Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018
Abstract
1. Introduction
2. Materials and Methods
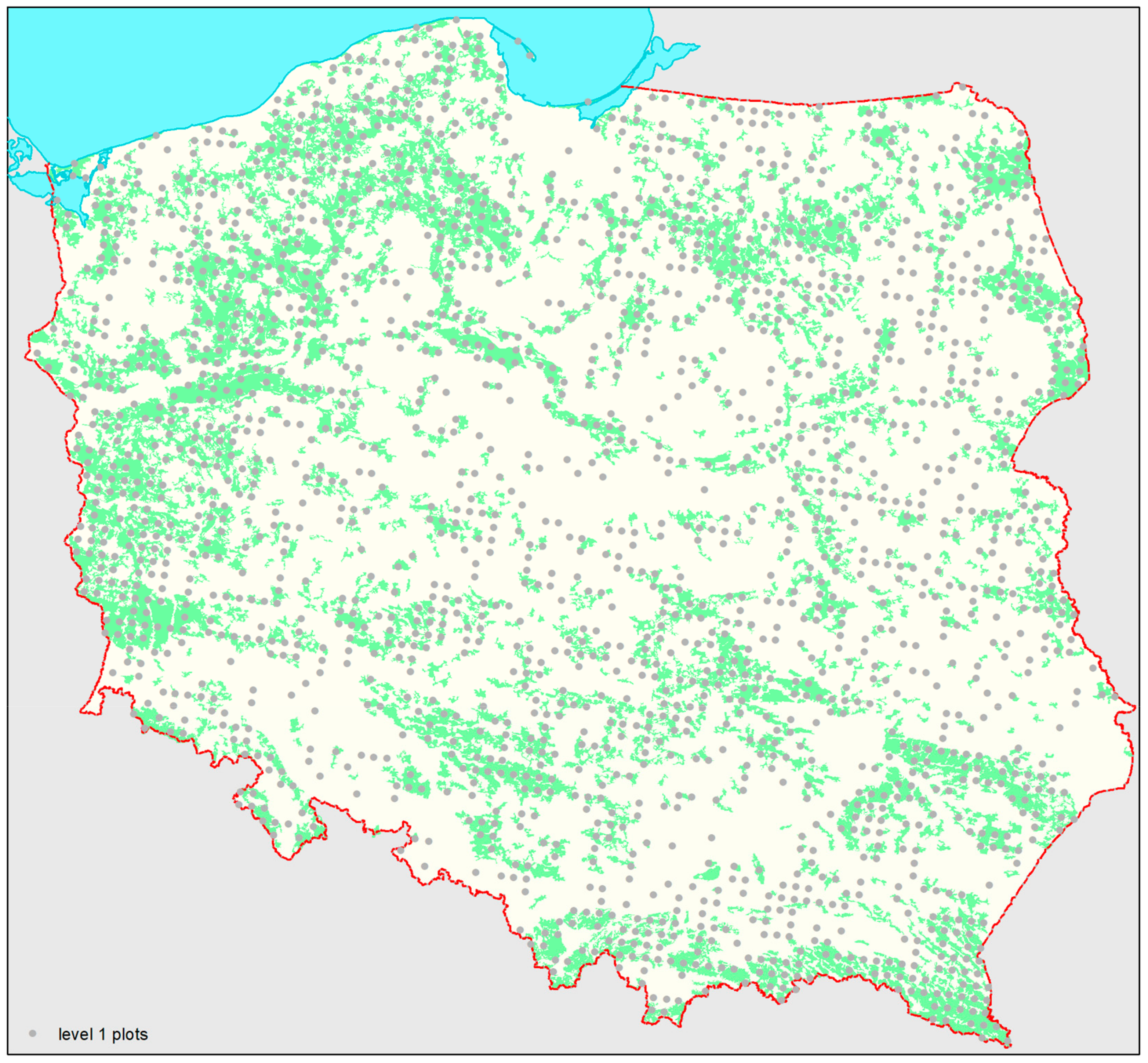
2.1. Data
2.2. Methods of Analysis
- F—coefficient of V. album appearance frequency;
