Abstract
Plant leaves respond to environmental sounds by vibration. This study aimed to examine such responses by evaluating the influences of physical properties on vibrational amplitude, velocity and frequency before and during sound stimulation. Nine plant species with a wide range of leaf sizes, qualities and thicknesses and petiole lengths, widths and thicknesses were selected. In the absence of external sound, the leaf amplitude was ~1 μm, the vibrational velocity was ~0.05 mm s-1 and the vibrational frequency was ~0–15 Hz. After sound stimulation, however, the amplitude increased by 1–5.4×, the velocity was 1.75–14.1× higher and produced another spectral peak at ~80–95 Hz. Nevertheless, the amplitude and velocity varied by up to 1–10× among species mainly because of differences in leaf texture. However, these factors did not markedly change in succulent leaves because their thick epidermal cuticles and high water content buffered vibrations. In contrast, leathery leaves and papery and membranous leaves were highly responsive to sound stimuli. Leaf size, mass and thickness and petiole length, width and thickness also influenced leaf vibration. There is a positive correlation between noise reduction and leaf velocity. Noise reduction effect increases with the increase in leaf velocity until about 0.6 mm s−1 and then decreases. The relationship between leaf physical properties and leaf vibration may be used to study sound response and noise reduction in different plant species.
1. Introduction
Plants respond to various natural stimuli, including light, temperature, sound, wind and rain [1,2,3]. The environment in which plants live has many different types of sounds, including bird chirping, wind blowing through leaves and flowing water. Plant leaves can reduce and respond to various sounds by vibration.
Urban tree belts can reduce traffic noise. In Europe, green space coverage varies significantly and ranges from 1.9% (Reggio di Calabria, Italy) to 46% (Ferrol, Spain) with an average of 18.6% [4]. The proportion of green space in most Chinese cities is 25%–42% (General Administration of Mass Supervision, Inspection and Quarantine of the People’s Republic of China, 2002). In the studies on noise reduction by tree belts, it has been found that the width, length, height and visibility of tree belts all contributed to the noise reduction effect. In one study, the noise reduction efficacy of 35 tree belts was tested. It was found that visibility was negatively correlated with noise attenuation, whereas width, height and length were positively correlated with it [5]. Measurement of the reduction of noise at different frequencies by tree belts showed that the attenuation effect increased with noise frequency [6]. Recent reports by Renterghem et al. [7,8] showed that tree belts most effectively attenuated noise when the gaps in them were <3 m wide and the tree diameters were >0.11 m. Pathak et al. [9] found that of all the trees belts they studied, banyan and mango most effectively reduced noise. The arrangement density was proportional to the attenuation in different arrangement modes. However, excessively high density had the adverse effect of attenuating low-frequency sound [10]. For interlaced arrangements at the same planting density, the effect of noise reduction was the strongest, followed by alignment and scatter point. When the planting density was very low, the noise reduction effect of the scatter point was the strongest, whilst that of the alignment type was the weakest. Renterghem et al. [8,11] investigated the noise reduction efficacy of pseudorandom tree belt planting patterns. When all plants had the same physical properties, interlaced Ficus were the most effective at noise reduction. Recent studies have explored the relative influences of tree belt physical properties (length, width and visibility), tree planting pattern and tree species on noise reduction.
Research has also been carried out in terms of responses of plants to sound at the scale of individual plants. Yang et al. [12] found that soil depth and vegetation coverage were directly proportional to the sound absorption coefficient. It has been proposed but not yet confirmed that leaf size also affects the sound absorption coefficient. Tree crown size may be the most important factor influencing noise reduction. The presence of plant leaves increased noise attenuation at high frequencies [13]. Horoshenkov et al. [14] found that the leaf area density and the dominant leaf orientation angle were two key morphological characteristics that could be used in the accurate prediction of plant flow resistivity and tortuosity. The ground effect was also the main factor influencing plant noise reduction. Perforated soil tended to reduce low-frequency noise. The ground effect strongly attenuated noise at frequencies <500 Hz and >1000 Hz by trunk, branch, and leaf scattering and by branch and leaf absorption. Liu [15] found that oval leaves more strongly attenuated noise than long narrow leaves. Renterghem et al. [16] reported that the canopy substantially attenuated high-frequency noise, whereas the trunk markedly attenuated medium-frequency noise. However, neither structure had any obvious effect on low-frequency noise.
