Key Community Assembly Processes Switch between Scales in Shaping Beta Diversity in Two Primary Forests, Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Census Data
2.2. Environmental Data
2.3. Tree DBH Size Class
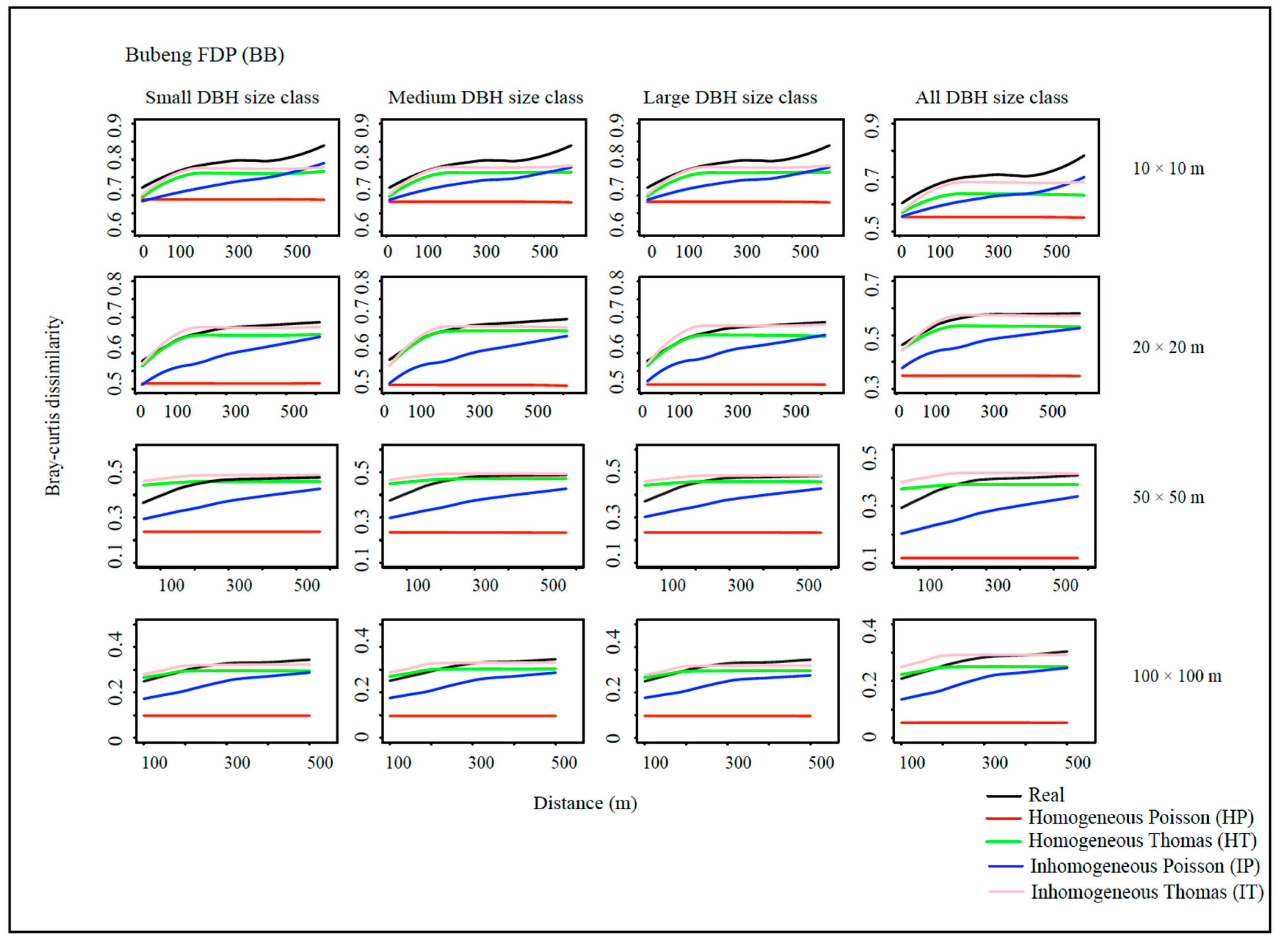
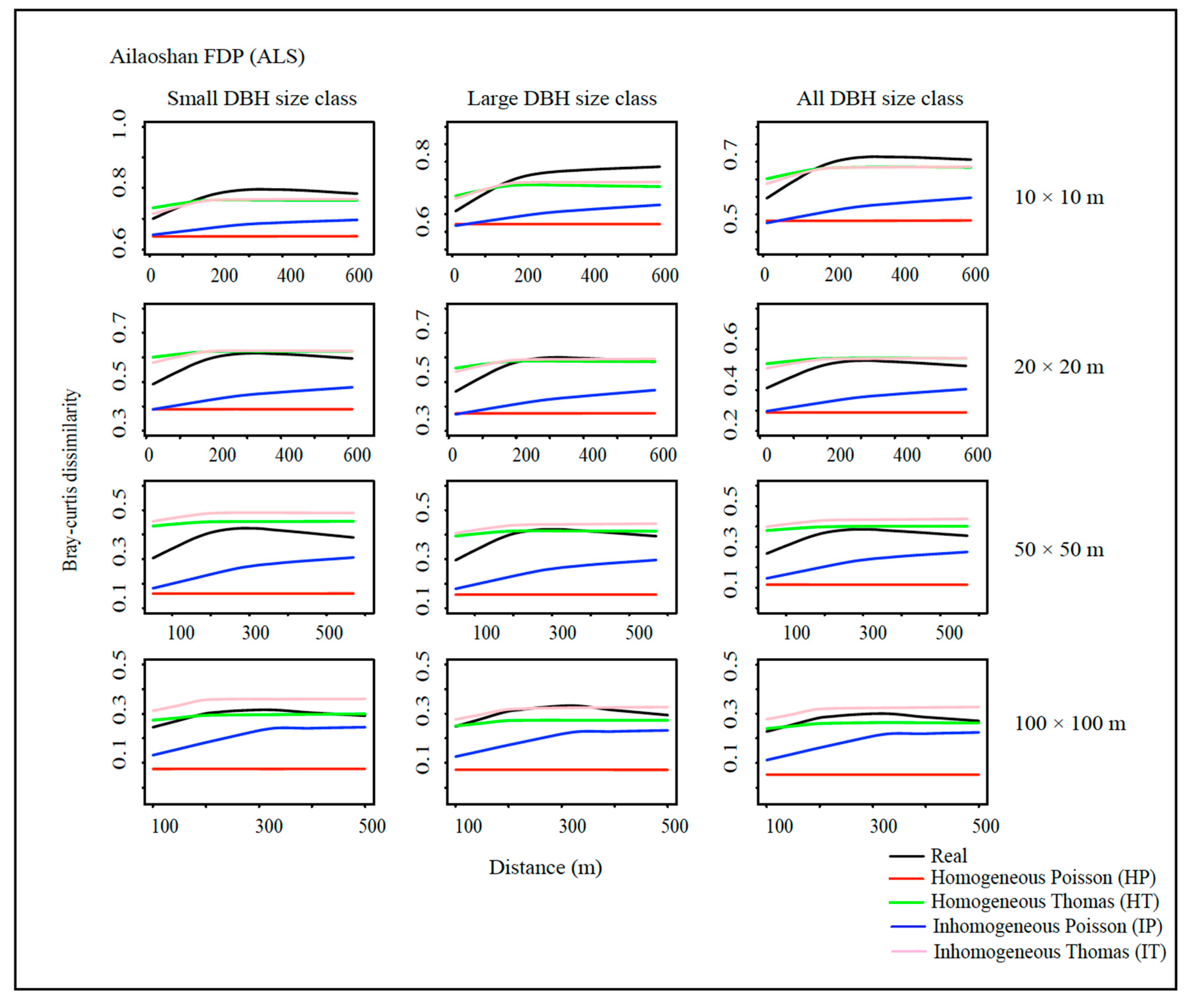
2.4. Spatial Point Pattern Analysis and Null Communities
2.5. Species Beta Diversity
2.6. Goodness-of-Fit of the Models
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Accessibility
References
- Chave, J. Spatial variation in tree species composition across tropical forests: Pattern and process. Trop. For. Community Ecol. 2008, 11–30. [Google Scholar]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Qian, H.; Ricklefs, R.E. A latitudinal gradient in large-scale beta diversity for vascular plants in North America. Ecol. Lett. 2007, 10, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Mi, X.; Ren, H.; Ma, K.; Yu, M.; Sun, I.; He, F. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 2009, 90, 663–674. [Google Scholar] [CrossRef]
- Shen, G.; Yu, M.; Hu, X.-S.; Mi, X.; Ren, H.; Sun, I.-F.; Ma, K. Species–area relationships explained by the joint effects of dispersal limitation and habitat heterogeneity. Ecology 2009, 90, 3033–3041. [Google Scholar] [CrossRef]
- Hu, Y.H.; Johnson, D.J.; Mi, X.C.; Wang, X.G.; Ye, W.H.; De Li, Y.; Lian, J.Y.; Cao, M. The relative importance of space compared to topography increases from rare to common tree species across latitude. J. Biogeogr. 2018, 45, 2520–2532. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Guo, H.; Fan, W.; Lv, H.; Duan, R. Distinguishing the importance between habitat specialization and dispersal limitation on species turnover. Ecol. Evol. 2013, 3, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- De Caáceres, M.; Legendre, P.; Valencia, R.; Cao, M.; Chang, L.-W.; Chuyong, G.; Condit, R.; Hao, Z.; Hsieh, C.-F.; Hubbel, S.; et al. The variation of tree beta diversity across a global network of forest plots. Glob. Ecol. Biogeogr. 2012, 21, 1191–1202. [Google Scholar] [CrossRef]