- n—number of injuries caused by V. album on trees of a given species or all species together; and
- N—total number of trees of a given species or all species together.
3. Results
3.1. Mistletoe Occurrence on Tree Species
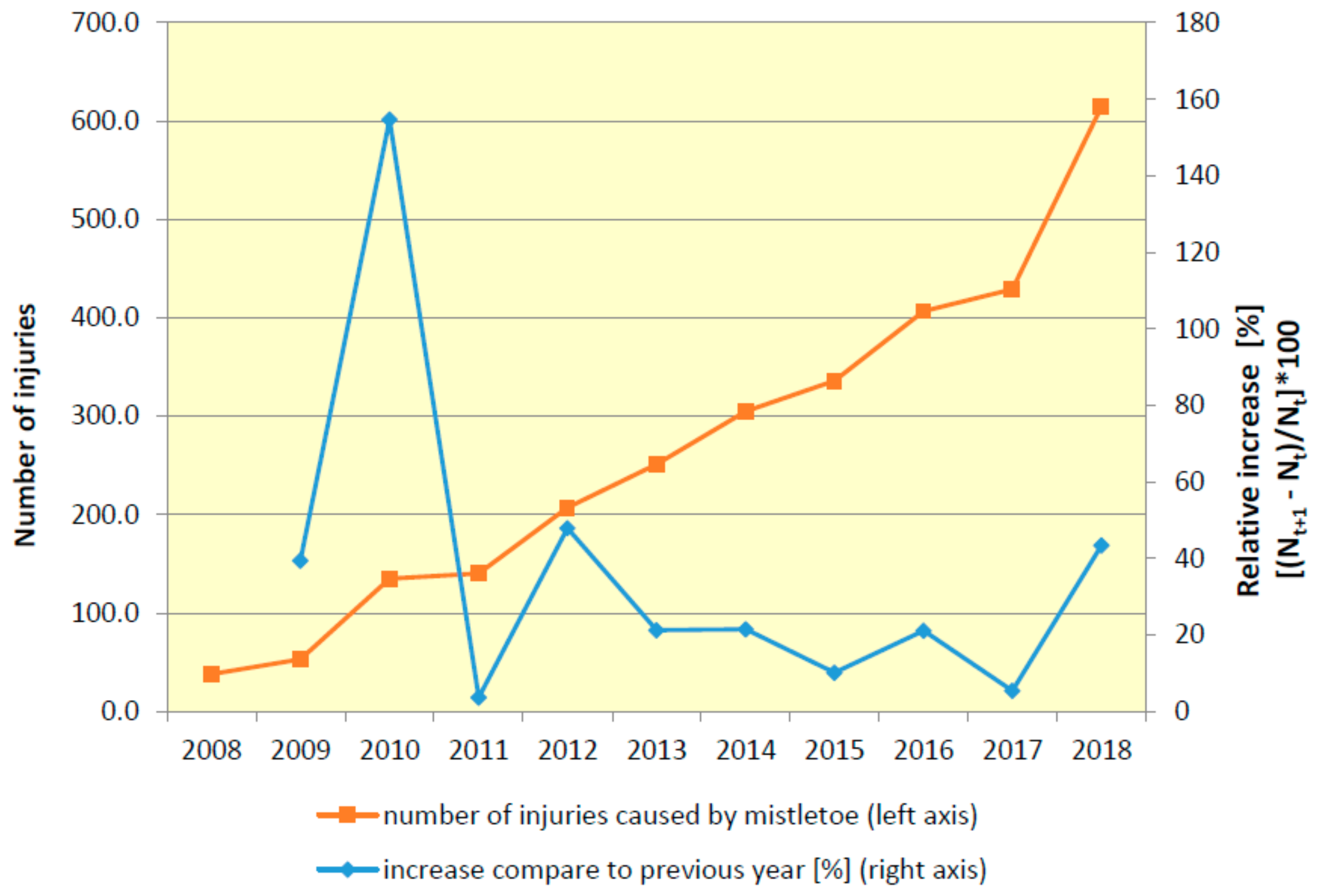
