Modeling Migratory Flight in the Spruce Budworm: Temperature Constraints
Abstract
:1. Introduction
2. Model Construction
2.1. Modeling Approach
2.2. Morphometric Relationships
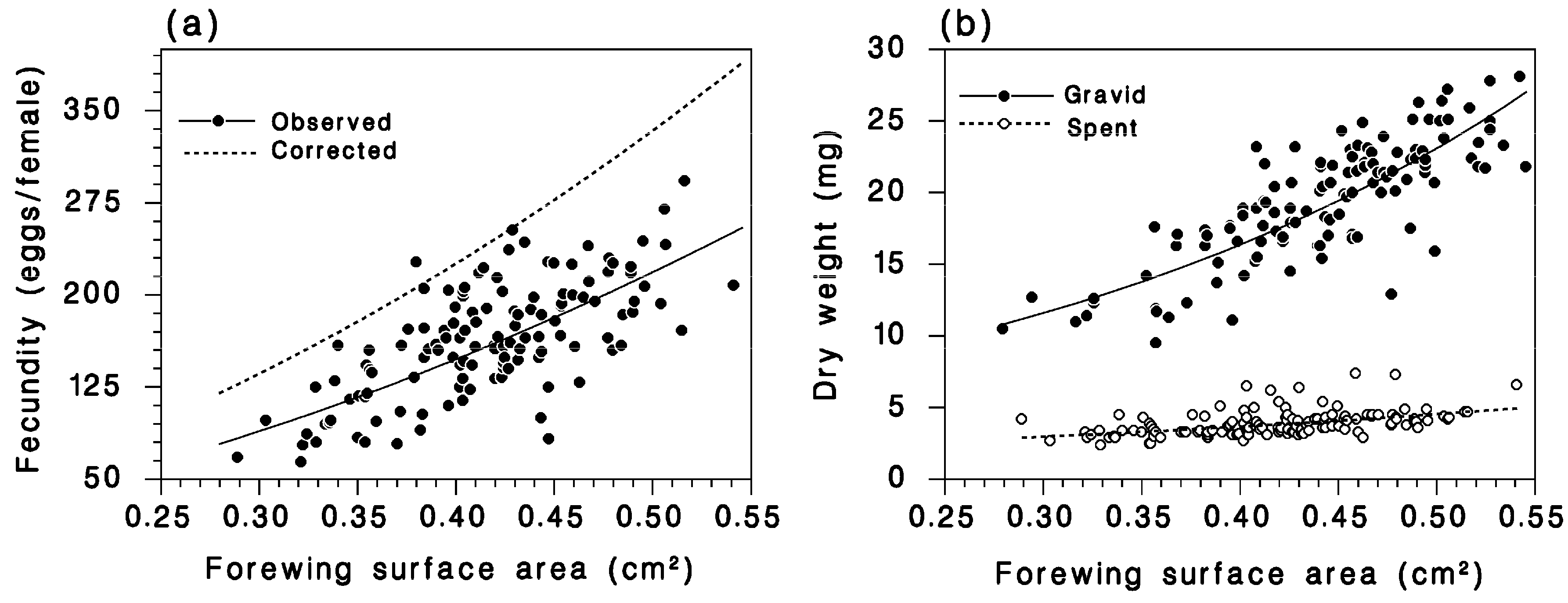
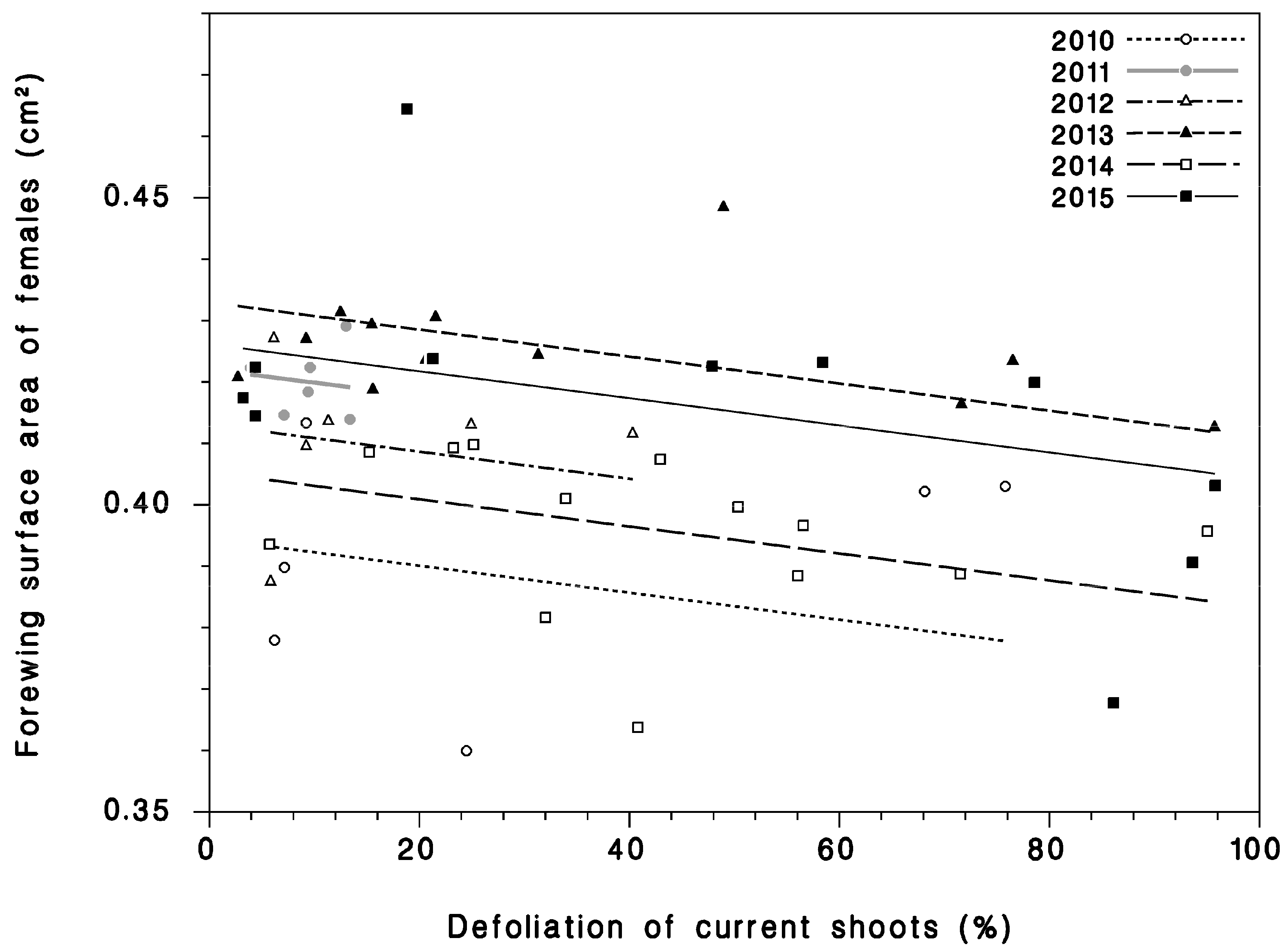
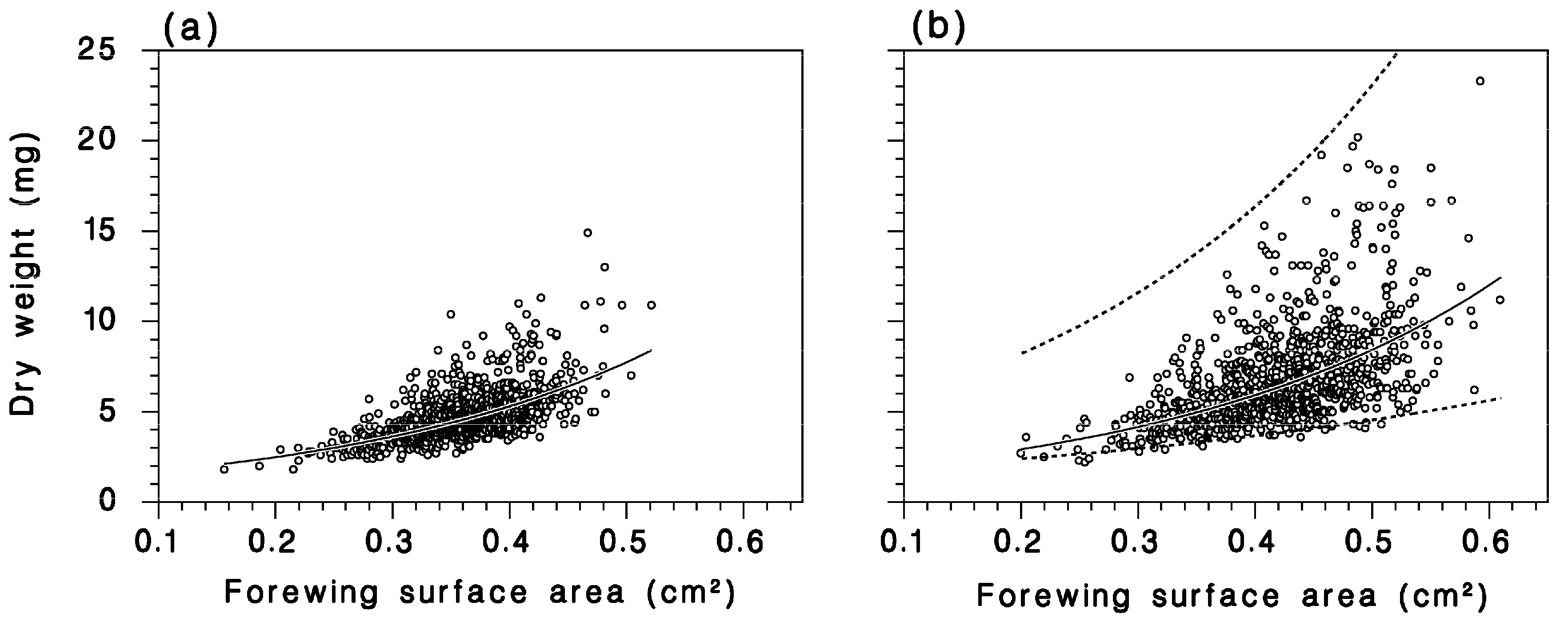
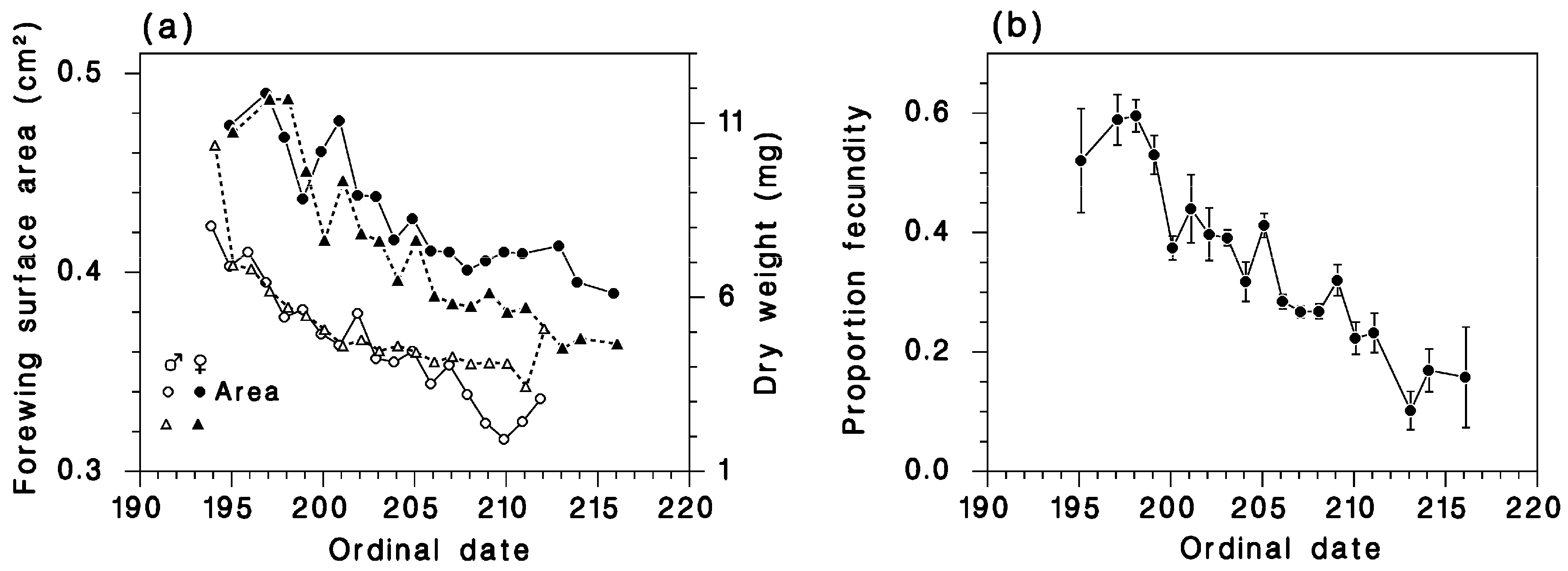
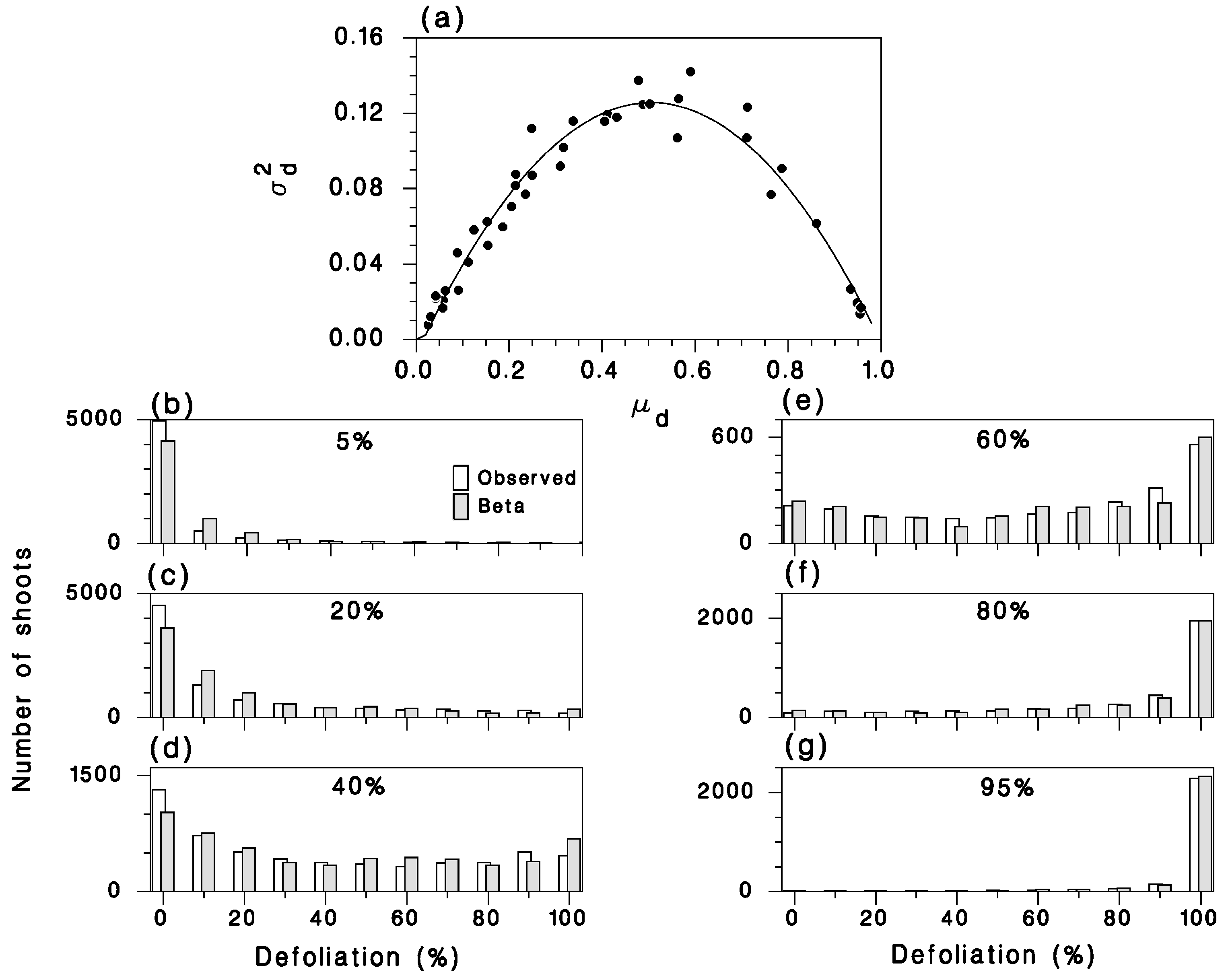
2.2.1. Female Fecundity, Weight, Wing Area, and Influence of Defoliation
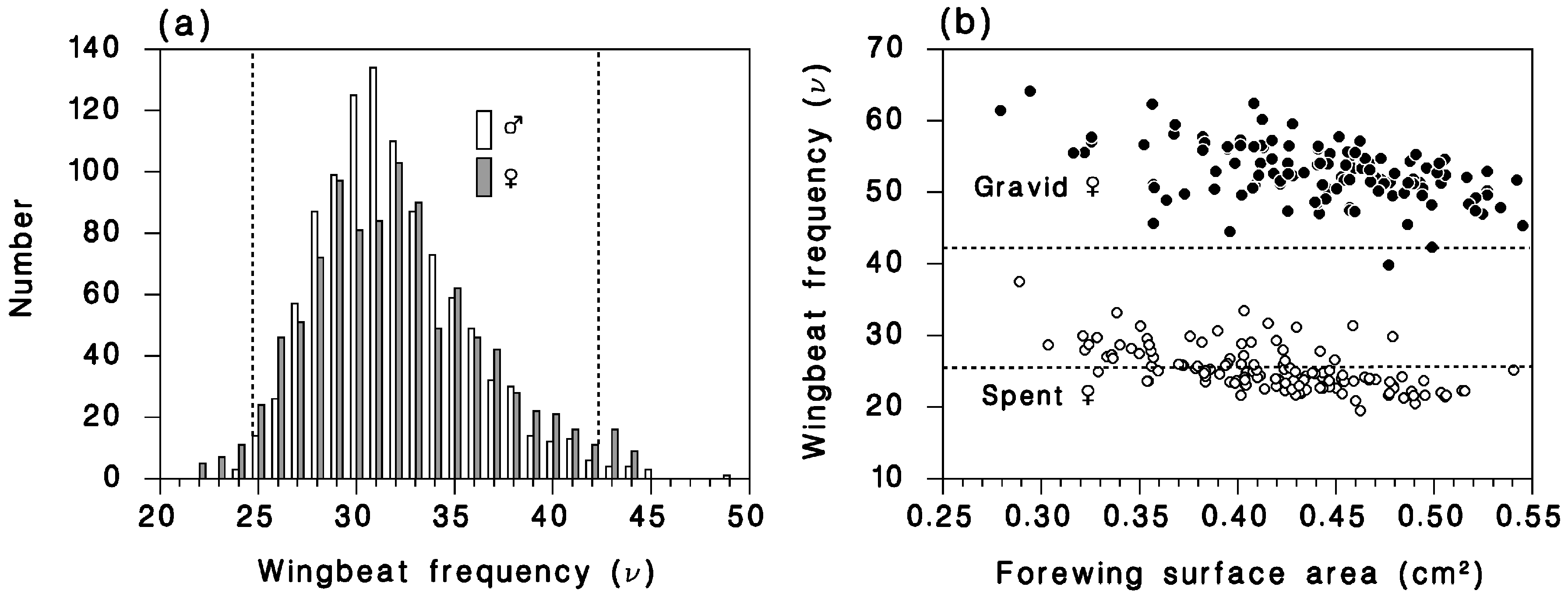
2.2.2. Moths Captured in Canopy Traps
2.2.3. Defoliation at the Individual Level
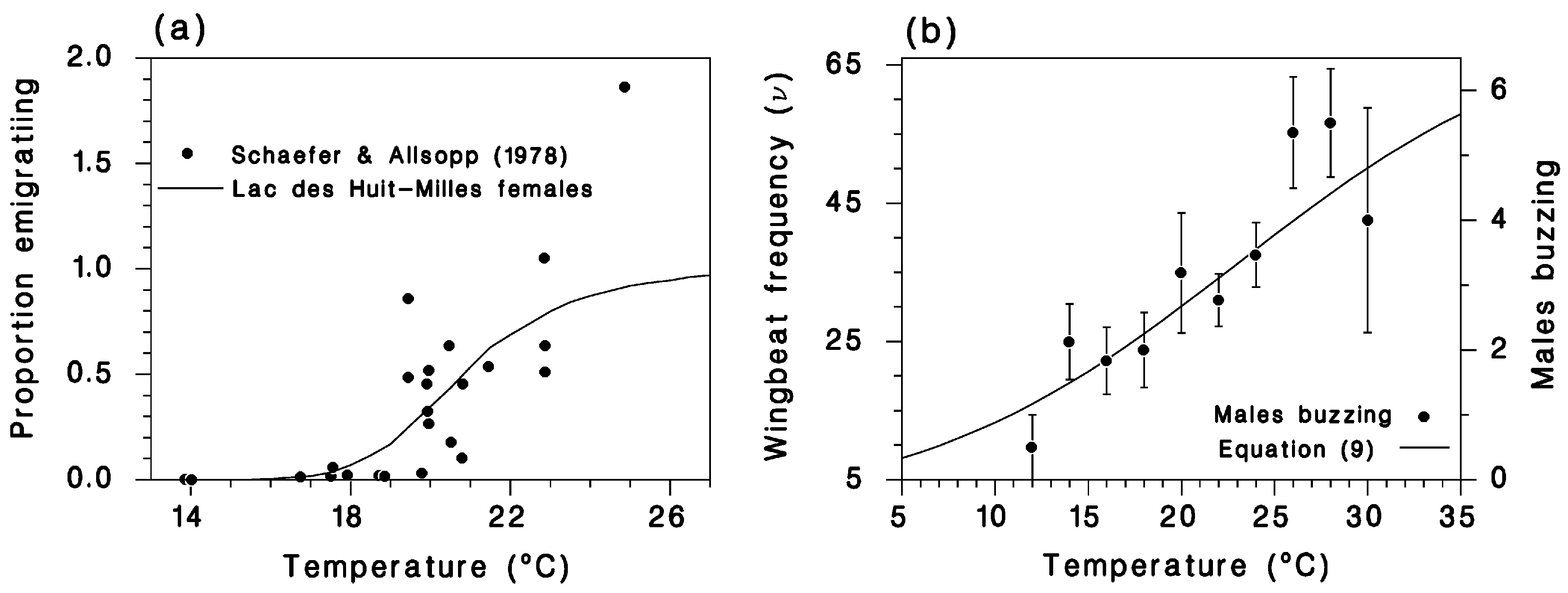
2.3. Flight Model
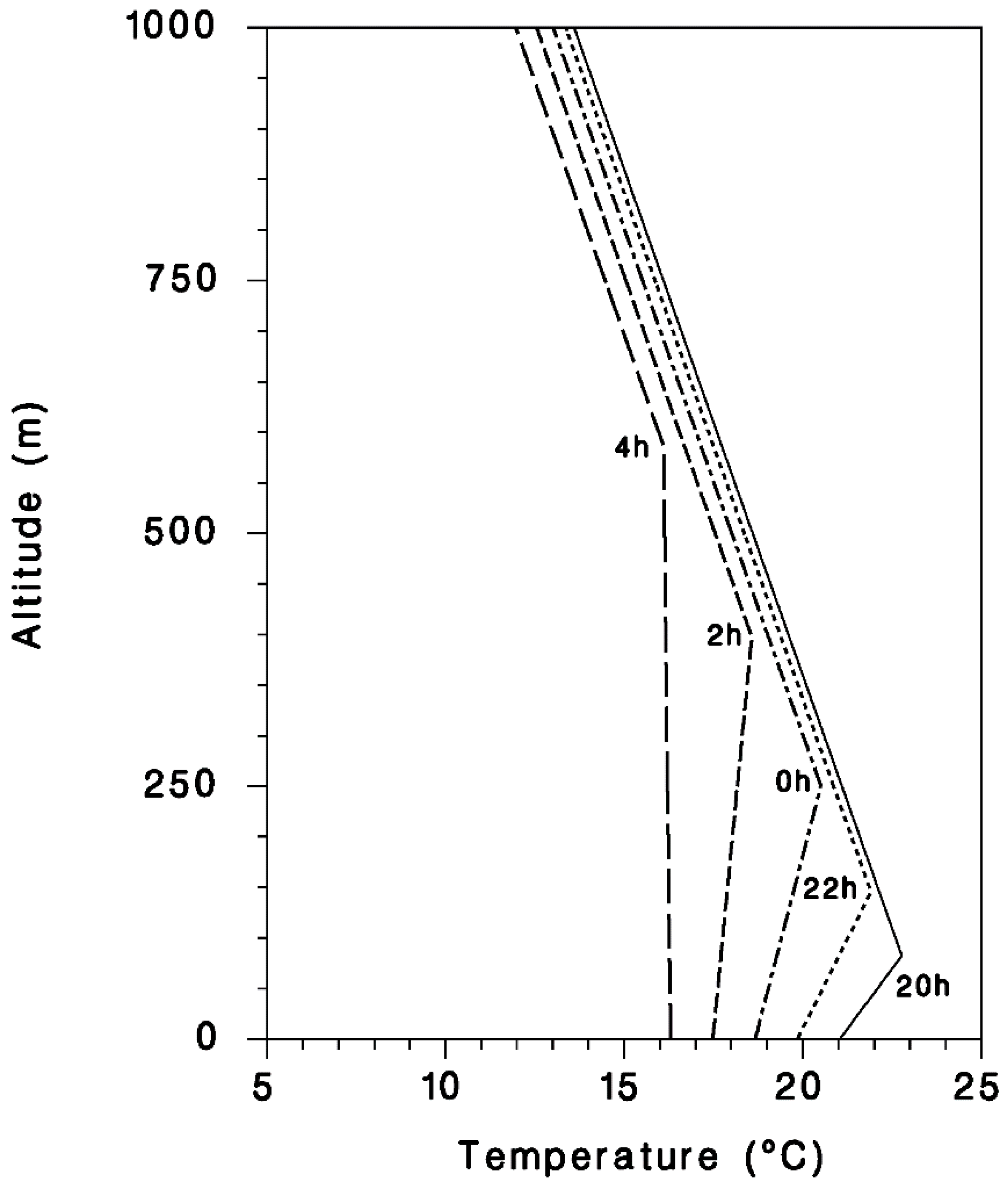
2.4. Flight Model Simulations
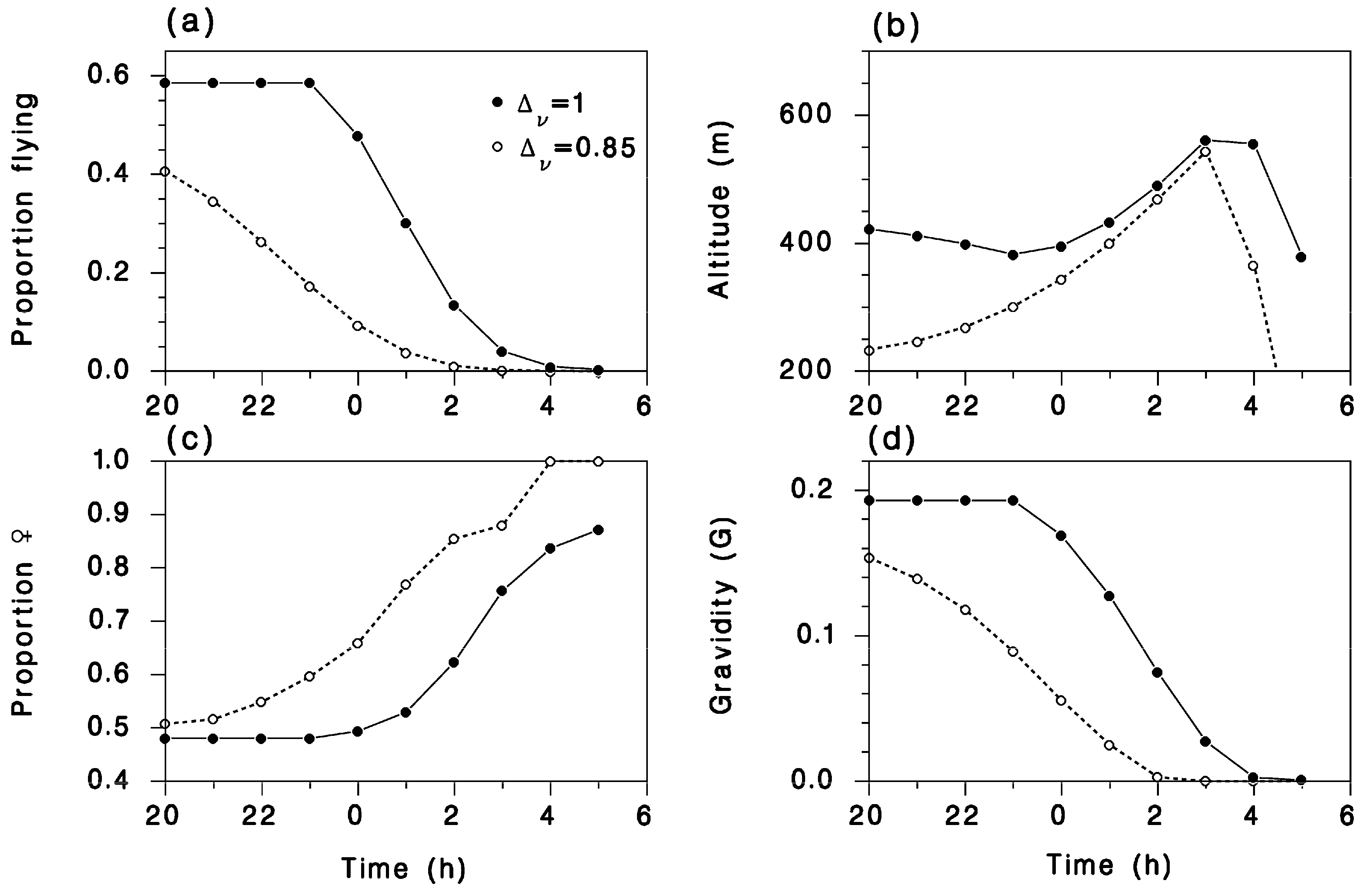
3. Simulation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen & Co. Ltd.: London, UK, 1969; p. 763. [Google Scholar] [CrossRef]
- Schowalter, T. Insect Ecology: An Ecosystem Approach, 4th ed.; Academic Press: London, UK, 2016; p. 774. [Google Scholar]
- Den Boer, P.J. Spreading of risk and stabilization of animal numbers. Acta Biotheor. 1968, 18, 165–194. [Google Scholar] [CrossRef] [PubMed]
- Dingle, H.; Drake, V.A. What is migration? Bioscience 2007, 57, 113–121. [Google Scholar] [CrossRef]
- Chapman, J.W.; Drake, V.A.; Reynolds, D.R. Recent insights from radar studies of insect flight. Ann. Rev. Entomol. 2011, 56, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Drake, V.A. The vertical distribution of macro-insects migrating in the nocturnal boundary layer: A radar study. Bound.-Layer Meteorol. 1984, 28, 353–374. [Google Scholar] [CrossRef]