To determine how plants respond to sound, researchers examined plant sound scattering paths, sound absorption coefficients and vibrations. It was found that plants respond to sound mainly through friction between the leaves and the air and by leaf vibrations. Martens [17] used a laser-Doppler-vibrometer system to measure acoustic energy absorption by plant leaves. All leaves behaved as linear mechanical systems in response to sound fields. Moreover, vibrational velocities were lower at the leaf veins than the leaf areas immediately adjacent to them. In that study, however, the leaves were excised from the plants and cut into discs to accommodate the measuring equipment [18]. Luan and Hou [19] found that the vibrational frequency of plants after sound stimulation produced a spectral peak at both the frequency of the external stimulus and at twice that frequency. Moreover, the irrigation time of the plants was correlated with the spectral peak of the vibrational frequency. To the best of our knowledge, there has been no previous study on the responses of complete, intact leaves to sound stimuli. In addition, earlier researches focused only on high- or medium-frequency noise reduction by leaves.
The aim of this study was to examine a single leaf responses to sound vibrations by assessing the influences of leaf physical properties on leaf vibrational amplitude, velocity and frequency before and during sound stimulation, and to explore the reduction of noise by leaf vibration. Ninety complete, intact leaves of nine plant species with a wide range of leaf sizes, qualities and thicknesses and petiole lengths, widths and thicknesses were selected to test the influencing factor of leaf vibration. Five complete leaves were cut from one live tree to measure the effect of noise reduction by a single leaf.
2. Materials and Methods
Noise is reduced by vibrational leaves through the way that sound energy is converted into kinetic energy when leaves are stimulated by sound. Energy consumption of vibration body is affected by the mass, amplitude and frequency of the vibration body [20]. To investigate plant leaf vibration in response to sound stimuli, its amplitude, velocity and frequency were measured before and during sound stimulation in a closed environment with zero wind velocity.
2.1. Experimental Settings
Martens [17] indicated that plant leaf vibration varies with different parts of the leaf surface. Six points were selected to find the differences in vibration between different parts of a leaf. Compared with different leaves, the differences between different measuring points on the same leaf can be neglected. In this experiment, the measurement point was the intersection of the midline from the leaf centre to the top and the midline from the petiole to the leaf edge. To test the influence of sound directivity, the distance between the speaker and the measuring point (h) was changed. When h = 14–22 cm, it has little influence on leaf vibration in the sound field. The experimental setup is shown in Figure 1. The speaker was placed 14–22 cm directly below the measurement point. A laser micrometer (IG-1000/CCD; KEYENCE Corporation, Osaka, Japan) was fixed directly above it. Plants were set on a marble isolation table. A sealed cover was put in place to eliminate vibrations caused by human activity. After the experimental setup was fully assembled, the plants were allowed to rest for five min to eliminate the vibrations created during the setup.
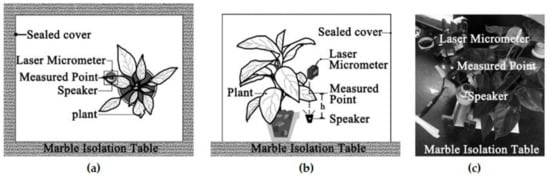
Figure 1.
Experimental Setup: (a) Overhead view of the measurement; (b) Lateral view of the measurement; (c) Photograph of the layout.
2.2. Sound Source Selection
Several earlier studies showed that plant leaves can effectively reduce noise at high frequency [8,21,22]. Plant leaves also respond to sounds at mid-frequency [18]. Nevertheless, comparatively little is known about how plants behave in the presence of low-frequency noise. To understand plant response to sound, a point source with a wide frequency range from 0 to 8 kHz was used (93.5 dBA) in this study. Spectral characteristics are shown in Figure 2.
