Relationships between Structural Indices and Conventional Stand Attributes in an Old-Growth Forest in Southeast Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Measurements
2.3. Data Analysis
3. Results
3.1. Descriptive Statistics and Frequency Distributions of All Examined Indices
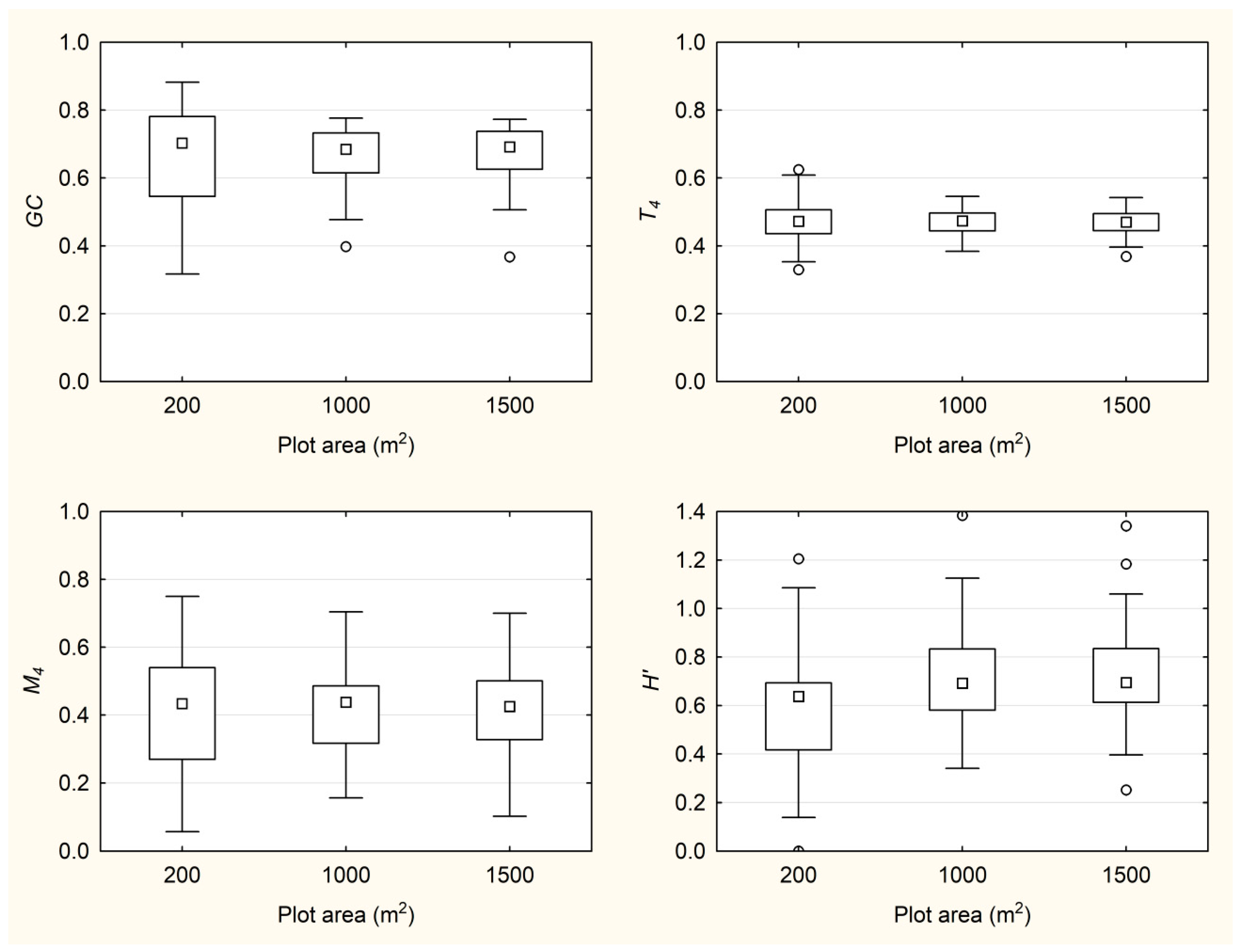
3.2. Behavior of Indices with the Change of Plot Size
3.3. Associations among Indices and Their Correlations with Stand Attributes
4. Discussion
4.1. Local DBH Differentiation, Species Mingling, and Diversity in Perućica
4.2. Relationships between the Evaluated Indices and Conventional Stand Attributes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McElhinny, C.; Gibbons, P.; Brack, C.; Bauhus, J. Forest and woodland stand structural complexity: Its definition and measurement. For. Ecol. Manag. 2005, 218, 1–24. [Google Scholar] [CrossRef]
- Schall, P.; Schulze, E.D.; Fischer, M.; Ayasse, M.; Ammer, C. Relations between forest management, stand structure and productivity across different types of Central European forests. Basic Appl. Ecol. 2018, 32, 39–52. [Google Scholar] [CrossRef]
- Keeton, W.S. Managing for late-successional/old-growth characteristics in northern hardwood-conifer forests. For. Ecol. Manag. 2006, 235, 129–142. [Google Scholar] [CrossRef]
- Korpel, S. Die Urwälder der Westkarpaten; Gustav Fischer Verlag: Stuttgart, Germany; Jena: New York, NY, USA, 1995. [Google Scholar]
- Vrška, T.; Adam, D.; Hort, L.; Kolář, T.; Janík, D. European beech (Fagus sylvatica L.) and silver fir (Abies alba Mill.) rotation in the Carpathians—A developmental cycle or a linear trend induced by man? For. Ecol. Manag. 2009, 258, 347–356. [Google Scholar] [CrossRef]
- Jaworski, A.; Kołodziej, Z.; Łapka, M. Mortality, recruitment, and increment of trees in the Fagus-Abies-Picea stands of a primeval character in the lower mountain zone. Dendrobiology 2007, 57, 15–26. [Google Scholar]
- Diaci, J.; Rozenbergar, D.; Anic, I.; Mikac, S.; Saniga, M.; Kucbel, S.; Visnjic, C.; Ballian, D. Structural dynamics and synchronous silver fir decline in mixed old-growth mountain forests in Eastern and Southeastern Europe. Forestry 2011, 84, 479–491. [Google Scholar] [CrossRef]