- Riley, J.R.; Reynolds, D.R. A long-range migration of grasshoppers observed in the Sahelian zone of Mali by two radars. J. Anim. Ecol. 1983, 52, 167–183. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Greenbank, D.O.; Schaefer, G.W.; Rainey, R.C. Spruce budworm (Lepidoptera: Tortricidae) moth flight and dispersal: New understanding from canopy observations, radar, and aircraft. Mem. Entomol. Soc. Can. 1980, 112, 1–49. [Google Scholar] [CrossRef]
- Gray, D.R.; MacKinnon, W.E. Outbreak patterns of the spruce budworm and their impacts in Canada. For. Chron. 2006, 82, 550–561. [Google Scholar] [CrossRef] [Green Version]
- Régnière, J.; Delisle, J.; Pureswaran, D.S.; Trudel, R. Mate-finding Allee effect in spruce budworm population dynamics. Entomol. Exp. Appl. 2013, 146, 112–122. [Google Scholar] [CrossRef]
- Régnière, J.; Cooke, B.J.; Béchard, A.; Dupont, A.; Therrien, P. Dynamics and management of rising spruce budworm populations. Forests 2019, 10, 748. [Google Scholar] [CrossRef]
- Wellington, W.G.; Henson, W.R. Notes on the effects of physical factors on the spruce budworm, Choristoneura fumiferana (Clem.). Can. Entomol. 1947, 79, 168–170. [Google Scholar] [CrossRef]
- Rhainds, M.; Kettela, E.G. Oviposition threshold for flight in an inter-reproductive migrant moth. J. Insect Behav. 2013, 26, 850–859. [Google Scholar] [CrossRef]