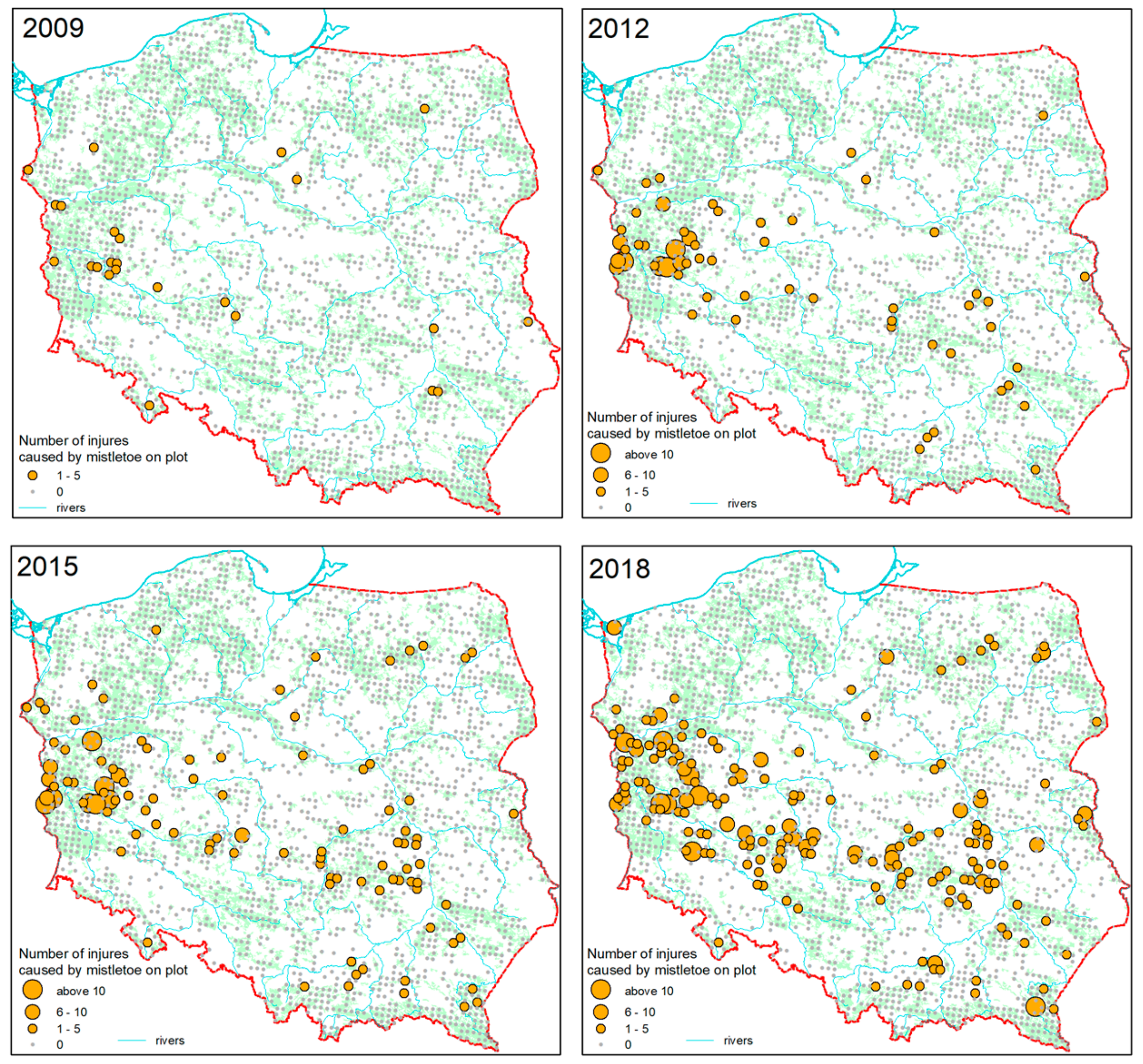
3.2. Spatial and Temporal Dynamics of Mistletoe Spread
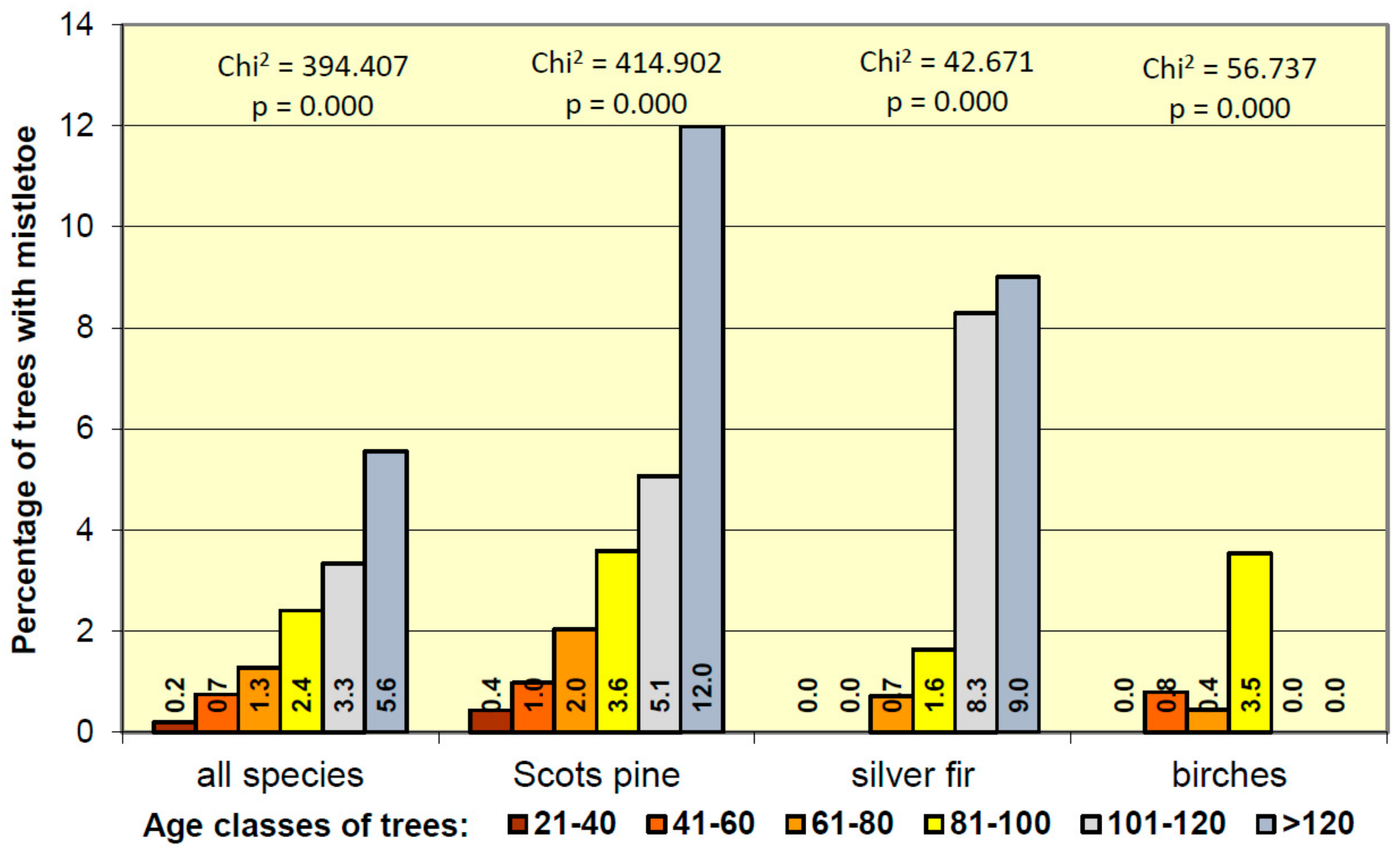
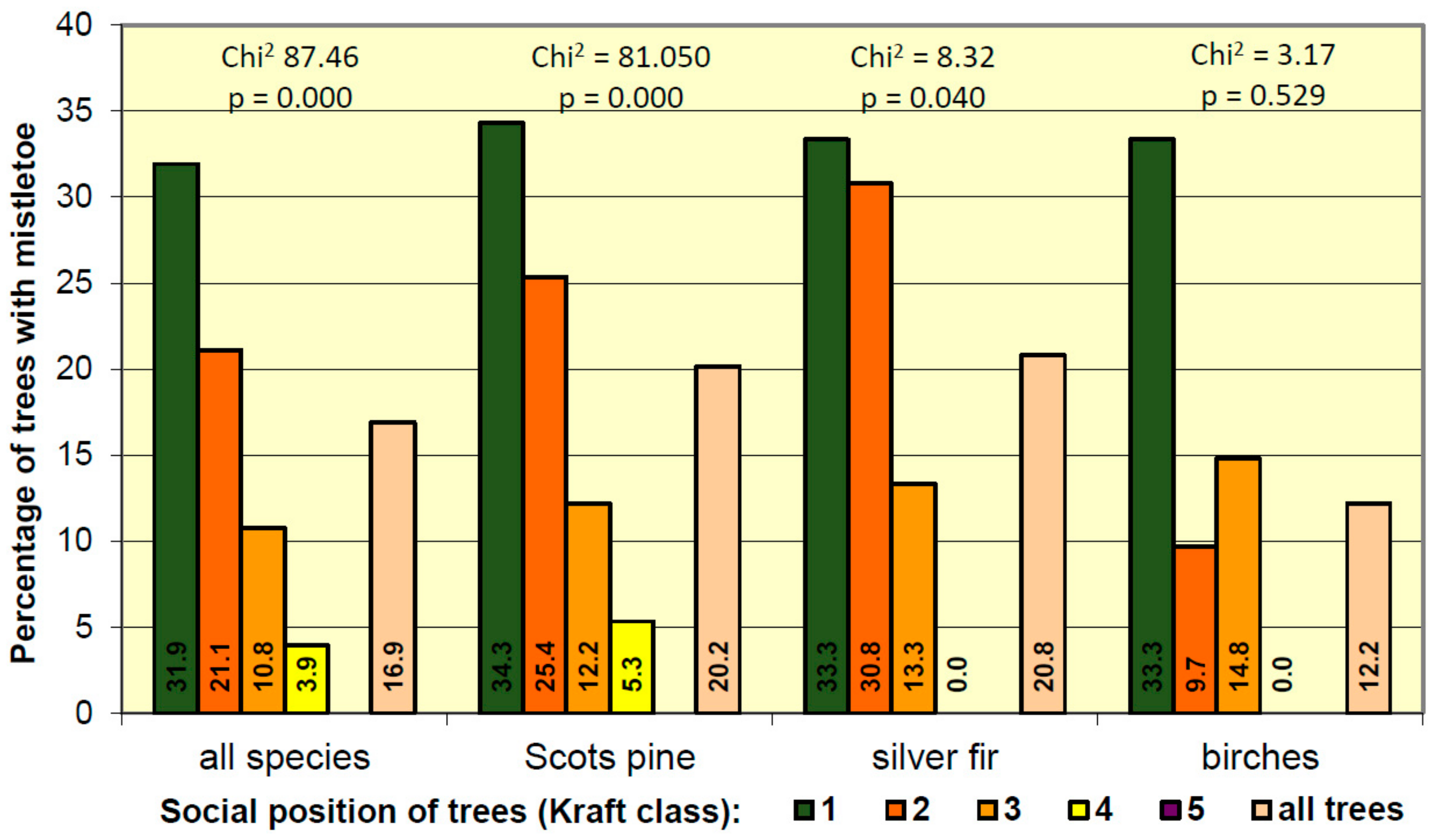
3.3. Mistletoe Occurrence in Relation to Age Classes of Trees