- Zenner, E.K.; Peck, J.L.E. Floating neighborhoods reveal contribution of individual trees to high sub-stand scale heterogeneity. For. Ecol. Manag. 2018, 412, 29–40. [Google Scholar] [CrossRef]
- Mauro, F.; Haxtema, Z.; Hailemariam, T. Comparison of sampling methods for estimation of nearest-neighbor index values. Can. J. For. Res. 2017, 47, 703–7015. [Google Scholar] [CrossRef]
- Pommerening, A.; Grabarnik, P. Individual-Based Methods in Forest Ecology and Management, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Neumann, M.; Starlinger, F. The significance of different indices for stand structure and diversity in forests. For. Ecol. Manag. 2001, 145, 91–106. [Google Scholar] [CrossRef]
- Li, Y.; Hui, G.; Yu, S.; Luo, Y.; Yao, X.; Ye, S. Nearest neighbour relationships in Pinus yunnanensis var. Tenuifolia forests along the Nanpan River, China. iForest 2017, 10, 746–753. [Google Scholar] [CrossRef]
- Nagel, T.A.; Mikac, S.; Dolinar, M.; Klopcic, M.; Keren, S.; Svoboda, M.; Diaci, J.; Boncina, A.; Paulic, V. The natural disturbance regime in forests of the Dinaric Mountains: A synthesis of evidence. For. Ecol. Manag. 2017, 388, 29–42. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Maciejewski, Z.; Maciejewska, E.; Tomski, A.; Gazda, A. Forest recovery in set-aside windthrow is facilitated by fast growth of advance regeneration. Ann. For. Sci. 2018, 75, 80. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M. Gap disturbance regime in an old-growth Fagus–Abies forest in the Dinaric Mountains, Bosnia-Herzegovina. Can. J. For. Res. 2008, 38, 2728–2737. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus-Abies forest of Bosnia-Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Kobal, M. Disturbance, life history traits, and dynamics in an old-growth forest landscape of southeastern Europe. Ecol. Appl. 2014, 24, 663–679. [Google Scholar] [CrossRef]
- Gadow, K.; Zhang, C.Y.; Wehenkel, C.; Pommerening, A.; Corral-Rivas, J.; Korol, M.; Myklush, S.; Hui, G.Y.; Kiviste, A.; Zhao, X.H. Forest structure and diversity. In Continuous Cover Forestry; Pukkala, T., Gadow, K., Eds.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2012; pp. 29–84. [Google Scholar]