- Blais, J.R. Effects of the destruction of the current year’s foliage of balsam fir on the fecundity and habits of flight of the spruce budworm. Can. Entomol. 1953, 85, 446–448. [Google Scholar] [CrossRef]
- Van Hezewijk, B.; Wertman, D.; Stewart, D.; Beliveau, C.; Cusson, M. Environmental and genetic influences on the dispersal propensity of spruce budworm (Choristoneura fumiferana). Agric. For. Entomol. 2018, 20, 433–441. [Google Scholar] [CrossRef]
- Régnière, J.; Garcia, M.; St-Amant, R. Modeling migratory flight in the spruce budworm: Circadian rhythm. Forests 2019. in revision. [Google Scholar]
- Reynolds, D.R.; Chapman, J.W.; Edwards, A.S.; Smith, A.D.; Wood, C.R.; Barlow, J.F.; Woiwod, I.P. Radar studies of the vertical distribution of insects migrating over southern Britain: The influence of temperature inversions on nocturnal layer concentrations. Bull. Entomol. Res. 2005, 95, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, Y.; Fabry, F.; Kilambi, A.; Pureswaran, D.S.; Sturtevant, B.R.; Saint-Amant, R. The use of weather surveillance radar and high-resolution three dimensional weather data to monitor a spruce budworm mass exodus flight. Agric. For. Meteorol. 2017, 234, 127–135. [Google Scholar] [CrossRef]
- Anderson, D.P.; Sturtevant, B.R. Pattern analysis of eastern spruce budworm Choristoneura fumiferana dispersal. Ecography 2011, 34, 488–497. [Google Scholar] [CrossRef]