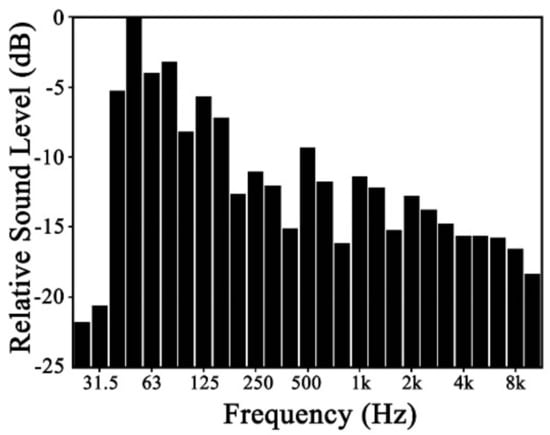
Figure 2.
Spectral characteristic of point source.
2.3. Plant Selection
In this study, nine different common plants were selected. They had various leaf physical properties (size, thickness, etc.) and textures (leathery, succulent, papery and membranous). The species included Gardenia jasminoides Ellis (shrub), Camellia japonica (L.) (shrub), Anthurium andraeanum Linden. (perennial herb), Zamioculcas zamiifolia Engl. (herbaceous plant), Echeveria pulvinata (succulent plant), Kalanchoe blossfeldiana Poelln. (succulent plant), Hibiscus syriacus Linn. (shrub), Rohdea roth (herbaceous plant) and Plectranthus scutellarioides (L.) R.Br. (perennial herb). The physical properties of their plant leaves are shown in Table 1, and their photographs appear in Figure 3. Three species were selected for each leaf texture type. Ten leaves substantially differing in size and thickness were selected from each plant species, and leaves from the same tree were marked with the same number, as shown in Figure 3.

Table 1.
Plant leaf physical properties.
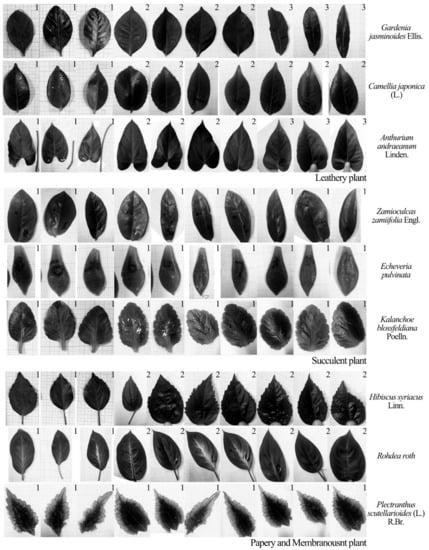
Figure 3.
Plant leaves used in the study.
Table 2 shows the Kendall correlation coefficients among leaf physical properties (analysed by SPSS software). After removing outliers, the Shapiro–Wilk test showed that leaf physical properties did not conform to the normal distribution (p < 0.05), and there no linear relationship existed between any two of these physical properties. So the Kendall correlation was adopted to analyse the relationship among leaf physical properties. As shown in Table 2, no significant correlation could be found between leaf thickness and all other leaf physical properties. Except for correlations with leaf thickness, petiole length and petiole thickness, the rest of correlations between leaf physical properties were significant.

Table 2.
Kendall correlation coefficients among plant leaf physical properties.
2.4. Data Extraction
Figure 4 shows the process of data extraction. The duration of each test was 24 s (sampling frequency: 50,000/s). Each leaf was measured 5×, and the averages were used in the subsequent analyses. To identify the differences in vibration before and during sound stimulation, the plant leaves were exposed to no sound for the first 10 s then stimulated by sound thereafter. Each test had 1.2 million measured data points. Of these, 500,000 were vibrations without sound stimulation, whilst the remaining 700,000 were the responses to sound stimuli. Each data extraction covered a cycle of plant leaf vibration to characterise the vibration amplitude. As shown in Figure 4, the amplitude of the plant leaf in the absence of any sound stimulus is ~1 μm. In contrast, the maximum amplitude was ~3 μm with sound stimulation.
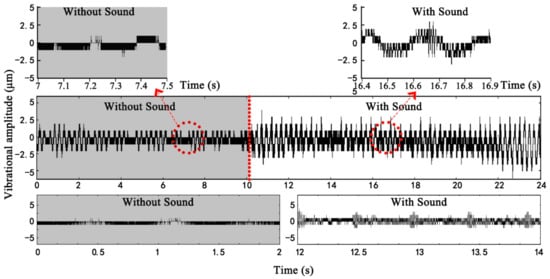
Figure 4.