- Motz, K.; Sterba, H.; Pommerening, A. Sampling measures of tree diversity. For. Ecol. Manag. 2010, 260, 1985–1996. [Google Scholar] [CrossRef]
- Pretzsch, H. Forest Dynamics, Growth and Yield; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- O’Hara, K.L.; Hasenauer, H.; Kindermann, G. Sustainability in multi-aged stands: An analysis of long-term plenter systems. Forestry 2007, 80, 163–181. [Google Scholar] [CrossRef]
- Pukkala, T.; von Gadow, K. Continuous Cover Forestry; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pommerening, A. Evaluating structural indices by reversing forest structural analysis. For. Ecol. Manag. 2006, 224, 266–277. [Google Scholar] [CrossRef]
- Peck, J.E.; Zenner, E.K.; Brang, P.; Zingg, A. Tree size distribution and abundance explain structural complexity differentially within stands of even-aged and uneven-aged structure types. Eur. J. For. Res. 2014, 133, 335–346. [Google Scholar] [CrossRef]
- Bončina, A. History, current status and future prospects of uneven-aged forest management in the Dinaric region: An overview. Forestry 2011, 84, 467–478. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Bončina, A.; Diaci, J.; Anić, I.; Boydak, M.; Curovic, M.; Govedar, Z.; Grigoriadis, N.; Ivojevic, S.; Keren, S.; et al. Culture and Silviculture: Origins and Evolution of Silviculture in Southeast Europe. Int. For. Rev. 2018, 20, 130–143. [Google Scholar] [CrossRef]
- Fukarek, P.; Stefanović, V. Prasuma Perucica i njena vegetacija. Rad. Poljopr. Fak. 1958, 3, 93–146. [Google Scholar]
- Stupar, V.; Milanović, Đ. Istorijat Zaštite Prirode Na Području Nacionalnog Parka Sutjeska. Glas. Sumar. Fak. Univ. Banjoj Luci 2017, 113–128. [Google Scholar] [CrossRef]
- Drinić, P. Taksacioni Elementi Sastojina Jele, Smrce i Bukve Prasumskog Tipa u Bosni. Rad. Poljopr. Fak. Sarajevo B 1956, 1, 107–160. [Google Scholar]