- Sturtevant, B.R.; Achtemeier, G.L.; Charney, J.J.; Anderson, D.P.; Cooke, B.J.; Townsend, P.A. Long-distance dispersal of spruce budworm (Choristoneura fumiferana Clemens) in Minnesota (USA) and Ontario (Canada) via the atmospheric pathway. Agric. For. Meteorol. 2013, 168, 186–200. [Google Scholar] [CrossRef]
- Dudley, R. The Biomechanics of Insect Flight; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Harvey, G.T. Mean weight and rearing performance of successive egg clusters of eastern spruce budworm (Lepidoptera: Tortricidae). Can. Entomol. 1977, 109, 487–496. [Google Scholar] [CrossRef]
- Eveleigh, E.S.; Lucarotti, C.J.; McCarthy, P.C.; Morin, B.; Royama, T.; Thomas, A.W. Occurrence and effects of Nosema fumiferanae infections on adult spruce budworm caught above and within the forest canopy. Agric. For. Entomol. 2007, 9, 247–258. [Google Scholar] [CrossRef]
- Nealis, V.G.; Régnière, J. Fecundity and recruitment of eggs during outbreaks of the spruce budworm. Can. Entomol. 2004, 136, 591–604. [Google Scholar] [CrossRef]
- Anderson, T.W.; Darling, D.A. Asymptotic theory of certain “goodness-of-fit” criteria based on stochastic processes. Ann. Math. Stat. 1952, 23, 193–212. [Google Scholar] [CrossRef]
- Sanders, C.J. A summary of current techniques used for sampling spruce budworm populations and estimating defoliation in eastern Canada. Can. For. Serv. Info. Rep. 1980, O-X-306. [Google Scholar]