Data extraction diagram.
2.5. Experimental Setting of Noise Reduction by Leaf
To examine the reduction of noise by a single leaf vibration, five leaves of common street trees (leathery trees) were tested in an anechoic room. Figure 5 shows the experimental setup and the photos of leaves measured in this study. It can be seen that the leaf was placed in the middle of two microphones, and these two microphones were used to test the sound pressure level (SPL) of both sides of the leaf, respectively. The speaker (pink noise; frequency range of 50–8000 Hz) was put along the line of the two microphones, and the laser [23] was placed in the front of the leaf near the microphone 1. The distance between the two microphones was 5.4 cm, and the distance between the speaker and microphone 1 was 80 cm. Each leaf was tested five times, and each test time was 60 s. After the experimental setup was fully assembled, the leaf was allowed to rest for five minutes to eliminate the vibrations created during the setup.
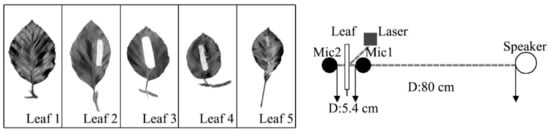
Figure 5.
Experimental Setup and the photos of leaves and paper tested.
3. Results
3.1. Vibrational Amplitude
Figure 6 shows the changes in vibrational amplitudes of the leaves from three kinds of leaf textures after being stimulated by sound. The vibrational amplitude of a plant leaf in the absence of any sound stimulus was ~1 μm. Compared with the unstimulated condition, the leaf vibrational amplitude increased by 1–5.4× in response to the sound field. Under the same sound field, the leaf vibrational amplitudes of different plants varied several fold mainly because of relative differences in leaf texture. The size of leathery leaves and the thickness of succulent leaves were large, leading to higher amplitudes. After sound stimulus, the vibrational amplitudes of leathery leaves and papery and membranous leaves significantly increased compared with those for the unstimulated leaves. The vibrational amplitudes of the succulent leaves in the sound field were only slightly higher than those of the unstimulated because they contained more water and have greater thickness. Note that the Zamioculcas zamiifolia Engl. leaf was a unique succulent resembling a leathery plant, which might be the reason that some succulent leaves had a clear increase in vibrational amplitude. Except for succulent leaves, the vibrational amplitude increased with the increase in leaf size and mass whilst it decreased with the increase in leaf thickness.
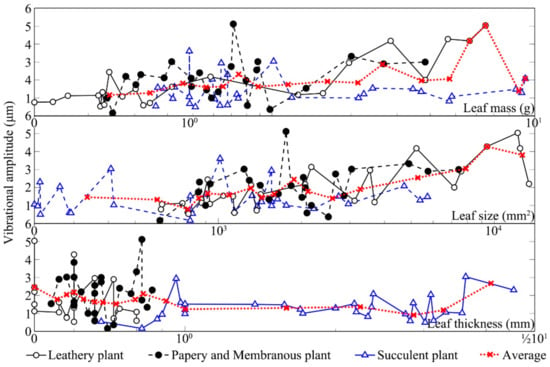
Figure 6.
Vibrational amplitudes of three different kinds of textures.
Leaf vibrational amplitude varied with texture and physical properties. Measurement of the vibrational amplitudes of 90 leaves from nine plants in the sound field indicated that leaf size, thickness and mass, and petiole length, width and thickness all influenced the increase in leaf vibrational amplitude, as shown in Table 3. According to the analysis of the vibrations of leaves with different textures, none of the six factors was significantly correlated with the increase in the vibrational amplitude of the papery and membranous leaves (p < 0.05). Moreover, there was no significant difference in the Kendall correlation coefficients between leaf physical characteristics and increased vibration amplitude of succulent plants without petioles. For leathery plants, there were significant correlations between leaf vibrational amplitude increase and leaf size, leaf mass, petiole length and petiole thickness (p < 0.01). For all plant leaves, leaf size, leaf mass, petiole length and petiole thickness were weakly positively correlated with leaf vibrational amplitude increase (p < 0.05).

Table 3.
Kendall correlation coefficients between the increase in plant leaf vibrational amplitude and leaf physical properties.