- Keren, S.; Motta, R.; Govedar, Z.; Lucic, R.; Medarevic, M.; Diaci, J. Comparative structural dynamics of the Janj mixed old-growth mountain forest in Bosnia and Herzegovina: Are conifers in a long-term decline? Forests 2014, 5, 1243–1266. [Google Scholar] [CrossRef]
- Motta, R.; Garbarino, M.; Berretti, R.; Bjelanovic, I.; Borgogno Mondino, E.; Čurović, M.; Keren, S.; Meloni, F.; Nosenzo, A. Structure, spatio-temporal dynamics and disturbance regime of the mixed beech–silver fir–Norway spruce old-growth forest of Biogradska Gora (Montenegro). Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2015, 149, 966–975. [Google Scholar] [CrossRef]
- Kozák, D.; Mikolá, M.; Svitok, M.; Ba, R.; Paillet, Y.; Larrieu, L.; Nagel, T.A.; Diku, A.; Frankovic, M.; Janda, P.; et al. Profile of tree-related microhabitats in European primary beech-dominated forests. For. Ecol. Manag. 2018, 429, 363–374. [Google Scholar] [CrossRef]
- Lexerød, N.L.; Eid, T. An evaluation of different diameter diversity indices based on criteria related to forest management planning. For. Ecol. Manag. 2006, 222, 17–28. [Google Scholar] [CrossRef]
- Sterba, H.; Zingg, A. Abstandsabhängige und abstandsunabhängige Bestandesstrukturbeschreibung. Allg. Forst Jagdztg. 2006, 177, 169–176. [Google Scholar]
- Füldner, K. Zur Strukturbeschreibung in Mischbeständen. Forstarchiv 1995, 66, 235–240. [Google Scholar]
- Füldner, K. Die “Strukturelle Vierergruppe”—Ein Stichprobenverfahren zur Erfassung von Strukturparametern in Wäldern. In Beiträge zur Waldinventur; von Gadow, K., Beisch, T., Eds.; Cuvillier Verlag: Göttingen, Germany, 1996; pp. 13–30. [Google Scholar]
- Pommerening, A.; Uria-Diez, J. Do large forest trees tend towards high species mingling? Ecol. Inform. 2017, 42, 139–147. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Leinonen, K.; Nygren, M.; Penttinen, A. Statistical opportunities for comparing stand structural heterogeneity in managed and primeval forests: An example from boreal spruce forest in southern Finland. Silva Fenn. 1996, 30, 315–328. [Google Scholar] [CrossRef]