- Gupta, A.K.; Nadarajah, S. Handbook of Beta Distribution and Its Applications; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Byrne, D.N.; Buchmann, S.L.; Spangler, H.G. Relationship between wing loading, wingbeat frequency and body mass in homopterous insects. J. Exp. Biol. 1988, 135, 9–23. [Google Scholar]
- Deakin, M.A.B. Formulae for insect wingbeat frequency. J. Insect Sci. 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, E.G. Effects of ambient temperature, humidity, and age on wing-beat frequency of Periplaneta species. J. Insect Physiol. 1972, 18, 827–839. [Google Scholar] [CrossRef]
- Farmery, M.J. The effect of air temperature on wingbeat frequency of naturally flying armyworm moth (Spodoptera exempta). Entomol. Exp. Appl. 1982, 32, 193–194. [Google Scholar] [CrossRef]
- Unwin, D.M.; Corbet, S.A. Wingbeat frequency, temperature and body size in bees and flies. Physiol. Entomol. 1984, 9, 115–121. [Google Scholar] [CrossRef]
- Oertli, J.J. Relationship of wing beat frequency and temperature during takeoff flight in temperate-zone beetles. J. Exp. Biol. 1989, 145, 321–338. [Google Scholar]
- Foster, J.A.; Robertson, R.M. Temperature dependency of wing-beat frequency in intact and deafferented locusts. J. Exp. Biol. 1992, 162, 295–312. [Google Scholar]
- Snelling, E.P.; Seymour, R.S.; Matthews, G.D.; White, C.R. Maximum metabolic rate, relative lift, wingbeat frequency and stroke amplitude during tethered flight in the adult locust Locusta migratoria. J. Exp. Biol. 2012, 215, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, G.; Wang, Y. Effects