- Myers, J.A.; Chase, J.M.; Jimenez, I.; Jorgensen, P.M.; Araujo-Murakami, A.; Paniagua-Zambrana, N.; Seidel, R. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 2013, 16, 151–157. [Google Scholar] [CrossRef]
- Tang, Z.; Fang, J.; Chi, X.; Yang, Y.; Ma, W.; Mohhamot, A.; Guo, Z.; Liu, Y.; Gaston, K.J. Geography, environment, and spatial turnover of species in China’s grasslands. Ecography 2012, 35, 1103–1109. [Google Scholar] [CrossRef]
- Xu, W.; Chen, G.; Liu, C.; Ma, K. Latitudinal differences in species abundance distributions, rather than spatial aggregation, explain beta-diversity along latitudinal gradients. Glob. Ecol. Biogeogr. 2015, 24, 1170–1180. [Google Scholar] [CrossRef]
- Page, N.V.; Shanker, K. Environment and dispersal influence changes in species composition at different scales in woody plants of the Western Ghats, India. J. Veg. Sci. 2018, 29, 74–83. [Google Scholar] [CrossRef]
- Asefa, M.; Wen, H.D.; Brown, C.; Cao, M.; Xu, K.; Hu, Y.H. Ecological drivers of tree assemblage in tropical, subtropical and subalpine forests. J. Veg. Sci. 2020, 31, 107–117. [Google Scholar] [CrossRef]
- Comita, L.S.; Condit, R.; Hubbell, S.P. Developmental changes in habitat associations of tropical trees. J. Ecol. 2007, 95, 482–492. [Google Scholar] [CrossRef]
- Seidler, T.G.; Plotkin, J.B. Seed dispersal and spatial pattern in tropical trees. PLoS Biol. 2006, 4, e344. [Google Scholar] [CrossRef]
- Milberg, P.; Lamont, B.B. Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol. 1997, 137, 665–672. [Google Scholar] [CrossRef]