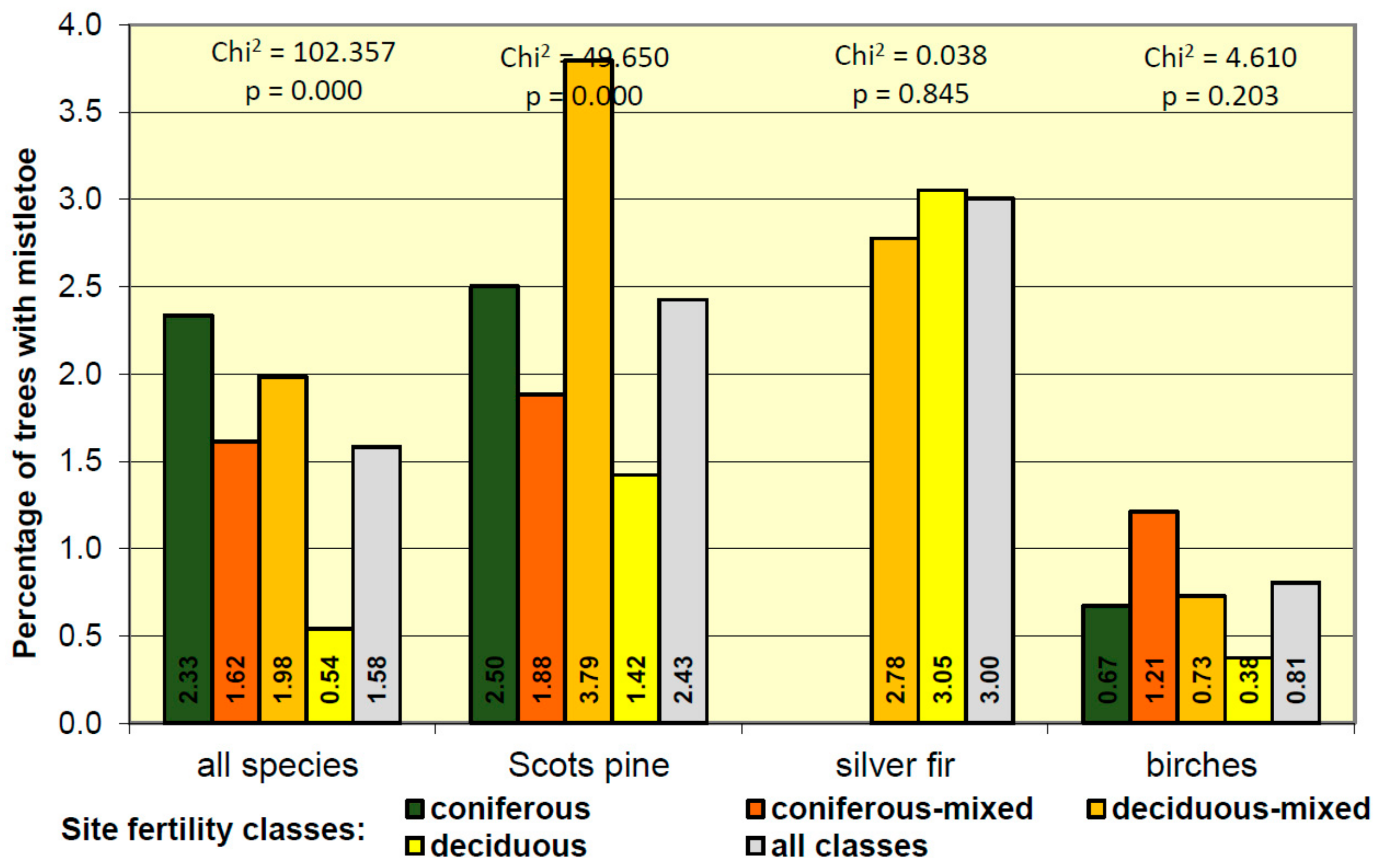
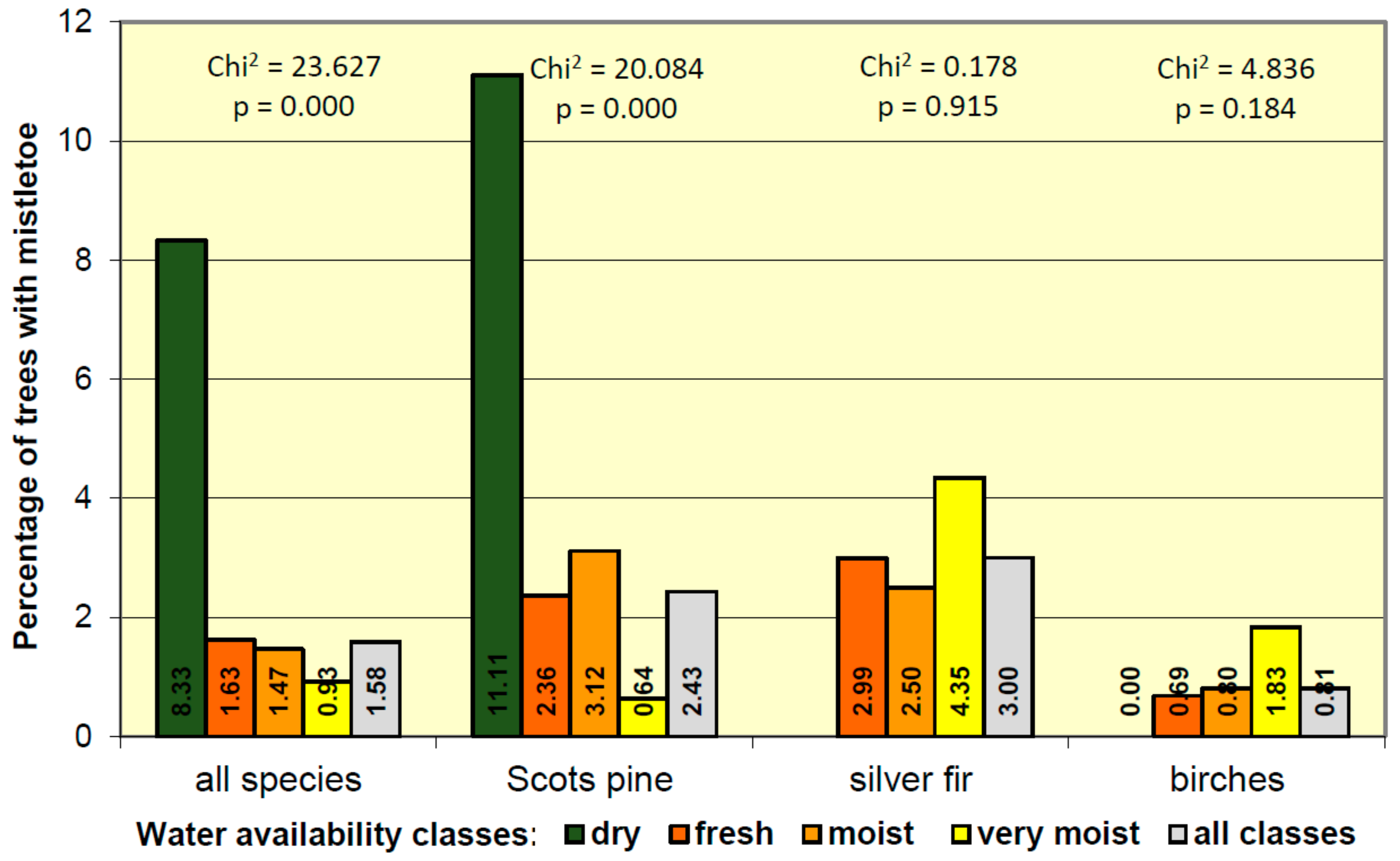
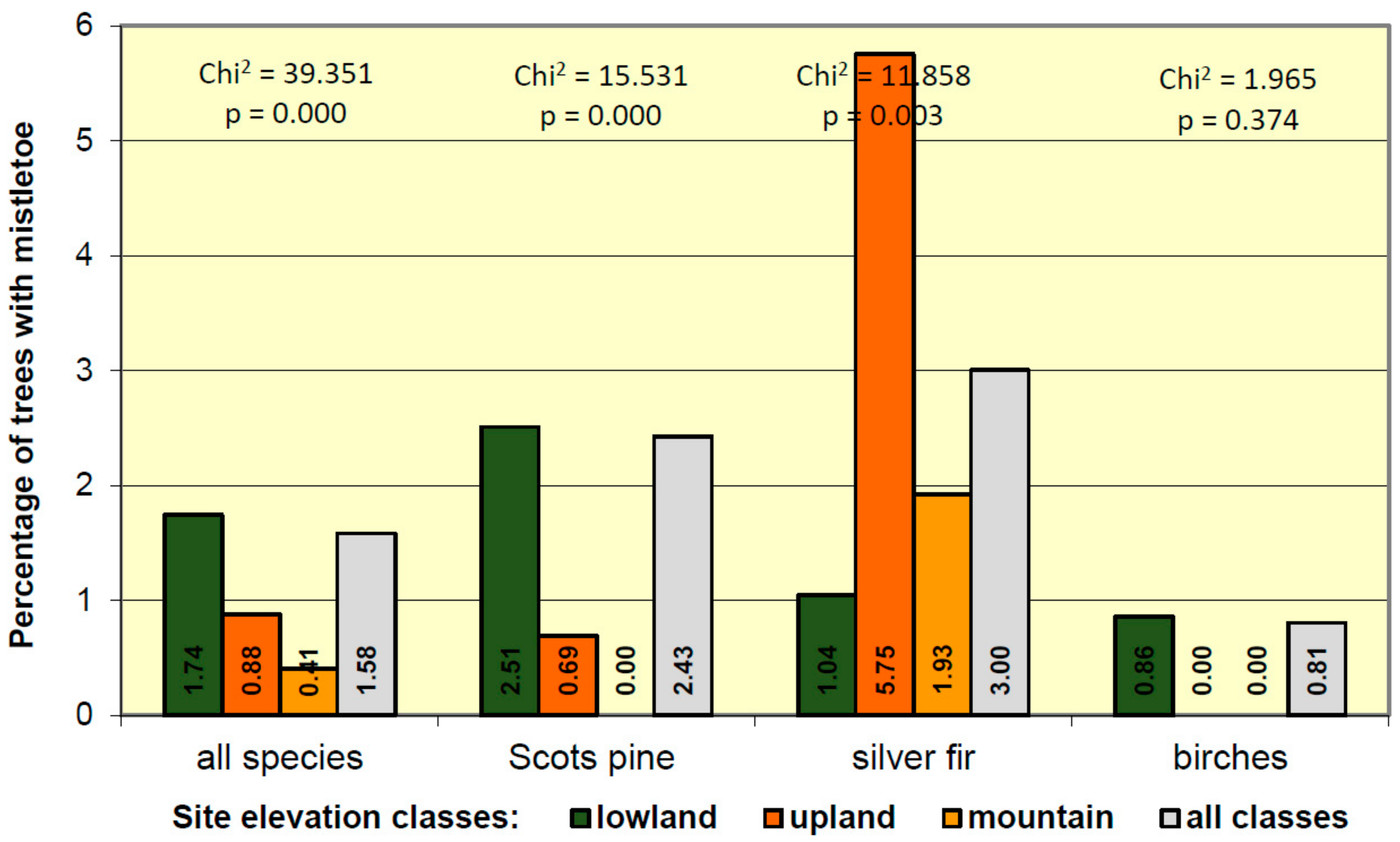
3.4. Mistletoe Occurrence in Relation to Site Condition
3.5. Mistletoe Occurrence in Relation to Size of Tree
