Abstract
Carbon assimilation is reduced by stress. Under such conditions, the trade-off between growth and non-structural carbohydrate (NSC) storage becomes crucial for plant survival and continued growth. However, growth and NSC responses to drought and shading in Pinus massoniana Lamb. remain unclear. Here, we investigated the effects of drought, shading, and combined drought and shading on leaf gas exchange parameters, stem basal diameter, plant height, biomass accumulation, and NSC concentration in 2-year old seedlings after a 2 month treatment. The results showed that (1) both drought and shading significantly reduced photosynthetic rate, increment of stem basal diameter and plant height, and biomass accumulation, while NSC concentration increased under drought but decreased under shading; (2) the combined drought-shading treatment had a stronger effect on photosynthetic rate and growth than either stress factor individually, whereas the concentration of NSC did not change significantly; and (3) drought, shading, and their combination had a lower effect on biomass than on NSC partitioning, in which case clear effects were observed. Drought increased NSC proportion in roots by 5.4%; conversely, shading increased NSC proportion in leaves by 3.7%, while the combined treatment increased NSC proportion in roots by 5.1% but decreased it in the leaves by 5.4%. These results suggest that the mechanism inhibiting P. massoniana growth is different under drought and shading conditions according to carbon partitioning. Furthermore, complex environmental stress may lead to different mechanisms of carbon partitioning compared with either dry or shaded environments. Our findings will be helpful in predicting the impact of climate change on P. massoniana growth.
1. Introduction
Water and light are important environmental factors that influence plant growth. The irregular distribution of precipitation and variable light conditions often cause drought and shading stress [1,2], thereby limiting growth [3,4,5,6,7]. Plants can alter their biomass distribution to improve their ability to capture resources and mitigate the damage caused by environmental stress [8,9,10]. According to the functional equilibrium hypothesis [11], resources are preferentially partitioned to the organs responsible for acquiring the most limiting resource. For example, under water deficit conditions, plants allocate more biomass to roots to increase water absorption capacity [4,12,13,14] On the other hand, under low-light conditions, plants increase biomass allocation to leaves in order to increase light absorption [15,16,17]. However, biomass partitioning varies greatly among different plant species [18,19,20]. Studies on the response of plant biomass distribution to drought and shading in different species may help to predict trends in plant growth and guide the development of afforestation strategies.
Non-structural carbohydrate (NSC), a carbon reserve for energy and biosynthesis, provides energy for physiological processes (e.g., metabolism, cell turgor maintenance, and embolism repair) [21,22] and plant growth [23]. Plants enriched with higher NSC concentrations can maintain metabolic functions for survival and long-term growth [22,24,25], and their resistance to stressful environments is increased [26]. Both drought and shading cause an imbalance in organic carbon supply and demand that results in a trade-off between plant growth and NSC storage [27,28,29,30,31], which in turn affects plant growth and stress tolerance [32,33]. Therefore, monitoring changes in NSC concentration should be informative for predicting trends in plant growth. However, evidence on the trade-off between plant growth and NSC storage is inconclusive, as it depends on species [34], ontogeny [27], and stress type [35]. According to the photosynthate allocation mechanism proposed by Brouwer [36], we can infer that NSC allocation to corresponding functional organs might be enhanced under stressful conditions. However, there is a dearth of evidence on the variation of NSC allocation, especially under conditions of drought and shading stress.
Plant growth under field conditions is affected not only by a single environmental factor but by multiple environmental factors, often acting simultaneously [37,38]. According to previous studies, the effect of combined drought and shading on plant growth is complex [39,40,41,42,43]. For example, Holmgren [44] considered that shading may lessen the effect of drought by reducing evapotranspiration. However, Smith and Huston [45] reported that shading increased the effect of drought because, under shaded conditions, the plant prioritized energy partitioning to the shoot and leaves while decreasing allocation to their roots, thereby diminishing their ability to absorb water, which was inconsistent with the results obtained by Sack and Grubb [46]. Furthermore, whether the effects of a combined drought–shading stress would aggravate or mitigate the negative effect by drought reportedly depends on species and shading intensity [47].
Pinus massoniana Lamb., which is native to China, is the main species used for afforestation. Previous studies on this species have mainly focused on water relations [48], root characteristics [49], and antioxidant activity [50,51]. However, the nature of NSC metabolism and photoassimilate partitioning in P. massoniana under drought and shading conditions remain unclear. Therefore, in this study, we simulated drought, shading, and their combination to monitor growth and NSC concentration in all organs of the tree. Our aim was to answer the following three questions: (1) How do drought and shading affect growth and NSC concentration in P. massoniana? (2) How does the variation in NSC and biomass partitioning respond to drought and shading stress, and are these responses similar? (3) Does the combined drought–shading stress aggravate or mitigate the effects of either single factor on P. massoniana? We hypothesized that both drought and shading inhibit the growth of P. massoniana, but NSC concentration and biomass allocation respond differently under different stress conditions. Specifically, we hypothesized that (1) compared with controls, seedlings subjected to drought stress will show higher NSC concentrations, while seedlings grown under shading stress will show lower NSC concentrations. (2) The variation of NSC and biomass allocation will be similar, with their proportion increasing in leaves under shading, while decreasing in roots under drought. (3) The combined drought–shading stress will aggravate the negative effects of either single stress factor on P. massoniana.
2. Materials and Methods
2.1. Experimental Site
A controlled pot experiment was conducted at the Forest Ecosystem State Positioning Observation Station in the Three Gorges Reservoir area, Zigui County, Hubei province (30°53′ N, 110°54′ E), China. The region has a subtropical continental monsoon climate with a mean annual precipitation (1973–2017) of 1245 mm, which mainly falls between April and September; and a mean air temperature (1973–2017) of 16.5 °C.