- Wright, S.J. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Murrell, D.J. On the emergent spatial structure of size-structured populations: When does self-thinning lead to a reduction in clustering? J. Ecol. 2009, 97, 256–266. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Harms, K.E.; Yavitt, J.B.; John, R.; Turner, B.L.; Valencia, R.; Navarrete, H.; Bunyavejchewin, S.; Kiratiprayoon, S.; Yaacob, A.; et al. Habitat filtering across tree life stages in tropical forest communities. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130548. [Google Scholar] [CrossRef]
- Kanagaraj, R.; Wiegand, T.; Comita, L.S.; Huth, A. Tropical tree species assemblages in topographical habitats change in time and with life stage. J. Ecol. 2011, 99, 1441–1452. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Kitching, R.L.; Lan, G.-Y.; Zhang, J.-L.; Sha, L.-Q.; Cao, M. Size-class effect contributes to tree species assembly through influencing dispersal in tropical forests. PLoS ONE 2014, 9, e108450. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M. Spatial scale resolves the niche versus neutral theory debate. J. Veg. Sci. 2014, 25, 319–322. [Google Scholar] [CrossRef]
- Catano, C.P.; Dickson, T.L.; Myers, J.A. Dispersal and neutral sampling mediate contingent effects of disturbance on plant beta-diversity: A meta-analysis. Ecol. Lett. 2017, 20, 347–356. [Google Scholar] [CrossRef]
- Cheng, J.; Mi, X.; Nadrowski, K.; Ren, H.; Zhang, J.; Ma, K. Separating the effect of mechanisms shaping species-abundance distributions at multiple scales in a subtropical forest. Oikos 2012, 21, 236–244. [Google Scholar] [CrossRef]
- Yuan, Z.; Gazol, A.; Wang, X.; Lin, F.; Ye, J.; Bai, X.; Li, B.; Hao, Z. Scale specific determinants of tree diversity in an old growth temperate forest in China. Basic Appl. Ecol. 2011, 12, 488–495. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods and Results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer: Berlin/Heidelberg, Germany; R.G. Landes Georg: Austin, TX, USA, 1998. [Google Scholar]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.C.; Hernandez, C.; Romoleroux, K.; Losos, E.; Magard, E.; Balslev, H. Tree species distributions and local habitat variation in the Amazon: Large forest plot in eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Sha, L.