of age, ambient temperature and reproductive status on wing beat frequency of the rice leafroller Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Crambidae). Appl. Entomol. Zool. 2013, 48, 499–505. [Google Scholar] [CrossRef]
- Chuine, I.; Régnière, J. Process-based models of phenology for plants and animals. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 159–182. [Google Scholar] [CrossRef]
- Sanders, C.J.; Wallace, D.R.; Lucuik, G.S. Flight activity of female spruce budworm (Lepidoptera: Tortricidae) at constant temperatures in the laboratory. Can. Entomol. 1978, 110, 627–632. [Google Scholar] [CrossRef]
- Schaefer, G.W.; Allsopp, K. Analysis and Interpretation of Ground-Based and Airborne Radar Data Collection for the Spruce Budworm Dispersal Studies 1973–1976 Inclusive; Final Report No. 4. Part B. The Effects of Canopy Temperature and Meteorological Factors on the Number of Moths Entering the Air Space; Ecological Physics Research Group, Cranfield Institute of Technology: Bedfordshire, UK, 1978. [Google Scholar]
- Malingowski, J.; Atkinson, D.; Fochesatto, J.; Cherry, J.; Stevens, E. An observational study of radiation temperature inversions in Fairbanks, Alaska. Polar Sci. 2014, 8, 24–39. [Google Scholar] [CrossRef] [Green Version]
- Wellington, W.G. The light reactions of the spruce budworm, Choristoneura fumiferana Clemens (Lepidoptera: Tortricidae). Can. Entomol. 1948, 80, 56–82. [Google Scholar] [CrossRef]