2.2. Sapling Preparation and Stress Treatments
Generally, coniferous afforestation adopts 2 year old seedlings. The growth and survival of seedlings during afforestation are of great significance to the success of the afforestation. Therefore, we used 2 year old seedlings to study the physiological mechanisms of adaptation to drought and shading stress conditions. On 5 March 2018, 400 2 year old P. massoniana seedlings were cultivated in separate pots (32 cm in diameter, 27 cm in height), each filled with 10 kg of soil. The soil was collected from the P. massoniana forest stands within 2 km of the experimental site. Five replicate soil samples were collected and sieved after air-drying. Basic soil properties were determined. Total nitrogen (TN) was determined using the Kjeldahl acid-digestion method [52]. Total phosphorus (TP), total potassium (TK), available phosphorus (AP), and available potassium (AK) were measured using an inductively coupled plasma mass-spectroscopy (ICP-MS) analyzer (IRIS Intrepid II XSP, Thermo Fisher Scientific Co., Waltham, MA, USA) [53]. Soil pH was measured from a soil:water suspension (1:5 w/v) [54]. The basic physical and chemical properties of the experimental soil are shown in Table 1.

Table 1.
Basic physical and chemical properties of soil in pots. pH: soil pH; TN: total nitrogen; TP: total phosphorus; TK: total potassium; AP: availability phosphorus; AK: available potassium.
Our research aimed mainly to explore the impact of a more stressful environment on P. massoniana, and to illustrate the response and adaptability of P. massoniana to the experimental stress factors. Based on our own preliminary experiments and previous studies (e.g., Reference [43]), 30% shading and 30% of the soil moisture content at field capacity corresponds to moderately severe shading and moderately severe drought, respectively. Therefore, we used these two treatments in the study. Our experiments were carried out in July and August 2018. These two months comprise the period of rapid growth of P. massoniana during the growing season. Extreme drought events often occurred during this rapid growth period, whereby we extended the duration of the stress treatments and set it to 2 months.
On 1 July, 2018, 120 pots with uniform saplings at an average height of 65.52 cm and average stem diameter of 11.61 mm were randomly divided into four groups as follows: a control group (CK): full sunlight and 70% of field capacity for soil moisture treatment and three treatment groups: drought (DR): full sunlight and 30% of soil field capacity, shading (LL): 30% full sunlight and 70% of soil field capacity, and the combination of drought and shading (DRLL): 30% full sunlight and 30% of soil field capacity. Before the experiment, soil field capacity was determined as follows: in the evening, the soil in the pot was drenched with water, and the following morning the soil water content was determined on a 20 g wet soil sample and dried to a constant weight at 105 °C. Soil moisture at field capacity (FC) was calculated as follows:
where Gws is the weight of wet soil sample, Gds is the weight of dry soil sample, Gp is the weight of the pot, and Gw is the weight of the wet soil in the pot.
Throughout the experiment, a neutral density black nylon net (six pin shade net, Wenan Dinghao Plastic Products Co., LTD., Langfang, China) was used to reduce irradiance by 70% for the shading treatment. Pots were supplemented with water by weighing daily at 18:00 to maintain soil moisture levels. The average amount of water supplied to each treatment was different under different weather conditions. Thus, under full sunlight conditions, the average amount of water supplied was 4–5 and 1–2 times greater than under shading conditions on sunny and cloudy days, respectively. The experiment was terminated on 5 September 2018.
2.3. Growth Measurement and Sampling
Height and stem basal diameter of seedlings were measured 2 cm above the root collar at the beginning and at the end of the experiment. The increases in plant height and stem basal diameter were calculated as follows: value after treatment − value before treatment.
At the end of the treatment, 72 saplings, 18 from each treatment, were selected. Saplings were divided into current-year leaves, 1-year leaves, branches, stems, and roots. Upon organ separation, sample tissues were immediately dried at 105 °C for 5 min and then to constant mass at 65 °C before weighing. For NSC measurements, the tissues of three individually dried saplings from each treatment were sampled and ground to powder to pass through a 60 mesh sieve.
2.4. Gas Exchange Measurements
Photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (Tr), and water-use efficiency (WUE = Pn/Tr) were measured using a portable photosynthesis system (LI-6400, LI-COR Inc., Lincoln, NE, USA) fitted with a 2 × 3 cm2 cuvette and a red/blue light emitting diode (LED) source. Measurements were conducted on clear days of September between 09:00 and 11:30. Light conditions within the cuvette were controlled at a photosynthetic photon flux density of 1000 and 300 μmol m−2 s−1 at leaf level under full sunlight and 30% sunlight, respectively. All measurements were conducted with the cuvette temperature set at 25 °C and relative humidity at 60%. Clusters of current year leaves in the major branch of each sapling were selected for measurements of gas exchange, and the leaves were tiled side by side and covered the cuvette. Measurements were made twice per plant, and the average value of each plant was treated as a replicate. Four individuals were measured in each treatment.
2.5. Non-Structural Carbohydrate Measurements
The total concentration of NSC was defined as the sum of soluble sugar and starch concentrations. Measurements were made as described by Shi et al. [55] after slight modifications. Briefly, for soluble sugars, ~0.5 g dry powder sample was added into a conical flask with 50 mL distilled water. After boiling for 2 h in a steam cooker, the solution samples were allowed to cool and then filtered. The filtrate was injected into a Water 2695 High Performance Liquid Chromatograph (Waters-Millipore, Milford, MA, USA) equipped with a Sugar-Pak I column. The mobile phase was distilled water, flowing at 0.6 mL·min−1, with column temperature at 70 °C, and a parallax detector was used. As for starch, ~0.1 g dry power sample was added into a plug tube with 10 mL distilled water, and 1 mL 2:1 HCL was added before incubation at 100 °C for 8 h. After cooling, pH was adjusted to neutrality with 40% NaOH, and then distilled water was added to 15 mL. After filtrating, the filtrate was used for starch analysis. The determination procedure used for starch content was the same as that for soluble carbohydrates. The total concentration of NSC was defined as the sum of soluble sugar and starch concentrations.
2.6. Calculations and Data Analysis
The biomass (NSC, soluble sugars, starch) fraction in different organs was calculated as follows:
where T(Cmi) is the amount of substance in each organ, m stands for each different substance, i stands for each different organ, and T(Cmw) stands for the total substance in the whole plant.