-Q.; Blanchet, F.G.; Zhang, J.-L.; Tang, Y.; Lan, G.-Y.; Cao, M. Dominant species and dispersal limitation regulate tree species distributions in a 20-ha plot in Xishuangbanna, southwest China. Oikos 2012, 123, 952–960. [Google Scholar] [CrossRef]
- Bagchi, R.; Henrys, P.A.; Brown, P.E.; Burslem, D.F.R.P.; Diggle, P.J.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Kassim, A.R.; Law, R.; Noor, S.; et al. Spatial patterns reveal negative density dependence and habitat associations in tropical trees. Ecology 2011, 92, 1723–1729. [Google Scholar] [CrossRef]
- Baddeley, A.; Turner, R.; Møller, J.; Hazelton, M. Residual analysis for spatial point processes. J. R. Stat. Soc. B 2005, 67, 617–666. [Google Scholar] [CrossRef]
- Illian, J.; Stoyan, D.; Stoyan, H.; Penttinen, A. Statistical Analysis and Modelling of Spatial Point Patterns; John Wiley & Sons: Chichester, UK, 2008; ISBN 9780470014912. [Google Scholar]
- Wiegand, T.; Moloney, K.A. Handbook of spatial Point-Pattern Analysis in Ecology; Taylor Fr. Group, LLC: New York, NY, USA, 2014. [Google Scholar]
- Stoyan, D.; Stoyan, H. Fractals, Random Shapes and Point Fields: Methods of Geometrical Statistics; Wiley: Chichester, UK, 1994. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; W.H.Freeman & Co.: New York, NY, USA, 1995. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Shen, T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Wang, X.; Wiegand, T.; Wolf, A.; Howe, R.; Davies, S.J.; Hao, Z. Spatial patterns of tree species richness in two temperate forests. J. Ecol. 2011, 99, 1382–1393. [Google Scholar] [CrossRef]
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; Chapman and Hall/CRC Press: London, UK, 2015; Available online: http://www.crcpress.com/Spatial-Point-Patterns-Methodology-and-Applications-with-R/Baddeley-Rubak-Turner/9781482210200/ (accessed on 24 February 2016).
