11111184
Gazagne E, Pitance J-L, Savini T, Huynen M-C, Poncin P, Brotcorne F, Hambuckers A. Seed Shadows of Northern Pigtailed Macaques within a Degraded Forest Fragment, Thailand. Forests. 2020; 11(11):1184. https://doi.org/10.3390/f11111184
Chicago/Turabian StyleGazagne, Eva, Jean-Luc Pitance, Tommaso Savini, Marie-Claude Huynen, Pascal Poncin, Fany Brotcorne, and Alain Hambuckers. 2020. "Seed Shadows of Northern Pigtailed Macaques within a Degraded Forest Fragment, Thailand" Forests 11, no. 11: 1184. https://doi.org/10.3390/f11111184
APA StyleGazagne, E., Pitance, J.-L., Savini, T., Huynen, M.-C., Poncin, P., Brotcorne, F., & Hambuckers, A. (2020). Seed Shadows of Northern Pigtailed Macaques within a Degraded Forest Fragment, Thailand. Forests, 11(11), 1184. https://doi.org/10.3390/f11111184