The increase in the vibrational amplitude of the leathery leaves exposed to sound was significantly correlated with their physical properties. Figure 7 shows the factors influencing the increases in the vibration of leathery plant leaves. Leathery leaf vibrational amplitude varied directly with leaf mass. The latter was the most important factor affecting the increase in vibrational amplitude of leathery leaves in the sound field. Leaf size, petiole length and thickness were also positively correlated with the increase in leaf vibrational amplitude of leathery plants. Although leathery petiole width was also positively correlated with vibrational amplitude, its R2 was small, and no further analysis was conducted on it.
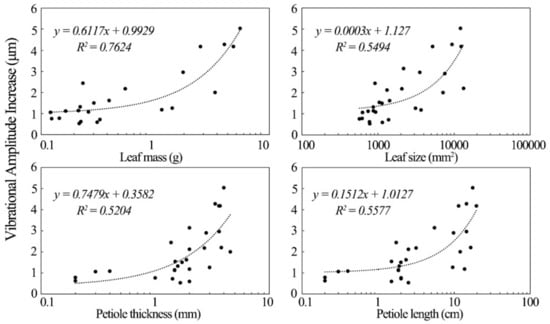
Figure 7.
Factors influencing increases in leathery plant leaf vibration.
3.2. Vibrational Velocity
Similar to vibrational amplitude, leaf vibrational velocity was also influenced by the leaf texture and physical property. Figure 8 shows the changes in leaf vibrational velocity from three different textures after sound stimulation. Both leathery leaves and papery and membranous leaves significantly increased with sound stimulation relative to those measured in the absence of sound. The vibrational velocity of all plant leaves was ~0.05 mm s−1 when there was no sound stimulus. When the leaves were exposed to sound, however, the vibrational velocity increased to 1–2 mm s−1. Nevertheless, the vibrational velocity of the leaves of succulent plants did not markedly increase after sound stimulation compared to that measured in the absence of sound. As shown in Figure 7, the vibrational velocity of leathery leaves and papery and membranous leaves increased with the increase in leaf size and mass. Except for succulent leaves, with the increase in leaf thickness, the leaf vibrational velocity decreased.
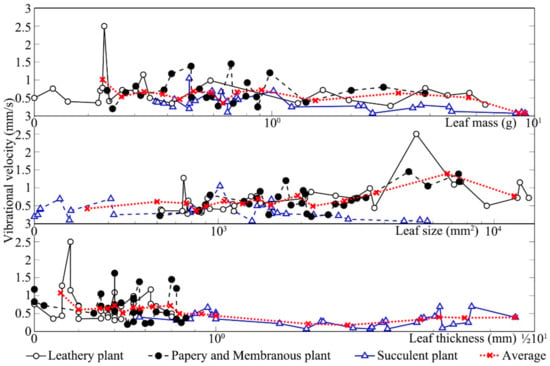
Figure 8.
Vibrational velocities of three different kinds of textures.
To evaluate the influence of leaf texture on the increases in vibrational velocity, measurements were taken on leathery leaves, succulent leaves and papery and membranous leaves before and during sound stimulation. Table 4 shows the influencing factors on the increase in leaf vibrational velocity analysed by Kendall correlation. For leathery plants, except for leaf thickness, all the other physical properties were positively correlated with the increase in vibrational velocity at the 5% statistical significance level (p < 0.01). The increase in vibrational velocities of papery and membranous leaves had no connection with all these six factors. In contrast, for succulent plants, only leaf size had a moderate correlation with the increase in vibrational velocity, while leaf mass and thickness had no correlation with the increase in vibrational velocity (p < 0.05).

Table 4.
Kendall correlation coefficients between the increase in plant leaf vibrational velocity and leaf physical properties.
3.3. Vibrational Frequency
For plant leaf, it can only respond to a specific range of frequencies, which is not correlated to the sound source. In this study, a wide frequency range between 0 Hz and 8K Hz sound source was used to test leaf vibration, which will not miss any important frequencies that leaves can respond to. Plant leaf vibration before and during sound stimulation was concentrated in the low-frequency range, as shown in Figure 9. Although vibrational frequencies differed among various plant species without stimuli, they tended to be concentrated in the range of 0 to15 Hz. During sound stimulation, however, their vibrational frequencies increased, producing another peak in the range of 80 to 95 Hz.