- Liu, Y.; Tang, Z.; Fang, J. Contribution of environmental filtering and dispersal limitation to species turnover of temperate deciduous broad-leaved forests in China. Appl. Veg. Sci. 2015, 18, 34–42. [Google Scholar] [CrossRef]
- Shen, G.; He, F.; Waagepetersen, R.; Sun, I.-F.; Hao, Z.; Chen, Z.-S.; Yu, M. Quantifying effects of habitat heterogeneity and other clustering processes on spatial distributions of tree species. Ecology 2013, 94, 2436–2443. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Ecological Niches; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Brown, C.; Burslem, D.F.R.P.; Illian, J.B.; Bao, L.; Brockelman, W.; Cao, M.; Chang, L.W.; Dattaraja, H.S.; Davies, S.; Gunatilleke, C.V.S.; et al. Multispecies coexistence of trees in tropical forests: Spatial signals of topographic niche differentiation increase with environmental heterogeneity. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130502. [Google Scholar] [CrossRef]
- Lai, J.; Mi, X.; Ren, H.; Ma, K. Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 2009, 20, 415–423. [Google Scholar] [CrossRef]
- Yang, Q.S.; Shen, G.C.; Liu, H.M.; Wang, Z.H.; Ma, Z.P.; Fang, X.F.; Zhang, J.; Wang, X.H. Detangling the Effects of Environmental Filtering and Dispersal Limitation on Aggregated Distributions of Tree and Shrub Species: Life Stage Matters. PLoS ONE 2016, 11, e0156326. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Plots | DBH-Size Class | Spatial Scale (m2) | Homogeneous Poisson (HP) | Homogeneous Thomas (HT) | Inhomogeneous Poisson (IP) | Inhomogeneous Thomas (IT) |
|---|---|---|---|---|---|---|
| Bubeng FDP | Small | 10 × 10 | 2251.77 | 367.72 | 991.38 | −174.68 |
| 20 × 20 | 475.98 | −288.18 | 81.86 | −395.03 | ||
| 50 × 50 | 49.26 | −107.56 | −18.95 | −94.76 | ||
| 100 × 100 | −1.61 | −32.59 | −9.65 | −17.54 | ||
| Medium | 10 × 10 | 2380.91 | 265.05 | 1002.02 | 9.77 | |
| 20 × 20 | 515.23 | −333.94 | 107.16 | −389.33 | ||
| 50 × 50 | 51.16 | −130.41 | −17.57 | −107.31 | ||
| 100 × 100 | −1.79 | −36.87 | −9.57 | −20.88 | ||
| Large | 10 × 10 | 2354.46 | 303.36 | 945.80 | −316.74 | |
| 20 × 20 | 485.178 | −279.02 | 3.62 | −387.11 | ||
| 50 × 50 | 50.33 | −107.56 | −26.45 | −96.16 | ||
| 100 × 100 | −1.51 | −32.64 | −9.12 | −16.63 | ||
| All | 10 × 10 | 2955.40 | 1585.51 | 1600.41 | 350.53 | |
| 20 × 20 | 675.14 | −32.64 | 238.58 | −276.99 | ||
| 50 × 50 | 64.98 | −100.18 | −6.09 | −90.62 | ||
| 100 × 100 | −1.47 | −32.04 | −10.29 | −18.22 | ||
| Ailaoshan FDP | Small | 10 × 10 | 2745.64 | −92.39 | 2082.93 | −33.11 |
| 20 × 20 | 648.49 | −334.51 | 428.44 | −327.46 | ||
| 50 × 50 | 55.47 | −85.18 | 4.23 | −40.44 | ||
| 100 × 100 | −1.91 | −45.5 | −15.61 | −27.55 | ||
| Large | 10 × 10 | 2803.81 | 517.95 | 2215.77 | 187.8 | |
| 20 × 20 | 656.93 | −224.04 | 451.78 | −234.99 | ||
| 50 × 50 | 56.22 | −112.52 | 9.10 | −89.89 | ||
| 100 × 100 | −1.24 | −33.77 | −12.56 | −32.97 | ||
| All | 10 × 10 | 3172.29 | 286.25 | 2500.18 | 291.82 | |
| 20 × 20 | 713.26 | −218.43 | 473.30 | −232.32 | ||
| 50 × 50 | 58.49 | −105.19 | 3.69 | −61.10 | ||
| 100 × 100 | −1.64 | −40.00 | −15.54 | −30.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asefa, M.; Wen, H.-D.; Cao, M.; Hu, Y.-H. Key Community Assembly Processes Switch between Scales in Shaping Beta Diversity in Two Primary Forests, Southwest China. Forests 2020, 11, 1106. https://doi.org/10.3390/f11101106
Asefa M, Wen H-D, Cao M, Hu Y-H. Key Community Assembly Processes Switch between Scales in Shaping Beta Diversity in Two Primary Forests, Southwest China. Forests. 2020; 11(10):1106. https://doi.org/10.3390/f11101106
Chicago/Turabian StyleAsefa, Mengesha, Han-Dong Wen, Min Cao, and Yue-Hua Hu. 2020. "Key Community Assembly Processes Switch between Scales in Shaping Beta Diversity in Two Primary Forests, Southwest China" Forests 11, no. 10: 1106. https://doi.org/10.3390/f11101106
APA StyleAsefa, M., Wen, H.-D., Cao, M., & Hu, Y.-H. (2020). Key Community Assembly Processes Switch between Scales in Shaping Beta Diversity in Two Primary Forests, Southwest China. Forests, 11(10), 1106. https://doi.org/10.3390/f11101106