- Henson, W.R. Mass flights of the spruce budworm. Can. Entomol. 1951, 83, 240. [Google Scholar] [CrossRef]
- Wood, C.R.; Clark, S.J.; Barlow, J.F.; Chapman, J.W. Layers of nocturnal insect migrants at high-altitude: The influence of atmospheric conditions on their formation. Agric. For. Entomol. 2010, 12, 113–121. [Google Scholar] [CrossRef]
- Angevine, W.M. Transitional, entraining, cloudy, and coastal boundary layers. Acta Geophys. 2008, 56, 2–20. [Google Scholar] [CrossRef]
- Angevine, W.M.; Tjernström, M.; Žagar, M. Modeling of the coastal boundary layer and pollutant transport in New England. J. Appl. Meteorol. Climatol. 2006, 45, 137–154. [Google Scholar] [CrossRef]
- Régnière, J.; St-Amant, R.; Duval, P. Predicting insect distributions under climate change from physiological responses: Spruce budworm as an example. Biol. Invasions 2012, 14, 1571–1586. [Google Scholar] [CrossRef]
- Pedgley, A.D.E. Concentration of flying insects by the wind. Philos. Trans. R. Soc. Lond. B 1990, 328, 631–653. [Google Scholar] [CrossRef]
- Schaefer, G.W. An airborne radar technique for the investigation and control of migrating pest species. Philos. Trans. R. Soc. Lond. B 1979, 287, 459–465. [Google Scholar] [CrossRef]
- Dickinson, R.B.B.; Haggis, M.J.; Rainey, R.C. Spruce budworm moth flight and storms: Case study of a cold front system. J. Clim. Appl. Meteorol. 1983, 22, 278–286. [Google Scholar] [CrossRef]