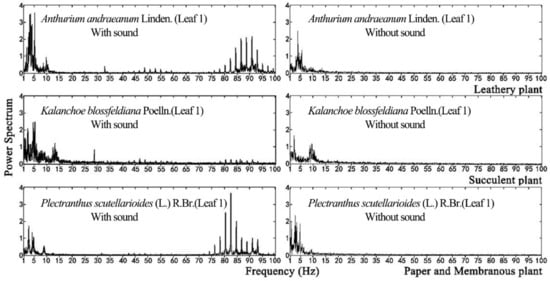
Figure 9.
Variations in leaf vibration with and without sound stimulation.
Within the same sound field, leaf vibrational frequency varied with the physical properties of the leaf. The leaf amplitude, vibrational velocity and vibrational frequency differed among plant species exposed to sound stimuli because their leaves had different textures. The physical properties of leaves with the same texture were the main factors affecting their vibration.
3.4. Noise Reduction by a Single Leaf
In addition to the influencing factors of leaf vibration, it is also important to know the relationship between leaf vibration and noise reduction. A one-way analysis of variance (ANOVA) was used to analyse the difference of the microphones, and the results showed that there was a significant difference between the mean values of microphone 1 and microphone 2, with the statistical significance level of 1% (p = 0.000). While there was no statistical significance in the repeated test of microphone 1 (p = 0.168) and microphone 2 (p = 0.347). To eliminate the influence of the sound pressure level difference between two microphones caused by the sound barrier (leaf), the method of the comparative experiment was adopted under the same setup as the situation of noise reduction experiment. The sound level of the compared test was determined by continuously decreasing the level of sound until the leaf nearly stopped vibrating (v < 0.1 mm s−1). SPL1 was the difference of sound level between two microphones in the situation that the leaf was stimulated to vibrate by sound. SPL2 was the difference of sound level between two microphones in the compared test, which meant that leaf was not stimulated to vibrate as the sound level decreased. The SPLdiff was the difference, namely SPL2 − SPL1, which indicates the SPL effect caused by leaf vibration.
Figure 10 shows the SPLdiff of five leaves influenced by leaf velocity. SPLdiff had a significantly positive correlation with leaf velocity according to Pearson correlation analysis (0.335 **; p = 0.004), which meant that the effect of noise reduction by leaf vibration increased with the increase in leaf velocity. Other factors, such as leaf textures and physical properties, may also impact SPLdiff. Leaf 1–5 came from the same tree, and the leaf size and mass decreased from leaf 1 to leaf 5. The SPLdiff of leaf 1 was the greatest, and the SPLdiff of leaf 5 was the smallest, which might be caused by the difference in leaf size and mass. Under the same velocity, the difference of SPLdiff between these five leaves was mainly caused by leaf physical properties because for leaf 1–5, leaf texture and physiology properties were the same. Compared with leaf velocity, leaf physical properties (leaf size, thickness and mass) had a greater influence on SPLdiff. For all five leaves, the SPLdiff started to fluctuate and decrease when the vibrational velocity approached the maximum value. According to the relationship between the average value of both velocity and SPLdiff, when the velocity was less than about 0.6 mm s-1, SPLdiff increased with the increase in velocity and decreased after that threshold. This phenomenon might mainly be caused by the fact that leaves vibrating with higher velocity can also make additional noise.
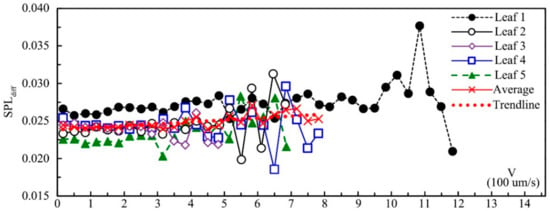
Figure 10.
The influence of leaf velocity on sound pressure level.
While noise reduction by a single leaf might be insignificant, as there are thousands of leaves in a tree, the overall effect of noise reduction by leaf vibration could be considerable, for which, in addition to leaf properties, the leaf density and the porosity, for example, should also be taken into account.