All statistical analyses were performed using the SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Data shown are means ± standard error (SE). After testing the assumptions of normality (Shapiro–Wilk test) and homoscedasticity, two-way analysis of variance (ANOVA) was used to test the effects of light and water on the variables under evaluation. One-way ANOVA was carried out comparing the four treatment combinations coupled with least significant difference (LSD) multiple comparison test to separate significantly different means. Figures were drawn using Origin 9.0 software (Origin Lab Corp., Northampton, MA, USA).
3. Results
3.1. Effects of Drought and Shading on Gas Exchange
Gas exchange parameters were differently affected by drought and shading (Table 2). Compared with CK, Pn, gs, Ci, and Tr were significantly reduced by 37.6%, 62.1%, 75.2%, and 31.1%, respectively, while WUE significantly increased by 68.0% under DR treatment. On the other hand, LL treatment significantly decreased Pn, gs, and Tr by 42.2%, 40.2%, and 49.1%, respectively, but had no effect on Ci or WUE in comparison with CK. Meanwhile, the variation in gas exchange parameters under DRLL was similar to that under DR treatment, with Pn, Tr, gs, and Ci significantly decreasing by 64.6%, 77.4%, 85.7%, and 30.5%, respectively, while WUE significantly increased by 60.8% (Figure 1).

Table 2.
Results of two-way analysis of variance (ANOVA) for P. massoniana on growth parameters, biomass partitioning, NSC content, and NSC partitioning, with water and light as fixed factors.
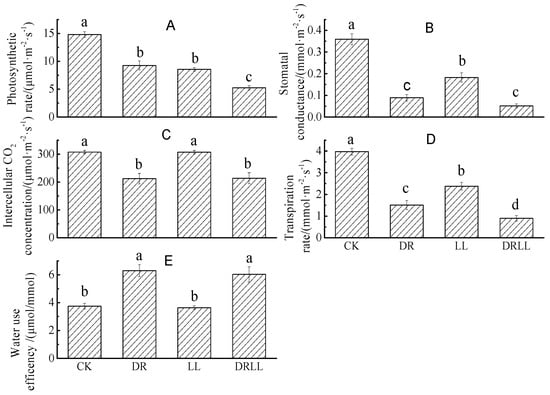
Figure 1.
Effects of drought and shading treatments on gas exchange parameter of Pinus massoniana seedlings (umol·m−2·s−1). CK: control (full sunlight and 70% field capacity); DR: drought (full sunlight and 30% field capacity); LL: shading (30% sunlight and 70% field capacity); DRLL: combined drought and shading (30% sunlight and 30% field capacity). The letters denote: (A) Photosynthetic rate, (umol·m−2·s−1); (B) Stomatal conductance, (mmol·m−2·s−1); (C) Intercellular CO2 concentration, (umol·m−2·s−1); (D) Transpiration rate, (mmol·m−2·s−1); (E) Water use efficency, (umol/mmol).
3.2. Effects of Drought and Shading on Growth
Compared with CK, basal diameter and height decreased by 52.7% and 28.1% under DR, respectively, and by 42.1% and 42.9% under LL, respectively (Table 3). Moreover, DRLL further decreased both parameters by up to 84.4% and 81.3%, respectively (Table 3). Biomass was also significantly reduced by 38.6%, 44.0%, and 48.7% in current leaves; 43.7%, 42.1%, and 47.5% in branches; 32.0%, 38.7%, and 45.3% in stems; 32.6%, 34.5%, and 41.8% in roots; and 32.1%, 34.2%, and 41.3% in the whole plant under DR, LL, and DRLL treatments, respectively. However, biomass of 1-year leaves did not significantly differ among treatments (Figure 2). The results showed that both drought and shading restricted growth of P. massoniana, and that the combination of the two stress conditions increased this restriction (Table 2).

Table 3.
Differences of growth increase in P. massoniana seedlings under drought and shading treatments. CK: control (full sunlight and 70% field capacity treatment); DR: drought (full sunlight and 30% field capacity); LL: shading (30% sunlight and 70% field capacity); DRLL: combined drought and shading (30% sunlight and 30% field capacity).
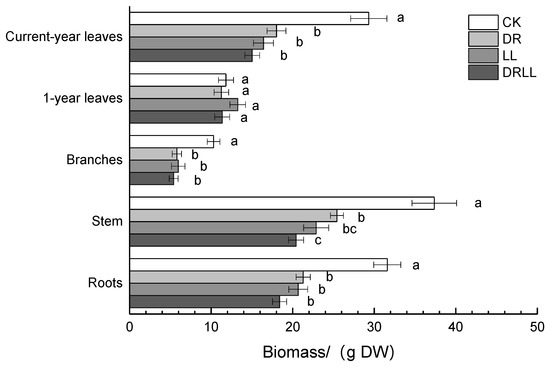
Figure 2.
Effects of drought and shading treatments on biomass of different organs in P. massoniana seedlings. Different letters indicate significant differences (p < 0.05) with LSD multiple range test. CK: control (full sunlight and 70% field capacity); DR: drought (full sunlight and 30% field capacity); LL: shading (30% sunlight and 70% field capacity); DRLL: combined drought and shading (30% sunlight and 30% field capacity). DW: dry weight.
Biomass allocation was barely affected by drought or shading (Table 2). Compared with CK treatment, biomass allocation to current-year leaves decreased by 2.5%, 3.5%, and 3.1%, whereas that in the 1-year leaves increased by 3.7%, 7.0%, and 6.1%, respectively, under DR, LL, and DRLL treatments. Most stems, branches, and roots differed little within each treatment, except for the branches under DR and stems under LL (Figure 3). These results indicated that the variation in leaf biomass was more sensitive to that of other organs in drought- and shade-stressed saplings.