- Pommerening, A.; Stoyan, D. Edge-correction needs in estimating indices of spatial forest structure. Can. J. For. Res. 2006, 36, 1723–1739. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Sterba, J.; Sterba, H. The semi-logarithmic stem number distribution and the Gini-index—Structural diversity in “balanced” dbh-distributions. Austrian J. For. Sci. 2018, 135, 19–31. [Google Scholar]
- Ponce, D.B.; Donoso, P.J.; Salas-Eljatib, C. Differentiating structural and compositional attributes across successional stages in chilean temperate rainforests. Forests 2017, 8, 329. [Google Scholar] [CrossRef]
- Balanda, M. Spatio-temporal structure of natural forest: A structural index approach. Beskydy 2012, 5, 163–172. [Google Scholar] [CrossRef]
- Parobeková, Z.; Pittner, J.; Kucbel, S.; Saniga, M.; Filípek, M.; Sedmáková, D.; Vencurik, J.; Jaloviar, P. Structural diversity in a mixed spruce-fir-beech old-growth forest remnant of the Western Carpathians. Forests 2018, 9, 379. [Google Scholar] [CrossRef]
- Keren, S.; Diaci, J.; Motta, R.; Govedar, Z. Stand structural complexity of mixed old-growth and adjacent selection forests in the Dinaric Mountains of Bosnia and Herzegovina. For. Ecol. Manag. 2017, 400, 531–541. [Google Scholar] [CrossRef]
- Seidling, W.; Travaglini, D.; Meyer, P.; Waldner, P.; Fischer, R.; Granke, O.; Chirici, G.; Corona, P. Dead wood and stand structure—Relationships for forest plots across Europe. iForest 2014, 7, 269–381. [Google Scholar] [CrossRef]
- Szmyt, J.; Dobrowolska, D. Spatial diversity of forest regeneration after catastrophic wind in northeastern Poland. iForest 2016, 9, 414–421. [Google Scholar] [CrossRef]
- Barbeito, I.; Cañellas, I.; Montes, F. Evaluating the behaviour of vertical structure indices in Scots pine forests. Ann. For. Sci. 2009, 66, 710. [Google Scholar] [CrossRef]
- Sterba, H. Diversity indices based on angle count sampling and their interrelationships when used in forest inventories. Forestry 2008, 81, 587–597. [Google Scholar] [CrossRef]
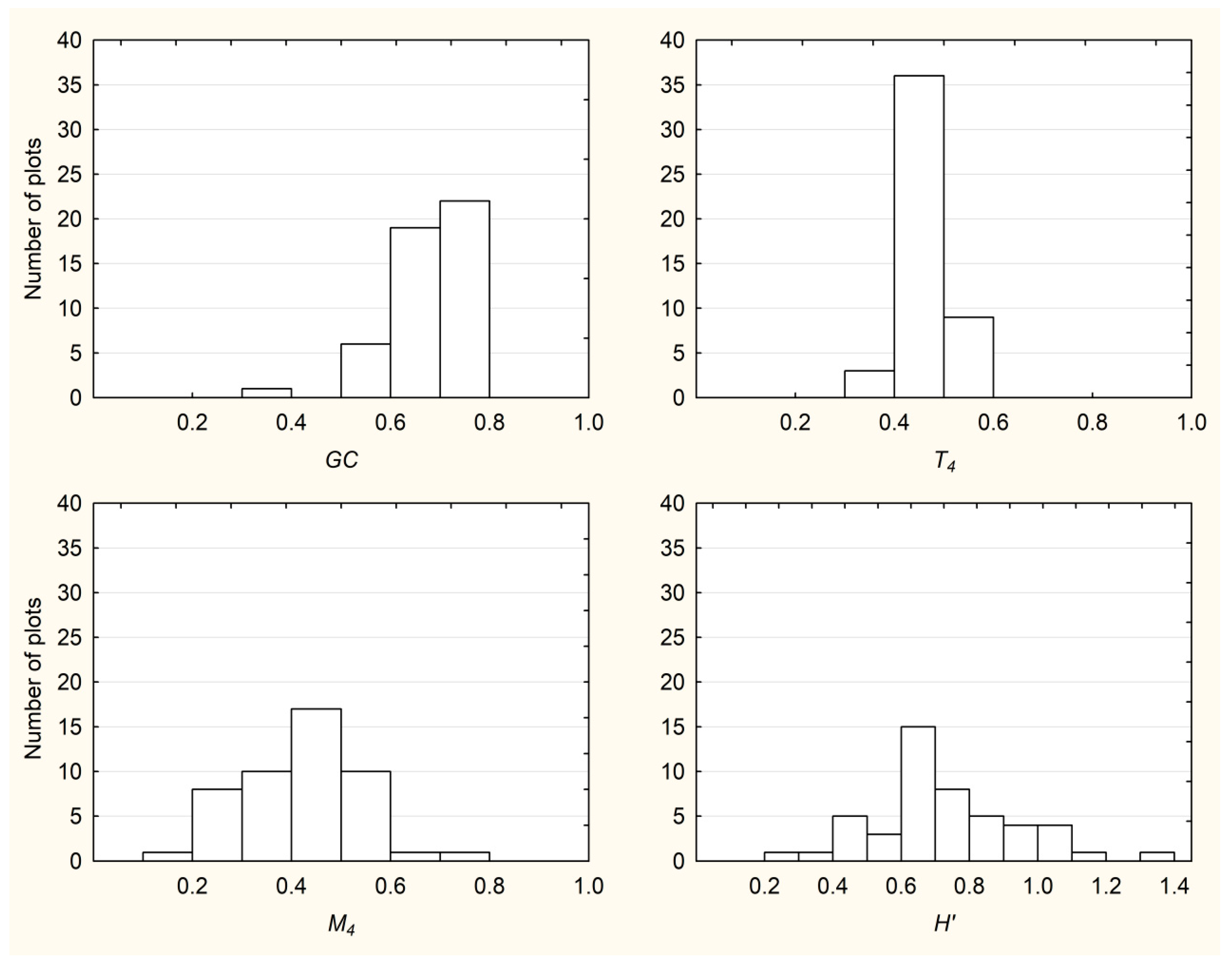
| Plot size | Index | Mean | Median | Min. | Max. | St. Dev. |
|---|---|---|---|---|---|---|
| 200 m2 | GC | 0.67 | 0.70 | 0.32 | 0.88 | 0.14 |
| T4 | 0.47 | 0.47 | 0.33 | 0.62 | 0.06 | |
| M4 | 0.41 | 0.43 | 0.06 | 0.75 | 0.17 | |
| H′ | 0.59 | 0.64 | 0.00 | 1.20 | 0.27 | |