4. Conclusions and Discussion
In the present study, vibrational experiments were conducted on the intact leaves of different plants in the sound field to identify leaf responses to sound stimuli. The parameters evaluated were leaf vibrational amplitude, velocity and frequency. The following observations were made:
(1) Without sound stimulation, leaf vibrational amplitude was small (~1 μm). When various plant species were stimulated by the same sound, their leaf vibrational amplitudes increased to ~2–6 μm. The main factor affecting this increase was leaf texture. The leaf vibrational amplitudes of succulent plants did not significantly increase in response to sound stimuli, whereas those of papery and membranous plants increased irregularly. The leaf vibrational amplitudes of leathery plants significantly increased with leaf size, leaf mass, petiole length, and petiole thickness (p < 0.01).
(2) The vibrational velocity of a plant leaf was ~0.05 mm s−1 in the absence of a sound stimulus. For all plants exposed to the same sound field, the leaf vibrational velocity significantly increased to 1–2 mm s−1. The main factor affecting the increase in leaf vibrational velocity was texture. The increase in vibrational velocities of papery and membranous leaves had no connection with leaf physical properties. Whereas, the increase in succulent leaf vibrational velocity in response to sound stimulation was negligible, it was significant in leathery leaves. Leaf size, leaf mass, petiole length, petiole width and petiole thickness were positively correlated with the increase in leaf vibrational velocity.
(3) Without sound stimulation, the vibrational frequency of a plant leaf was concentrated in the range of 0 to 15 Hz. Under the same sound stimulation, the leaf vibrational frequency of various plants had another peak in the vicinity of 80–95 Hz. Comparisons of the vibrational frequencies of leaves before and during sound stimulation indicated that all leaves emit vibrations within the same frequency range as that of the sound source used for the stimulation. Nevertheless, a vibrational frequency within the range of 0 to 15 Hz of the plant leaf remained constant.
In addition, noise reduction by leaf vibration differed significantly between leaves, which may be caused by different leaf physical properties, especially leaf size and mass. When the vibrational velocity was below 0.6 mm s−1, there was a positive correlation between noise reduction and leaf velocity. The noise reduction effect started to fluctuate and decrease when the vibrational velocity approached the maximum value.
In this study, some shortcomings still need to be mentioned. Only directed sound was taken into consideration, which is rare in real life. For a whole tree, the sound would repeatedly scatter between leaves, which may increase noise reduction values. In this study, the sound source used for the experiment was 93.5 dBA, which was high enough, but still failed to significantly stimulate succulent plant leaves. In the future study, it may be possible to raise the sound level of the experimental sound sources to study how succulent plants respond to the sound.
In addition, the plant specimen tested in this study were randomly selected from common plant species in northern China. This study provides a method to identify plants with higher noise reduction potential. To elucidate plant responses to sound, it would be reliable to apply the test method to other plant species. Yang’s [13] research indicated that the presence of leaves has an influence at high frequencies compared with no leaves, above about 2 kHz, which is mainly caused by the scattering between leaves. While this phenomenon also appeared at a low frequency below 160 Hz, which was mainly caused by leaf vibration. In addition, the sound response of a single whole plant should be assessed to expand our knowledge and understanding of plant acoustics.
Author Contributions
Conceptualization, M.L. and J.K.; methodology, M.L.; software, M.L.; validation, M.L. and J.K.; formal analysis, M.L.; investigation, M.L.; resources, M.L.; data curation, M.L.; writing—original draft preparation, M.L.; writing—review and editing, J.K.; visualization, J.K.; supervision, J.K.; project administration, J.K.; funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (NSFC), grant number 51778169.