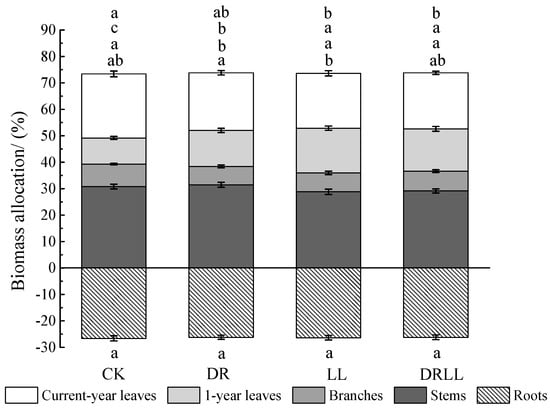
Figure 3.
Effects of drought and shading treatments on biomass allocation of different organs of P. massoniana seedlings (%). Different letters indicate significant differences (p < 0.05) as per the LSD multiple range test. CK: control (full-sunlight and 70% field capacity); DR: drought (full-sunlight and 30% field capacity); LL: shading (30% of full-sunlight and 70% field capacity); DRLL: combined drought and shading (30% of full-sunlight and 30% field capacity).
3.3. Effects of Drought and Shading on Non-Structural Carbohydrate Content in Different Organs
Changes in soluble sugar, starch, and NSC content are shown in Figure 4. Soluble sugar, starch, and NSC content responded differently to drought, shading, and the combination of both factors (Table 2). Although both starch and NSC content showed significant differences under drought and shading, soluble sugar only showed significant differences under drought relative to the control treatment. However, soluble sugar varied little under the combined drought–shading treatment.
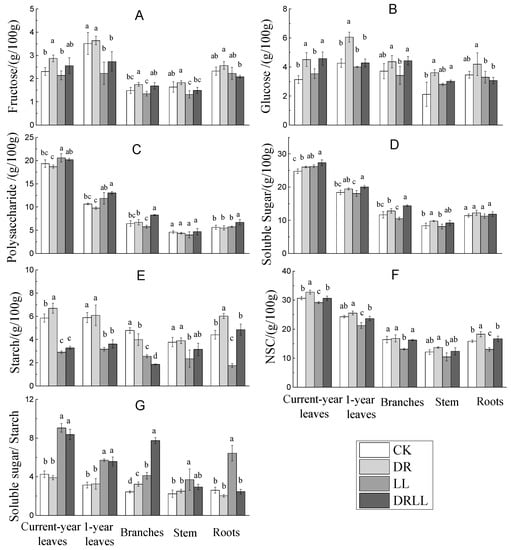
Figure 4.
Effects of drought and shading on the content of non-structural carbohydrates in different organs of P. massoniana. Different letters indicate significant differences (p < 0.05) as per the LSD multiple range test. CK: control (full sunlight and 70% field capacity); DR: drought (full sunlight and 30% field capacity); LL: shading (30% sunlight and 70% field capacity); DRLL: combined drought and shading (30% sunlight and 30% field capacity). The letters denote: (A) Fructose, (g/100 g); (B) Glucose, (g/100 g); (C) Polysaccharide, (g/100 g); (D) Soluble Sugar, (g/100 g); (E) Starch, (g/100 g); (F) NSC, (g/100 g); (G) Soluble sugar/Starch.
Soluble sugar showed an increasing trend in all organs of DR-treated saplings, compared with CK saplings (Figure 4). However, fructose, glucose, and polysaccharide concentrations responded differently. DR treatment significantly increased fructose in current-year leaves by 24.6% and in branches by 17.7%, glucose in current-year leaves by 43.9%, in 1-year leaves by 42.1%, and in stems by 70.7%; in contrast, no significant effect on polysaccharide was observed. Furthermore, starch content increased significantly by 14.4% and by 36.7% in current-year leaves and roots, respectively, whereas it decreased significantly by 16.6% in branches, but no change was observed in 1-year leaves or stems (Figure 4). The trend in NSC concentration was similar to that in starch concentration in all organs, except for branches.
Soluble sugar in current-year leaves significantly increased under LL treatment, compared with CKs (Figure 4). However, fructose, glucose, and polysaccharide were not significantly different between CK and LL groups, except that fructose significantly decreased by 36.6% in 1-year leaves, and polysaccharide significantly increased by 6.4% in current-year leaves. Furthermore, starch was significantly lower in all organs under LL treatment compared with CKs; a similar variation was observed for NSC in all organs, except for stems (Figure 4).
Under DRLL treatment, soluble sugar in current-year leaves, 1-year leaves, and branches increased significantly, but starch decreased significantly compared with CKs. However, no significant difference was observed in stems or roots (Figure 4). The combination of drought and shading did not affect NSC concentration in any organ.
The ratio of soluble sugar to starch differed with treatment. As can been seen in Table 2 and Figure 4, the ratio was more sensitive to LL compared with DR or DRLL treatments. Further, compared with CK, the ratio of soluble sugar to starch increased significantly in branches under DR treatment, and in current-year leaves, 1-year leaves, and branches in the DRLL treatment, and in all sapling organs in the LL treatment.
3.4. Effects of Drought and Shading on Non-Structural Carbohydrate Allocation
Soluble sugar, starch, and NSC partitioning responded differently to drought, shading, and the two combined (Figure 5). The proportion of soluble sugars under DR decreased by 8.01% in current-year leaves; it increased by 6.5% and 3.9% in 1-year leaves and roots, respectively, but it did not differ much in branches (up by 1.4%) or stems (up by 1.1%). The proportion of starch and NSC decreased by 5.4% and 7.5%, respectively, in current-year leaves, 3.5% and 1.9% in branches, and 5.9% and 2.2% in stems, but increased by 5.3% and 6.3% in 1-year leaves and by 9.4% and 5.4% in roots, respectively. The proportion of soluble sugars under LL decreased by 8.1% in current-year leaves but it increased by 10.5% in 1-year leaves, while it differed only slightly in other organs. The proportion of starch decreased by 9.1% and 3.8% in current-year leaves and roots, while it increased by 11.8% and 2.5% in 1-year leaves and stem, respectively, while it showed almost no variation in branches. The proportion of NSC only varied greatly in current-year leaves and 1-year leaves; decreasing by 7.0% in the first case, and increasing by 10.7% in the latter.