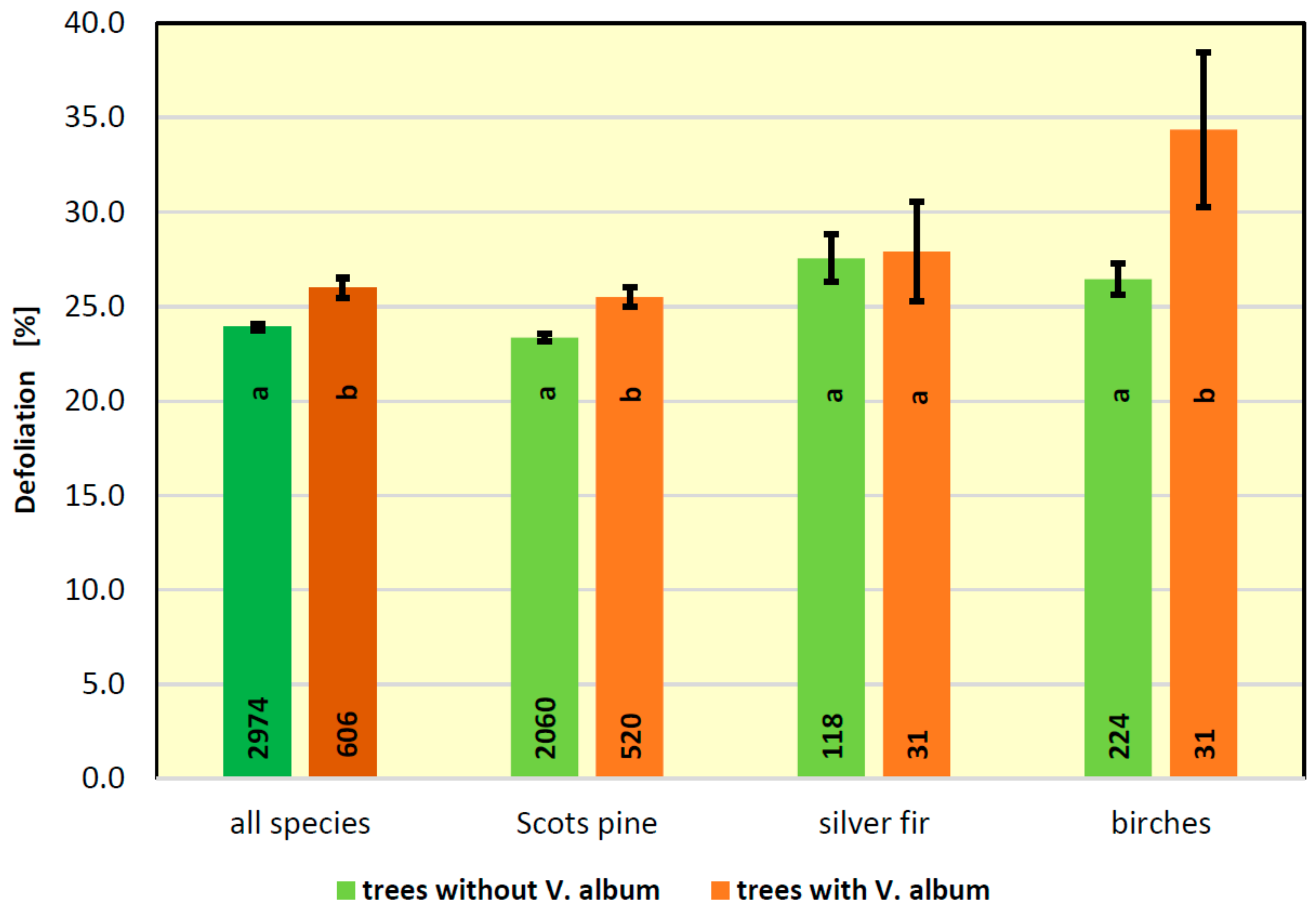
3.6. Effect of Mistletoe Infestation on Health Condition of Trees
4. Discussion
5. Conclusions
- ■
- The occurrence of mistletoe increased continuously throughout the entire period of 2008–2018.