Acknowledgments
The authors thank Dick Botteldooren, Timothy Van Renterghem, Qingliang Zhao, and Xiao Li, Yanlu Li, Yongcheng Pan, for useful discussions and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heggie, L.; Halliday, K.J. The highs and lows of plant life: Temperature and light interactions in development. Int. J. Dev. Biol. 2005, 49, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Telewski, F.W. A unified hypothesis of mechanoperception in plants. Am. J. Bot. 2006, 93, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.C.; Ghosh, R.; Bae, H. Plant acoustics: In the search of a sound mechanism for sound signaling in plants. J. Exp. Bot. 2016, 67, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.A.; Gaston, K.J. The scaling of green space coverage in European cities. Biol. Lett. 2009, 5, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.F.; Ling, D.L. Investigation of the noise reduction provided by tree belts. Landsc. Urban Plan. 2003, 63, 187–195. [Google Scholar] [CrossRef]
- Tyagi, V.; Kumar, K.; Jain, V.K. A study of the spectral characteristics of traffic noise attenuation by vegetation belts in Delhi. Appl. Acoust. 2006, 67, 926–935. [Google Scholar] [CrossRef]
- Van Renterghem, T.; Botteldooren, D.; Verheyen, K. Road traffic noise shielding by vegetation belts of limited depth. J. Sound Vib. 2012, 331, 2404–2425. [Google Scholar] [CrossRef]
- Van Renterghem, T. Guidelines for optimizing road traffic noise shielding by non-deep tree belts. Ecol. Eng. 2014, 69, 276–286. [Google Scholar] [CrossRef]
- Pathak, V.; Tripathi, B.D.; Mishra, V.K. Evaluation of anticipated performance index of some tree species for green belt development to mitigate traffic generated noise. Urban For. Urban Green. 2011, 10, 61–66. [Google Scholar] [CrossRef]
- Yang, F.; Bao, Z.Y.; Zhu, Z.J.; Liu, J.N. The investigation of noise attenuation by plants and the corresponding noise-reducing spectrum. J. Environ. Health 2010, 72, 8–15. [Google Scholar]
- Van Renterghem, T.; Botteldooren, D. View on outdoor vegetation reduces noise annoyance for dwellers near busy roads. Landsc. Urban Plan. 2016, 148, 203–215. [Google Scholar] [CrossRef]
- Yang, H.S.; Kang, J.; Cheal, C. Random-incidence absorption and scattering coefficients of vegetation. Acta Acust. United Acust. 2013, 99, 379–388. [Google Scholar] [CrossRef]
- Yang, H.S.; Kang, J.; Cheal, C.; Van Renterghem, T.; Botteldooren, D. Quantifying scattered sound energy from a single tree by means of reverberation time. J. Acoust. Soc. Am. 2013, 134, 264. [Google Scholar] [CrossRef]
- Horoshenkov, K.V.; Khan, A.; Benkreira, H. Acoustic properties of low growing plants. J. Acoust. Soc. Am. 2013, 133, 2554. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N. Research on Noise Reduction Function of Garden Plants. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2007. [Google Scholar]
- Van Renterghem, T.; Hornikx, M.; Smyrnova, Y.; Jean, P.; Kang, J.; Botteldooren, D.; Defrance, J. Road traffic noise reduction by vegetated low noise barriers in urban streets. In Proceedings of the 9th European Conference on Noise Control (Euronoise 2012), Prague, Czech Republic, 10–13 June 2012; pp. 944–948. [Google Scholar]
- Martens, M.J.M. Laser-doppler vibrometer measurements of leaves. In Physical Methods in Plant Sciences; Linskens, H.-F., Jackson, J.F., Eds.; Springer: Berlin, Germany, 1990; pp. 1–22. [Google Scholar]
- Martens, M.J.M.; Michelsen, A. Absorption of acoustic energy by plant leaves. J. Acoust. Soc. Am. 1981, 69, 303–306. [Google Scholar] [CrossRef]
- Luan, J.Y.; Hou, T.Z. Principle and design of plant acoustic vibration characteristic detector. Sci. Bull. 1995, 5, 266–271. [Google Scholar]
- Zhou, Y.B. Theoretical Mechanics Tutorial; Higher Education Press: Beijing, China, 2009. [Google Scholar]
- Smyrnova, Y.; Kang, J.; Hornikx, M.; Forssén, J. Effect of vegetation on noise propagation in streets and squares. In Proceedings of the Institute of Acoustics Environmental Noise Propagation Definitions Measuring & Control Aspects, London, UK, 21 March 2012; pp. 57–67. [Google Scholar]
- Pathak, V.; Tripathi, B.D.; Mishra, V.K. Dynamics of traffic noise in a tropical city varanasi and its abatement through vegetation. Environ. Monit. Assess. 2008, 146, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Duperron, M.; O’Brien, P.; Schüler, R.; Aasmul, S.; Melis, M.; Kersemans, M.; Baets, R. Six-beam homodyne laser Doppler vibrometry based on silicon photonics technology. Opt. Express 2018, 26, 3638–3645. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).