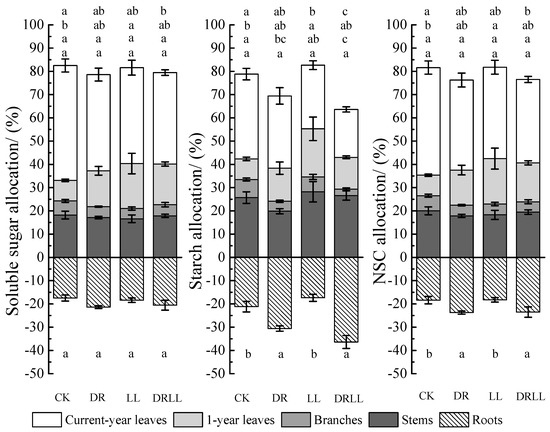
Figure 5.
Percentage of non-structural carbohydrates in various organs under drought and shading treatments (%). Different letters indicate significant differences (p < 0.05) as per the LSD multiple range test. CK: control (full sunlight and 70% field capacity); DR: drought (full sunlight and 30% field capacity); LL: shading (30% sunlight and 70% field capacity); DRLL: combined drought and shading (30% sunlight and 30% field capacity).
The proportions of soluble sugars, starch, and NSC under DRLL varied similarly to DR treatment in different organs. Compared with CKs, the proportion of soluble sugars decreased by 10.1% in current-year leaves, but it increased by 8.6% and 3.0% in 1-year leaves and roots, respectively. The proportion of starch and NSC decreased by 15.9% and 10.4% in current-year leaves and 5.0% and 2.1% in branches, and it increased by 4.8% and 7.9% in 1-year leaves and 15.2% and 5.1% in roots, respectively, while it did not differ much in the other organs. The above results demonstrated that saplings stored a larger proportion of NSC—including soluble sugars and starch—in roots under drought and under the combined DRLL treatment, whereas higher NSC was partitioned to the leaves under conditions of shading.
4. Discussion
4.1. Effects of Drought and Shading on Growth and NSC Concentration
Both drought and shading inhibited the growth of P. massoniana saplings. The variation in NSC concentration differed under drought and shading conditions, and drought significantly increased NSC content, while shading significantly reduced it. Concomitantly, photosynthetic rate tended to decrease significantly under both drought and shading (Figure 1). These findings indicated that the trade-off between plant growth and NSC storage remained different when carbon supply was limited by drought or shading conditions. Reportedly, cell division and expansion [56,57], and metabolic activity [58] are directly restricted by water deficit. This would cause reduced growth before a reduction in photosynthesis was perceived [6,21,59], and plant growth would be more sensitive to drought than to carbon availability [60], which indicates that the increase in NSC would occur at the expense of growth. Storage of NSC also prevents carbohydrate depletion and acute carbon starvation [61]; this is a reliable “conservative” mechanism when P. massoniana experiences drought stress. Conversely, under shading, both growth and NSC storage were restricted by the shortage of carbon supply, as respiratory losses were higher in shaded than in unshaded leaves [62], and relatively more carbon were incorporated into structural components [63], which was likely responsible for the slight increase in carbon partitioning to plant growth over NSC storage.
There was disparity in the response of the ratio of soluble sugar to starch to drought and shading. Under drought conditions, the ratio of soluble sugar to starch remained relatively stable, except for the increase observed in branches, and both soluble sugar and starch increased slightly. However, this result contrasts with previous studies which have reported that the ratio of soluble sugar to starch significantly increased [25], and the increase in soluble sugars was converted to starch [64]. This may have been due to the inhibition of growth and metabolism being much greater than photosynthesis rate in our study, which would result in the accumulation of starch in all organs. In addition, different soluble sugars play different metabolic roles, and monosaccharides are mainly involved in maintaining metabolic activity and osmotic potential [23]. In our study, together with a soluble sugar increase, monosaccharides (including fructose and glucose) increased while polysaccharides decreased. This indicates that, under drought conditions, P. massoniana only needs to convert polysaccharides to monosaccharides to increase osmotic potential and increase drought tolerance. Conversely, under shading conditions, the ratio of soluble sugar to starch significantly increased, and soluble sugars (including fructose, glucose, and monosaccharides) remained stable. However, starch significantly decreased. This result suggests that carbon becomes even more limited and the plant converts starch into soluble sugar to maintain growth and metabolism under shading conditions.
4.2. Effects of the Interaction between Drought and Shading on Growth and NSC Concentration
The shading mitigation of the negative effects of drought may depend on the shading intensity [43,47,65]; namely, moderate shading can mitigate the negative effects of drought, while severe shading can aggravate these effects. In this study, drought- or shading-induced reduction in growth was aggravated under the combined influence of shading and drought, which reduced Pn and gs to a greater extent than either stress condition separately. Compared with drought, Ci did not change significantly under the DRLL combination treatment. This may have been due to the decrease in Pn being aggravated by further biochemical limitations [66], which may have been caused by the severe reduction light in comparison with only DR treatment in this study. The result was consistent with the reports in Coffea arabica [66]—the reduction in Pn was aggravated under the DRLL combination treatment. However, according to the results of Duan et al. [39], shading mitigated the negative effects of drought in Picea asperata Mast. when the measurement of Pn was conducted under the same light intensity, while light intensity under combined drought–shading treatment was 70% less than that under the drought treatment in this study, which would cause a more severe limitation on Pn and growth. Thus further research is needed on the reasons for shading mitigating the negative effects of drought on P. massoniana.