- ■
- The highest concentration of parasite infestation was found in Western and Central Poland, gradually spreading to the East.
- ■
- Viscum album was found on 12 forest tree species confirming presence of three subspecies (subsp. album, subsp. austriacum, and subsp. abietis) in forests in Poland. Tree species with the highest infestation rates were silver fir, Scots pine, and birches.
- ■
- The oldest and biggest trees (predominant and dominant) were infested most frequently.
- ■
- Defoliation of trees infested by mistletoe was higher than in trees free from the parasite.
- ■
- The methodology of damage assessment used in forest monitoring may underestimate of mistletoe identification and endangerment to trees.
- ■
- Warm and dry conditions of vegetation period and mild winter months may have contributed to the spread of mistletoe in Polish forests in recent years.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nickkrent, D.L.; Malécot, V.; Vidal-Russell, R.; Der, J.R. A revised classification of Santalales. Taxon 2010, 59, 538–558. [Google Scholar] [CrossRef]
- Popp, M.; Richter, A. Ecophysiology of Xylem-Tapping Mistletoes. In Progress in Botany; Behnke, H.-D., Esser, K., Kadereit, J.W., Lüttge, U., Runge, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 659–674. ISBN 978-3-642-80448-9. [Google Scholar] [CrossRef]
- Zuber, D. Biological flora of Central Europe. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 181–203. [Google Scholar] [CrossRef]
- Schulze, E.D.; Turner, N.C.; Glatzel, G. Carbon, water and nutrient relations of two mistletoes and their hosts: An hypothesis. Plant Cell Environ. 1984, 7, 293–299. [Google Scholar]
- Glatzel, G.; Geils, B.W. Mistletoe ecophysiology: Host-parasite interactions. Botany 2009, 87, 10–15. [Google Scholar] [CrossRef]
- Sallé, G. Germination and Establishment of Viscum album L. In The Biology of Mistletoes; Calder, M., Bernhardt, P., Eds.; Academic Press Sydney: Sydney, NSW, Australia, 1983; pp. 154–159. [Google Scholar]
- Grundmann, B.M.; Pietzarka, U.; Roloff, A. Viscum album L. Enzyclopedia Woody Plants 2010, 56, 1–23. [Google Scholar]
- Frochot, H.; Sallé, G. Modalités de dissémination et d’implantation du gui. Revue Forestière Française 1980, XXXII, 505–519. [Google Scholar] [CrossRef]
- Hawksworth, F.G. Mistletoes as forest parasites. In The Biology of Mistletoes; Calder, M., Bernhardt, P., Eds.; Academic Press Sydney: Sydney, NSW, Australia, 1983; pp. 317–333. [Google Scholar]
- Stypiński, P.T. Biologia i ekologia jemioły pospolitej (Viscum album, Viscaceae) w Polsce. In Fragmenta Floristica et Geobotanica; Series Polonica. Supplementum 1; Instytut Botaniki im. W. Szafera; PAN: Kraków, Polônia, 1997; p. 115, (In Polish, abstract in English). [Google Scholar]
- Figarski, T. Wybrane aspekty zimowania paszkota Turdus viscivorus w Puszczy Kozienickiej (Selected aspects of wintering of Mistle Thrush Turdus viscivorus in the Kozienice Forest). Kulon 2009, 14, 1–7. [Google Scholar]
- Gill, L.S.; Hawksworth, F.G. Technical Bulletin No. 1242. In The Mistletoes: A Literature Review; Rocky Mountain Forest and Range Experiment Station Forest Service, United States Department of Agriculture: Washington, DC, USA, 1961; p. 87. [Google Scholar]
- Overton, J.M. Dispersal and infection in mistletoe metapopulation. J. Ecol. 1994, 82, 711–723. [Google Scholar] [CrossRef]
- Watson, D.M. Mistletoe—A keystone resource in forests and woodlands worldwide. Annu. Rev. Ecol. System. 2001, 32, 219–249. [Google Scholar] [CrossRef]
- Aukema, J.E.; Martínez del Rio, C. Where does a fruit eating bird deposit mistletoe seeds? Seed deposition patterns and an experiment. Ecology 2002, 83, 3489–3496. [Google Scholar] [CrossRef]
- Kołodziejek, J.; Kołodziejek, A. The Spatial Distribution of Pine Mistletoe Viscum album ssp. austriacum (Wiesb.) Volmann in Scots Pine (Pinus sylvestris L.) Stand in Central Poland. Pol. J. Ecol. 2013, 4, 705–714. [Google Scholar]
- Hawksworth, F.G.; Scharpf, R.F. Spread of European mistletoe (Viscum album) in California, U.S.A. Eur. J. For. Path. 1986, 16, 1–5. [Google Scholar] [CrossRef]
- Barney, C.W.; Hawksworth, F.G.; Geils, B.W. Hosts of Viscum album. Eur. J. For. Path. 1998, 28, 187–208. [Google Scholar] [CrossRef]
- Becker, H. European mistletoe: Taxonomy, Host trees, Parts used, Physiology. In Mistletoe: The Genus Viscum; Büssing, A., Ed.; Hardwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 31–44. [Google Scholar]
- Plagnat, F. Le gui du sapin. Ann. de l’École Natl. des eaux et Forêts 1950, 12, 155–231. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Böhling, N.; Greuter, W.; Raus, T.; Snogerup, B.; Songerup, S.; Zuber, D. Notes on the Cretan mistletoe, Viscum album subsp. creticum subsp. nova (Loranthaceae/Viscaceae). Israel J. Plant Sci. 2002, 50, 77–84. [Google Scholar]
- Potočić, N.; Timmermann, V.; Ognjević, M. Tree crown condition in 2017. In Forest Condition in Europe: 2018, Technical Report of ICP Forests, Report under the UNECE Convention on Long-Range Transboundary Air Pollution (Air Convention); Michel, A., Seidling, W., Prescher, A.-K., Eds.; BFW Austrian Research Centre for Forests: Vienna, Austrian, 2018; p. 92. [Google Scholar]
- Dobbertin, M.; Rigling, A. Pine mistletoe (Viscum album ssp. austriacum) contributes to Scots pine (Pinus sylvestris) mortality in the Rhone valley of Switzerland. For. Path. 2006, 36, 309–322. [Google Scholar] [CrossRef]
- MLU Waldzustandsbericht. Nordwestdeutsche Forstliche Versuchsanstalt; Sachsen-Anhalt Ministerium für Ländliche Entwicklung, Umwelt und Landwirtschaft (MLUL) des Landes Brandenburg Landesbetrieb Forst Brandenburg: Göttingen, Germany, 2015; p. 43. [Google Scholar]