| 1000 m2 | GC | 0.67 | 0.68 | 0.40 | 0.78 | 0.08 |
| T4 | 0.47 | 0.47 | 0.38 | 0.55 | 0.04 | |
| M4 | 0.42 | 0.44 | 0.15 | 0.70 | 0.12 | |
| H′ | 0.72 | 0.69 | 0.34 | 1.38 | 0.22 | |
| 1500 m2 | GC | 0.67 | 0.69 | 0.37 | 0.77 | 0.08 |
| T4 | 0.47 | 0.47 | 0.37 | 0.54 | 0.04 | |
| M4 | 0.42 | 0.42 | 0.10 | 0.70 | 0.12 | |
| H′ | 0.73 | 0.69 | 0.25 | 1.34 | 0.21 |
| Tree Species | European Beech | Silver Fir | Norway Spruce | |||
|---|---|---|---|---|---|---|
| Mingling Indices | Mean | Median | Mean | Median | Mean | Median |
| M1 | 0.30 | 0.00 | 0.45 | 0.00 | 0.74 | 1.00 |
| M2 | 0.31 | 0.00 | 0.46 | 0.50 | 0.79 | 1.00 |
| M3 | 0.31 | 0.33 | 0.46 | 0.33 | 0.81 | 1.00 |
| M4 | 0.31 | 0.25 | 0.47 | 0.50 | 0.81 | 1.00 |
| Plot Size 200 m2 | |||
| Index | T4 | M4 | H′ |
| GC | 0.25 | 0.09 | 0.15 |
| T4 | - | 0.19 | 0.08 |
| M4 | - | - | 0.87 *** |
| Plot Size 1000 m2 | |||
| Index | T4 | M4 | H′ |
| GC | 0.32 * | 0.05 | 0.05 |
| T4 | - | 0.36 * | 0.11 |
| M4 | - | - | 0.85 *** |
| Plot Size 1500 m2 | |||
| Index | T4 | M4 | H′ |
| GC | 0.32 ** | 0.15 | 0.16 |
| T4 | - | 0.43 *** | 0.14 |
| M4 | - | - | 0.86 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keren, S.; Svoboda, M.; Janda, P.; Nagel, T.A. Relationships between Structural Indices and Conventional Stand Attributes in an Old-Growth Forest in Southeast Europe. Forests 2020, 11, 4. https://doi.org/10.3390/f11010004
Keren S, Svoboda M, Janda P, Nagel TA. Relationships between Structural Indices and Conventional Stand Attributes in an Old-Growth Forest in Southeast Europe. Forests. 2020; 11(1):4. https://doi.org/10.3390/f11010004
Chicago/Turabian StyleKeren, Srđan, Miroslav Svoboda, Pavel Janda, and Thomas A. Nagel. 2020. "Relationships between Structural Indices and Conventional Stand Attributes in an Old-Growth Forest in Southeast Europe" Forests 11, no. 1: 4. https://doi.org/10.3390/f11010004
APA StyleKeren, S., Svoboda, M., Janda, P., & Nagel, T. A. (2020). Relationships between Structural Indices and Conventional Stand Attributes in an Old-Growth Forest in Southeast Europe. Forests, 11(1), 4. https://doi.org/10.3390/f11010004