Our results showed that the combination of drought and shading had no effect on NSC concentration, although photosynthesis rate decreased more compared with drought or shading alone (Figure 1). The trade-off between plant growth and NSC storage was changed under the drought × shading interaction. This may have been due to the following reasons. Firstly, the growth inhibition caused by the combined treatment would have reduced the consumption of NSC. Secondly, the reduction in carbon supply under the joint drought–shading treatment would have neutralized the accumulation of NSC; this might indicate that there remained a “neutralization” strategy for NSC storage under the combined stress treatment. However, the ratio of soluble sugar to starch was organ-specific in this case, significantly increasing in current-year leaves, 1-year leaves, and branches, and their variation was similar under shading treatment, while the ratio of soluble sugar to starch basically remained unchanged in stems and roots and was similar under drought treatment. According to Niinemets [67], for multiple stress conditions, plant response might depend on what stress comes first and whether the plant has acclimated to it. We can infer that under combined drought and shading stress, the aboveground organs will adapt to shading stress first, while the underground organs will preferentially adapt to drought stress. The results also suggest that under the joint treatment, aboveground organs still have a high metabolic capacity, and starch is consumed to maintain the content of soluble sugar. Secondly, considering the benefits of improving plant stress tolerance, starch remained stable in stems and roots.
4.3. Variation in NSC and Biomass Allocation
Under stressful conditions, plants can change their pattern of biomass distribution to compensate for shortages in resources, and thus increase their ability to obtain further resources [16]. Unlike previous studies [4,68,69,70], our results indicated that the three experimental treatments tested had no effect on biomass partitioning to roots, especially under drought conditions. This may have been due to the high drought tolerance that characterizes P. massoniana; acquisition of light under natural conditions to increase photosynthetic capacity and inherent growth rates would be the higher priority, whereas the increase in root biomass would be of greater significance under drought stress. The drought condition in this study was not severe enough to induce a significant change in biomass partitioning to roots, which is consistent with the results reported by Schall et al. [41] for Picea abies (L.) Karst. Biomass allocation is also determined by stress duration, prior stress history, and tree size [68]; therefore, further research is needed to explain why drought did not cause significant changes in biomass partitioning to roots under drought. However, the variation in biomass allocation in leaves was more sensitive, and the biomass proportion decreased in current-year leaves but increased in 1-year leaves under the three treatments. However, the biomass proportion sum of current-year and 1-year leaves increased only under shading or under the combined drought–shading treatment but remained stable under drought conditions. Greater biomass partitioning to the leaves may promote light capture under low-light conditions, whereby P. massoniana saplings might retain their original organs to support leaf biomass accumulation. Our findings suggest that the morphological variation in P. massoniana differed with drought and shading effects, and may have been more sensitive to shading than to drought stress.
In contrast to biomass partitioning, NSC partitioning varied significantly, especially with respect to starch. The proportional allocation of NSC, including soluble sugar and starch, to roots increased under drought, while the proportional allocation of NSC to the leaves increased under shading. This indicated that, although less carbon was incorporated into the leaves as stomata closed to minimize water loss under drought (Figure 1), NSC was preferentially allocated to the root to promote water absorption. However, severe drought would impair phloem transport of mobile forms of NSC, which would reduce the proportion of NSC allocated to roots [71]. The results suggest that our experimental drought treatment was not severe enough for P. massoniana. On the other hand, leaves retain more of the limiting amount of photoassimilate than roots under low irradiance [36]. Both these findings reflect that plants would increase NSC in the corresponding functional organs to increase their ability to supplement the resources most badly needed.
According to the aforementioned results, we concluded that the response of NSC and biomass partitioning was treatment-dependent in this study. Thus, allocation of NSC varied significantly, while none of the three treatments had any effect on biomass allocation. Variation in NSC and biomass allocation would depend on stress intensity, and NSC variation tends to precede any variation in biomass partitioning, thus indicating that the distribution of biomass changes with NSC allocation changes under prolonged environmental stress.
5. Conclusions
Our results showed that both drought and shading limited plant growth, and that the combined treatment aggravated negative effects on growth from either separate factor. The inhibitory mechanism of growth of P. massoniana differed with treatment. Under drought conditions, growth restriction was not caused by the lack of carbon, whereas under shading conditions, growth suffered for lack of a sufficient carbon supply. In the opposite responses of NSC concentration under drought and shading conditions, an intermediate response to the combined stress condition was observed. Under stress conditions, the variation in NSC partitioning was more highly significant than the variation in biomass partitioning, which would also increase the ability to acquire the most deficient resource. Our results clearly demonstrated that young P. massoniana trees improve their adaptation to drought and shading stress by changing the distribution of chemical energy. Our findings have shed novel insights into plant growth trends under stress conditions and will surely prove useful for developing more effective strategies for successful afforestation.
Author Contributions
X.D., W.X. and Z.S. designed the experiment; X.D. and Z.S. carried out the field experiment; X.D. performed the experiments in the lab; X.D. analyzed data and drafted the manuscript; W.X. and Z.S. revised and improved the manuscript; L.Z. and L.L. contributed to refining the ideas and discussing the results. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (No. 2016YFD0600201).