- Varga, I.; Poczai, P.; Tiborcz, V.; Aranyi, N.R.; Baltazár, T.; Bartha, D.; Pejchal, M.; Hyvönen, J. Changes in the Distribution of European Mistletoe (Viscum album) in Hungary During the Last Hundred Years. Folia Geobot. 2014, 49, 559–577. [Google Scholar] [CrossRef]
- Szmidla, H. Roślina pasożytnicza Viscum album L.—Jemioła pospolita. In Krótkoterminowa Prognoza Występowania Ważniejszych Szkodników Owadzich I Chorób Infekcyjnych Drzew Leśnych W 2019 Roku (Short-Term Forecast of The Occurrence of Major Pests and Infectious Diseases of Forest Trees in 2019); Jabłoński, T., Ed.; Analizy i Raporty nr 31; Instytut Badawczy Leśnictwa: Sękocin Stary, Poland, 2019; pp. 83–85. (In Polish) [Google Scholar]
- Szmidla, H.; Tkaczyk, M.; Plewa, R.; Tarwacki, G.; Sierota, Z. Impact of Common Mistletoe (Viscum album L.) on Scots Pine Forests—A Call for Action. Forests 2019, 10, 847. [Google Scholar] [CrossRef]
- Plagnat, F.; Brossier. J. Les sapiniares a gui. Rev. For. Fr. 1969, 6, 553–557. [Google Scholar] [CrossRef]
- Hofstetter, M. Über die Verbreitung der Mistel in der Schweiz. Schweiz. Z. Forstwes. 1988, 139, 97–127. [Google Scholar]
- Noetzli, K.P.; Müller, B.; Sieber, T.N. Impact of population dynamics of white mistletoe (Viscum album subsp. abietis) on European silver fir (Abies alba). Ann. For. Sci. 2003, 60, 773–779. [Google Scholar] [CrossRef]
- Idžojtić, M.; Pernar, R.; Glavaš, M.; Zebec, M.; Diminić, D. The incidence of mistletoe (Viscum album ssp. abietis) on silver fir (Abies alba) in Croatia. Biologia 2008, 63, 81–85. [Google Scholar]
- Barbu, C. The incidence and distribution of white mistletoe (Viscum album ssp. abietis) on Silver fir (Abies alba Mill.) stands from Eastern Carpathians. Ann. For. Res. 2010, 53, 27–36. [Google Scholar]
- Oliva, J.; Colinas, C. Decline of silver fir (Abies alba Mill.) stands in the Spanish Pyrenees: Role of management, historic dynamics and pathogens. For. Ecol. Manag. 2007, 252, 84–97. [Google Scholar] [CrossRef]
- Tsopelas, P.; Angelopoulos, A.; Economou, A.; Soulioti, N. Mistletoe (Viscum album) in the fir forest of Mount Parnis, Greece. For. Ecol. Manag. 2004, 202, 59–65. [Google Scholar] [CrossRef]
- Hawksworth, F.G. The 6-Class Dwarf Mistletoe Rating System. In USDA Forest Service General Technical Report; RM-48; Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1977; p. 7. [Google Scholar]
- Hawksworth, F.G.; Shaw, C.G.; Tkacz, B. Damage and control of diseases of Southwestern ponderosa pine. In Multiresource Management of Ponderosa Pine Forests; USDA Forest Service General Technical Report RM-185; USDA Forest Service: Fort Collins, CO, USA, 1989; pp. 116–129. [Google Scholar]
- Varga, I.; Taller, J.; Baltazar, T.; Hyvonen, J.; Poczai, P. Leaf-spot disease on European mistletoe (Viscum album) caused by Phaeobotryosphaeria visci: A potential candidate for biological control. Biotechnol. Lett. 2012, 34, 1059–1065. [Google Scholar] [CrossRef]
- Fischer, J.T. Water relations of mistletoes and their hosts. In The Biology of Mistletoes; Calder, M., Bernhardt, P., Eds.; Academic Press: San Diego, CA, USA, 1983; pp. 161–183. [Google Scholar]
- Durand-Gilmann, M.; Cailleret, M.; Boivin, T.; Nageleisen, L.M.; Davi, H. Individual vulnerability factors of Silver fir (Abies alba Mill.) to parasitism by two contrasting biotic agents: Mistletoe (Viscum album L. ssp. abietis) and bark beetles (Coleoptera: Curculionidae: Scolytinae) during a decline process. Ann. Forest Sci. 2012, 71, 659–673. [Google Scholar]
- Ozturk, M.; Coskuner, K.A.; Usta, Y.; Serdar, B.; Bilgili, E. The effect of mistletoe (Viscum album) on branch wood and needle anatomy of Scots pine (Pinus sylvestris). IAWA J. 2019, 40, 352–365. [Google Scholar] [CrossRef]
- Nanu, N. Viscum album L., a parasite in Abies alba stands on the calcareous Anina-ravita plateau. Rev. Padur. 1969, 84, 177–178. [Google Scholar]
- Barbu, C. Impact of mistletoe attack (Viscum album ssp. abietis) on radial growth of silver fir. A case study in the North of Eastern Carpathians. Ann. Forest Res. 2009, 52, 89–96. [Google Scholar]
- Dobbertin, M.; Mayer, P.; Wohlgemuth, T.; Feldmeyer-Christe, E.; Graf, U.; Zimmermann, N.E.; Rigling, A. The decline of Pinus sylvestris L. forests in the Swiss Rhonne valley—A result of drought stress? Phyton 2005, 45, 153–156. [Google Scholar]
- Barbu, C. Aspects regarding mistletoe (Viscum album abietis) attack on silver fir stands. In Proceedings of the Lucrările sesiunii ştiinţifice biennale cu participare internaţională Pădurea şi Dezvoltarea Durabilă, Braşov, Romania, 27–28 October 2006; Transilvania University of Braşov: Braşov, Romania, 2007; pp. 183–188. [Google Scholar]
- Bukowiec, G.; Bednarz, B. Wpływ jemioły pospolitej jodłowej (Viscum album ssp. abietis) na przyrosty roczne jodły pospolitej (Abies alba). Acta Sci. Pol. Silv. Colendar. Ratio Ind. Lignar 2017, 16, 77–83. [Google Scholar] [CrossRef]
- Luther, P.; Becker, H. Die Mistel-Botanik, Lektine, Medizinische Anwendung; VEB Verlag Volk Und Gesundheit: Berlin, Germany, 1986. [Google Scholar]
- Tubeuf, K.F.; Neckel, G.; Marzell, H. Monographie Der Mistel; Oldenbourg: München, German; Berlin, Germany, 1923; p. 832. [Google Scholar]
- Skre, O. Regional distribution of vascular plants in Scandinavia with requirements for high summer temperatures. Norw. J. Bot. 1979, 26, 295–318. [Google Scholar]
- Iversen, J. Viscum, Hedera and Ilex as climate indicators. Geol. Fören. Stockh. Förh. 1944, 66, 463–1483. [Google Scholar] [CrossRef]