- Pedgley, D.E.; Reynolds, D.R.; Riley, J.R.; Tucker, M.R. Flying insects reveal small-scale wind systems. Weather 1982, 37, 295–306. [Google Scholar] [CrossRef]
- Isard, S.A.; Gage, S.H.; Comtois, P.; Russo, J.M. Principles of the atmospheric pathway for invasive species applied to soybean rust. BioScience 2005, 55, 851–861. [Google Scholar] [CrossRef]
- Benjamin, S.G.; Weygandt, S.S.; Brown, J.M.; Hu, M.; Alexander, C.R.; Smirnova, T.G.; Olson, J.B.; James, E.P.; Dowell, D.C.; Grell, G.A.; et al. A North American hourly assimilation and model forecast cycle: The Rapid Refresh. Mon. Weather Rev. 2016, 144, 1669–1694. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Régnière, J.; Delisle, J.; Sturtevant, B.R.; Garcia, M.; Saint-Amant, R. Modeling Migratory Flight in the Spruce Budworm: Temperature Constraints. Forests 2019, 10, 802. https://doi.org/10.3390/f10090802
Régnière J, Delisle J, Sturtevant BR, Garcia M, Saint-Amant R. Modeling Migratory Flight in the Spruce Budworm: Temperature Constraints. Forests. 2019; 10(9):802. https://doi.org/10.3390/f10090802
Chicago/Turabian StyleRégnière, Jacques, Johanne Delisle, Brian R. Sturtevant, Matthew Garcia, and Rémi Saint-Amant. 2019. "Modeling Migratory Flight in the Spruce Budworm: Temperature Constraints" Forests 10, no. 9: 802. https://doi.org/10.3390/f10090802
APA StyleRégnière, J., Delisle, J., Sturtevant, B. R., Garcia, M., & Saint-Amant, R. (2019). Modeling Migratory Flight in the Spruce Budworm: Temperature Constraints. Forests, 10(9), 802. https://doi.org/10.3390/f10090802