Acknowledgments
The authors thank all those who provide helpful suggestions and critical comments on this manuscript and anonymous reviewers. We also thank the Zigui Forest Ecosystem Research Station for their support in our field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Potts, M.D. Drought in a Bornean everwet rain forest. J. Ecol. 2003, 91, 467–474. [Google Scholar] [CrossRef]
- Phillips, O.L.; van der Heijden, G.; Lewis, S.L.; López-González, G.; Aragão, L.E.O.C.; Lloyd, J.; Malhi, Y.; Monteagudo, A.; Almeida, S.; Dávila, E.A.; et al. Drought–mortality relationships for tropical forests. New Phytol. 2010, 187, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Gansert, D.; Sprick, W. Storage and mobilization of nonstructural carbohydrates, and biomass development of beech seedlings (Fagus sylvatica L.), under different light regimes. Trees 1998, 12, 247–257. [Google Scholar] [CrossRef]
- Wu, F.; Bao, W.; Li, F.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef]
- Kuehne, C.; Nosko, P.; Horwath, T.; Bauhus, J. A comparative study of physiological and morphological seedling traits associated with shade tolerance in introduced red oak (Quercus rubra) and native hardwood tree species in southwestern Germany. Tree Physiol. 2014, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Hoch, G.; Yakir, D.; Körner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, W.; Du, N.; Xu, Z.; Guo, X. Nitrogen deposition does not affect the impact of shade on Quercus acutissima seedlings. PLoS ONE 2018, 13, 1–17. [Google Scholar] [CrossRef]
- Ammer, C. Growth and biomass partitioning of Fagus sylvatica L. and Quercus robur L. seedlings in response to shading and small changes in the R/FR-ratio of radiation. Ann. For. Sci. 2003, 60, 163–171. [Google Scholar] [CrossRef]
- Markestei, L.; Poorter, M.L. Seedling Root Morphology and Biomass Allocation of 62 Tropical Tree Species in Relation to Drought- and Shade-Tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Xue, S.L.; Wang, Q.C.; Sun, X.X.; Zhang, M.J. Effects of Shading on the Photosynthetic Characteristics, Growth, and Biomass Allocation in Fraxinus mandshurica and Quercus mongolica. Bull. Bot. Res. 2012, 32, 354–359. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Wang, X.; Duan, B.L.; Luo, J.X.; Li, C.Y. Early growth, dry matter allocation and water use efficiency of two sympatric Populus species as affected by water stress. Environ. Exp. Bot. 2005, 53, 315–322. [Google Scholar] [CrossRef]
- Martin, P.J.; Stephens, W. Willow growth in response to nutrients and moisture on a clay landfill cap soil. I. Growth and biomass production. Bioresour. Technol. 2006, 97, 437–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villagra, P.; Cavagnaro, J.B. Water stress effects on the seedling growth of Prosopis argentina and Prosopis alpataco. J. Arid Environ. 2006, 64, 390–400. [Google Scholar] [CrossRef]
- Shirley, H.L. The influence of light intensity and light quality upon the growth of plants. Am. J. Bot. 1929, 16, 354–390. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar]
- Chmura, D.J.; Modrzyński, J.; Chmielarz, P.; Tjoelker, M.G. Plasticity in seedling morphology, biomass allocation and physiology among ten temperate tree species in response to shade is related to shade tolerance and not leaf habit. Plant Biol. 2017, 19, 172–182. [Google Scholar] [CrossRef]
- Prieto, P.; Peñuelas, J.; Llusià, J.; Asensio, D.; Estiarte, M. Effects of experimental warming and drought on biomass accumulation in a Mediterranean shrubland. Plant Ecol. 2009, 205, 179–191. [Google Scholar] [CrossRef]
- Letícia, L.; Boeger, M.R.T.; Marques, M.C.M. Biomass allocation and shade tolerance in tree species of the Atlantic Forest. Botanique 2012, 90, 830–838. [Google Scholar]
- Modrzyński, J.; Chmura, D.J.; Tjoelker, M.G. Seedling growth and biomass allocation in relation to leaf habit and shade tolerance among 10 temperate tree species. Tree Physiol. 2015, 35, 879–893. [Google Scholar] [CrossRef]
- Muller, B.; Pantin, F.; Genard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.L.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Ann. Rev. Plant Biol. 2013, 65, 667–687. [Google Scholar] [CrossRef]
- Yang, B.; Peng, C.; Harrison, S.P.; Wei, H.; Wang, H.; Zhu, Q.; Wang, M. Allocation mchanisms of nn-sructural crbohydrates of Robinia pseudoacacia L. sedlings in rsponse to dought and werlogging. Forests 2018, 9, 754. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Poorter, L.; Kitajima, K. Crbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology 2007, 88, 1000–1011. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef]
- Huang, J.; Hammerbacher, A.; Weinhold, A.; Reichelt, M.; Gleixner, G.; Behrendt, T.; van Dan, N.M.; Sala, A.; Gershenzon, J.; Trumbore, S.; et al. Eyes on the future—Evidence for trade-offs between growth, storage and defense in Norway spruce. New Phytol. 2019, 222, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Reyes-Díaz, M.; Corcuera, L.J.; Lusk, C.H. Carbohydrate storage, survival, and growth of two evergreen Nothofagusspecies in two contrasting light environments. Ecol. Res. 2009, 24, 1233–1241. [Google Scholar] [CrossRef]
- Pons, T.L.; Poorter, H. The effect of irradiance on the carbon balance and tissue characteristics of five herbaceous species differing in shade-tolerance. Front. Plant Sci. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Genet, H.; Breda, N.; Dufrene, E. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiol. 2010, 30, 177–192. [Google Scholar] [CrossRef]
- Palacio, S.; Hoch, G.; Sala, A.; Körner, C.; Millard, P. Does carbon storage limit tree growth? New Phytol. 2013, 120, 1096–1100. [Google Scholar] [CrossRef]
- Brouwer, R. Nutritive influences on the distribution of dry matter in the plant. Neth. J. Agric. Sci. 1962, 10, 361–376. [Google Scholar]
- Logan, J.A.; Régnière, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137. [Google Scholar] [CrossRef]
- Netherer, S.; Schopf, A. Potential effects of climate change on insect herbivores in European forests—General aspects and the pine processionary moth as specific example. For. Ecol. Manag. 2010, 259, 831–838. [Google Scholar] [CrossRef]