- Skre, O. High temperature demands for growth and development in Norway Spruce (Picea abies (L.) Karst.) in Scandinavia. Nor. Landbr. Meld 1971, 51, 1–29. [Google Scholar]
- Dobbertin, M.; Hilker, N.; Rebetez, M.; Zimmermann, N.E.; Wohlgemuth, T.; Rigling, A. The upward shift in altitude of pine mistletoe (Viscum album ssp. austriacum) in Switzerland—The result of climate warming? Int. Biometeorol. 2005, 50, 40–47. [Google Scholar]
- Bojarczuk, T. Wyniki Dalszych Obserwacji Nad Występowaniem Jemioły Pospolitej (Viscum Album L.) W Arboretum Kórnickim; Arboretum Kórnickie: Poznań, Polônia, 1970. (In Polish) [Google Scholar]
- Bojarczuk, T. Żywiciele jemioły pospolitej (Viscum album L.) w Polsce. Rocz. Dendrol. 1971, 25, 189–199. (In Polish) [Google Scholar]
- Stypiński, P.T. Występowanie Jemioły Pospolitej Na Pojezierzu Mazurskim; Wyższa Szkoła Pedagogiczna: Olsztyn, Polônia; Warszawa, Polônia, 1978. (In Polish) [Google Scholar]
- Gołabek, E.; Sławiński, J. The Infestation Degree of Trees with Common Mistletoe Viscum album L. and their Health Status (on the Example of Praszka City). J. Econom. Eng. 2017, 18, 80–85. [Google Scholar] [CrossRef]
- Boratyńska, K.; Boratyński, A. Viscum album L.—jemioła pospolita, Viscum laxum Boiss—jemioła rozpierzchła. In Atlas Rozmieszczenia Drzew I Krzewów W Polsce, 19; Browicz, K., Ed.; PWN: Warszawa, Poland; Poznań, Poland, 1976; pp. 9–19. (In Polish) [Google Scholar]
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schröck, H.-W.; et al. Part IV: Visual Assessment of Crown Condition and Damaging Agents. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-Ordinating Centre, Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; p. 49. Available online: http://www.icp-forests.net/page/icp-forests-manual (accessed on 30 November 2019).
- StatPoint Inc. Statgraphics Centurion XV User Manual; StatPoint Inc.: Warrenton, VA, USA, 2005; p. 287. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- McLeod, A.I. R package version 2.2. Kendall: Kendall Rank Correlation and Mann-Kendall Trend Test. 2011. Available online: https://cran.r-project.org/web/packages/Kendall/Kendall.pdf (accessed on 30 September 2019).
- Boczoń, A.; Hildebrand, R. Susze na terenie Polski w latach 2010–2017 i ich wpływ na defoliację (Droughts in Poland in the years 2010–2017 and their influence on defoliation of trees). In Stan Zdrowotny Lasów W Polsce W 2018 Roku Na Podstawie Badań Monitoringowych (Health Condition of Forests in Poland in 2018—Results of Forests Monitoring); Wawrzoniak, J., Ed.; Forest Research Institute: Sękocin Stary, Poland, 2018; pp. 72–76. (In Polish) [Google Scholar]
- Boczoń, A.; Hildebrand, R. Warunki wodne panujące w glebach na terenie Polski (Water conditios in soils in Poland). In Stan Zdrowotny Lasów W Polsce W 2018 Roku Na Podstawie Badań Monitoringowych (Health Condition of Forests in Poland in 2018—Results of Forests Monitoring); Wawrzoniak, J., Ed.; Forest Research Institute: Sękocin Stary, Poland, 2019; pp. 73–77. (In Polish) [Google Scholar]
- Rigling, A.; Eilmann, B.; Koechli, R.; Dobbertin, M. Mistletoe-induced crown degradation in Scots pine in a xeric environment. Tree Physiol. 2010, 30, 845–852. [Google Scholar] [CrossRef]
- Diminić, D.; Potočić, N.; Jazbec, A.; Županić, M. Infestation of common mistletoe and nutrition status of silver fir in gorski Kotar (Croatia). Croat. J. For. Eng. 2011, 32, 223–237. [Google Scholar]
- Barbu, C.O. Impact of White Mistletoe (Viscum album ssp abietis) Infection on Needles and Crown Morphology of Silver Fr (Abies alba Mill.). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 152–158. [Google Scholar] [CrossRef]

| Year | Unit/Coefficient | Scots Pine | Silver Fir | Birches | Black Alder | Other Deciduous | Total |
|---|---|---|---|---|---|---|---|
| 2008 | count | 29 | 5 | 4 | 38 | ||
| F | 0.13 | 0.13 | 0.22 | 0.07 | |||
| 2009 | count | 38 | 4 | 7 | 4 | 53 | |
| F | 0.17 | 0.42 | 0.18 | 0.18 | 0.14 | ||
| 2010 | count | 110 | 8 | 15 | 2 | 135 | |
| F | 0.49 | 0.83 | 0.38 | 0.08 | 0.35 | ||
| 2011 | count | 111 | 10 | 13 | 6 | 140 | |
| F | 0.50 | 1.05 | 0.33 | 0.24 | 0.36 | ||
| 2012 | count | 182 | 11 | 8 | 6 | 207 | |
| F | 0.82 | 1.12 | 0.20 | 0.24 | 0.53 | ||
| 2013 | count | 213 | 11 | 5 | 3 | 19 | 251 |
| F | 0.96 | 1.11 | 0.12 | 0.12 | 0.74 | 0.64 | |
| 2014 | count | 250 | 13 | 15 | 2 | 25 | 305 |
| F | 1.11 | 1.31 | 0.35 | 0.08 | 0.94 | 0.76 | |
| 2015 | count | 283 | 16 | 23 | 1 | 13 | 336 |
| F | 1.25 | 1.60 | 0.54 | 0.04 | 0.48 | 0.84 | |
| 2016 | count | 337 | 20 | 27 | 1 | 22 | 407 |
| F | 1.51 | 1.97 | 0.64 | 0.04 | 0.82 | 1.02 | |
| 2017 | count | 363 | 21 | 29 | 16 | 429 | |
| F | 1.63 | 2.02 | 0.67 | 0.58 | 1.07 | ||
| 2018 | count | 521 | 36 | 42 | 16 | 615 | |
| F | 2.35 | 3.46 | 0.97 | 0.56 | 1.52 | ||
| M–K trend | I | I | I | PI | I | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lech, P.; Żółciak, A.; Hildebrand, R. Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018. Forests 2020, 11, 83. https://doi.org/10.3390/f11010083
Lech P, Żółciak A, Hildebrand R. Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018. Forests. 2020; 11(1):83. https://doi.org/10.3390/f11010083
Chicago/Turabian StyleLech, Paweł, Anna Żółciak, and Robert Hildebrand. 2020. "Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018" Forests 11, no. 1: 83. https://doi.org/10.3390/f11010083
APA StyleLech, P., Żółciak, A., & Hildebrand, R. (2020). Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018. Forests, 11(1), 83. https://doi.org/10.3390/f11010083