- Duan, B.; Lu, Y.; Yin, C.; Junttila, O.; Li, C. Physiological responses to drought and shade in two contrasting Picea asperata populations. Physiol. Plant. 2005, 124, 476–484. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Marañón, T.; Zamora, R. Interactions of drought and shade effects on seedlings of four Quercus species: Physiological and structural leaf responses. New Phytol. 2006, 170, 819–834. [Google Scholar] [CrossRef]
- Schall, P.; Lödige, C.; Beck, M.; Ammer, C. Biomass allocation to roots and shoots is more sensitive to shade and drought in European beech than in Norway spruce seedlings. For. Ecol. Manag. 2012, 266, 246–253. [Google Scholar] [CrossRef]
- Guo, X.; Guo, W.; Luo, Y.; Tan, X.; Du, N.; Wang, R. Morphological and biomass characteristic acclimation of trident maple (Acer buergerianum Miq.) in response to light and water stress. Acta Physiol. Plant. 2013, 35, 1149–1159. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Su, Y.; He, Q.; Wang, J.; Qiu, Q.; Ma, J. A morphophysiological analysis of the effects of drought and shade on Catalpa bungei plantlets. Acta Physiol. Plant. 2017, 39, 80. [Google Scholar] [CrossRef]
- Holmgren, M. Combined effects of shade and drought on tulip poplar seedlings: Trade-off in tolerance or facilitation? Oikos 2000, 90, 67–78. [Google Scholar] [CrossRef]
- Smith, T.; Huston, M. A theory of the spatial and temporal dynamics of plant communities. Vegetatio 1989, 83, 49–69. [Google Scholar] [CrossRef]
- Sack, L.; Grubb, P.J. The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 2002, 131, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, M.; Gómez-Aparicio, L.; José Luis Quero, J.L.; Valladares, F. Non-linear effects of drought under shade: Reconciling physiological and ecological models in plant communities. Oecologia 2012, 169, 293–305. [Google Scholar] [CrossRef]
- Yu, X.T.; Lu, J.H.; Wang, J.S. A Comparative study on the water physiological ecology of different provenances of Masson Pine. Acta Phytoecol. Geobot. Sin. 1991, 15, 355–365. [Google Scholar]
- Song, P.; Zhang, R.; Zhang, Y.; Zhou, Z.C.; Feng, Z.P. Effects of simulated nitrogen deposition on fine root morphology, nitrogen and phosphorus efficiency of Pinus massoniana clone under phosphorus deficiency. Chin. J. Plant Ecol. 2016, 40, 1136–1144. [Google Scholar]
- Han, W.P.; Ding, G.J.; Bao, B. Physiological and ecological responses of Pinus massoniana seedling from different provenances to drought stress. J. Cent. South Univ. For. Technol. 2012, 32, 25–29. [Google Scholar]
- Du, M.F.; Ding, G.J.; Zhao, X.Z. Responses to continuous drought dtress and drought resistance of different Masson Pine families. Sci. Silvae Sin. 2017, 53, 21–29. [Google Scholar]
- Zhu, T.B.; Zhang, J.B.; Meng, T.Z.; Zhang, Y.C.; Yang, J.J.; Müller, C.; Cai, Z.C. Tea plantation destroys soil retention of NO3—And increases N2O emissions in subtropical China. Soil Biol. Biochem. 2014, 73, 106–114. [Google Scholar] [CrossRef]
- Zhang, W.R.; Yang, G.C.; Tu, X.N. Adiministration Forestry Standard of People’s Republic of China—Method of Forest Soil Analysis; Chinese Standard Press: Beijing, China, 1999. [Google Scholar]
- Shen, Y.F.; Cheng, R.M.; Xiao, W.F.; Yang, S.; Guo, Y.; Wang, N.; Zeng, L.X.; Lei, L.; Wang, X.R. Labile organic carbon pools and enzyme activities of Pinus massoniana plantation soil as affected by understory vegetation removal and thinning. Sci. Rep. 2018, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Bai, D.Z.; Zhang, W.C.; Xiao, W.F. Variation of nonstructural carbohydrate (NSC) in Picea crassifolia at the alpine treeline of Qilian Mountains before and after dormancy. For. Res. 2017, 30, 908–915. [Google Scholar]
- Boyer, J.S. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970, 46, 233–235. [Google Scholar] [CrossRef]
- Dosio, G.A.A.; Tardieu, F.; Turc, O. Floret initiation, tissue expansion and carbon availability at the meristem of the sunflower capitulum as affected by water or light deficits. New Phytol. 2011, 189, 94–105. [Google Scholar] [CrossRef]
- Hasibeder, R.; Fuchslueger, L.; Richter, A.; Bahn, M. Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol. 2015, 205, 1117–1127. [Google Scholar] [CrossRef]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrates sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Lacointe, A.; Deleens, E.; Ameglio, T.; Saint-Joanis, B.; Lelarge, C.; Vandame, M.; Song, G.C.; Daudef, F.A. Testing the branch autonomy theory: A 13C/14C double-labelling experiment on differentially shaded branches. Plant Cell Environ. 2004, 27, 1159–1168. [Google Scholar] [CrossRef]
- Maguire, A.J.; Kobe, R.K. Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol. Evol. 2016, 5, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.X.; Yin, C.Y.; Duan, B.L.; Li, C.Y. Interactions between drought and shade on growth and physiological traits in two Populus cathayana populations. Can. J. For. Res. 2008, 38, 1877–1887. [Google Scholar] [CrossRef]
- Cavatte, P.C.; Oliveira, Á.A.G.; Morais, L.E.; Martins, S.C.V.; Sanglard, L.M.V.P.; DaMatta, F.M. Could shading reduce the negative impacts of drought on coffee? A morphophysiological analysis. Physiol. Plant. 2012, 144, 111–122. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Abbas, A.M.; Rubio-Casal, A.E.; De Cires, A.; Grewell, B.J.; Castillo, J.M. Differential tolerance of native and invasive tree seedlings from arid African deserts to drought and shade. S. Afr. J. Bot. 2019, 123, 228–240. [Google Scholar] [CrossRef]
- Li, F.L.; Bao, W.K.; Wu, N. Effects of water stress on growth, dry matter allocation and water-use efficiency of a leguminous species, Sophora davidii. Agrofor. Syst. 2009, 77, 193–201. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhou, X.H.; Nie, Y.Y.; Bai, S.H.; Zhou, L.Y.; Shao, J.J.; Cheng, W.S.; Wang, J.W.; Hu, F.Q.; Fu, Y.L. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, K.; Jiang, T.; Cao, P.; Sun, J.; Wang, D.H. Staged Responses of Non-structural Carbohydrates of Seedlings to Drought Stress. Bull. Bot. Res. 2018, 38, 460–466. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).