Monitoring of a Seedling Planting Restoration in a Permanent Preservation Area of the Southeast Atlantic Forest Biome, Brazil
Abstract
1. Introduction
2. Materials and Methods
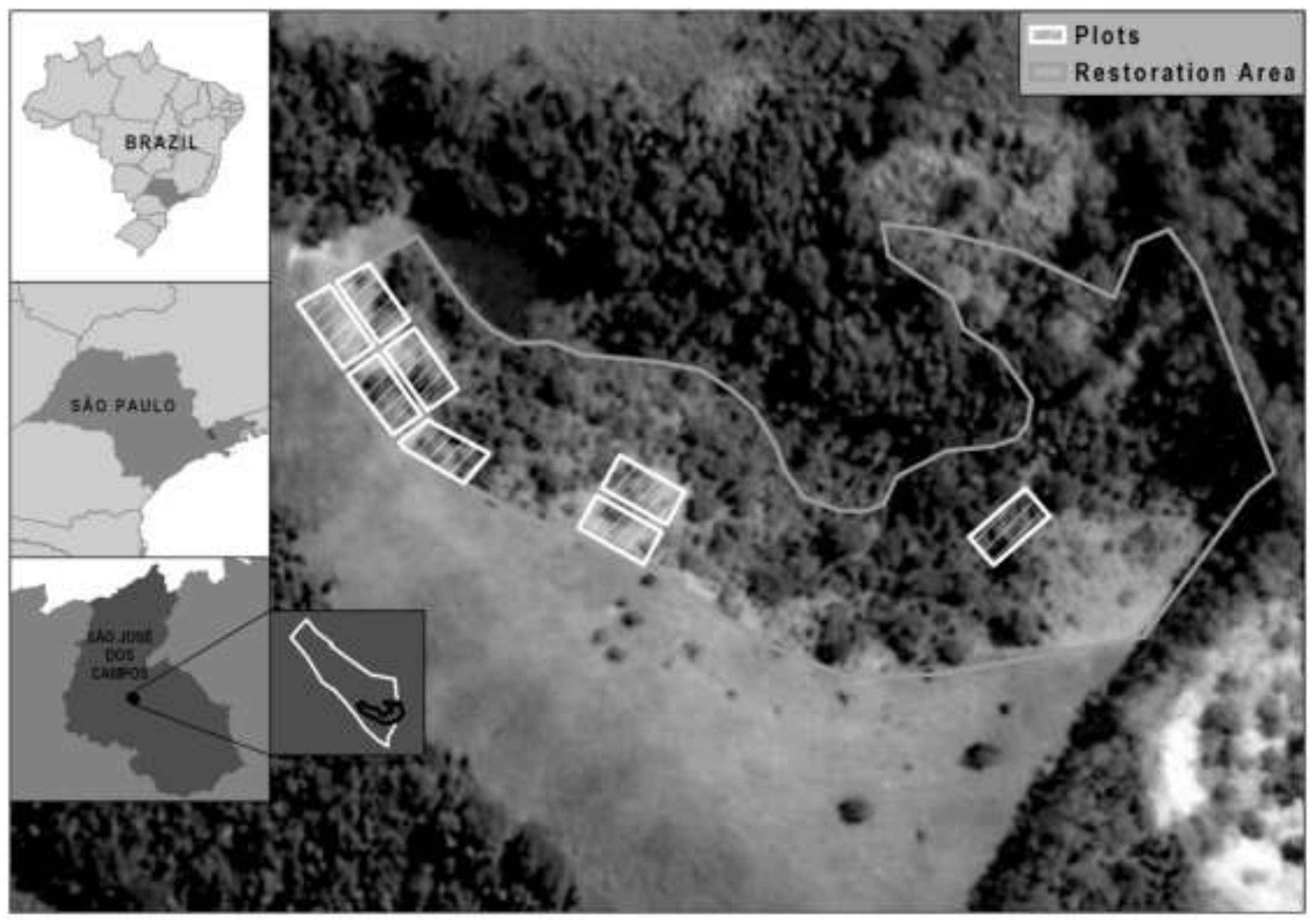
2.1. Study Area
2.2. Plant Measurements
2.3. Statistical Analysis
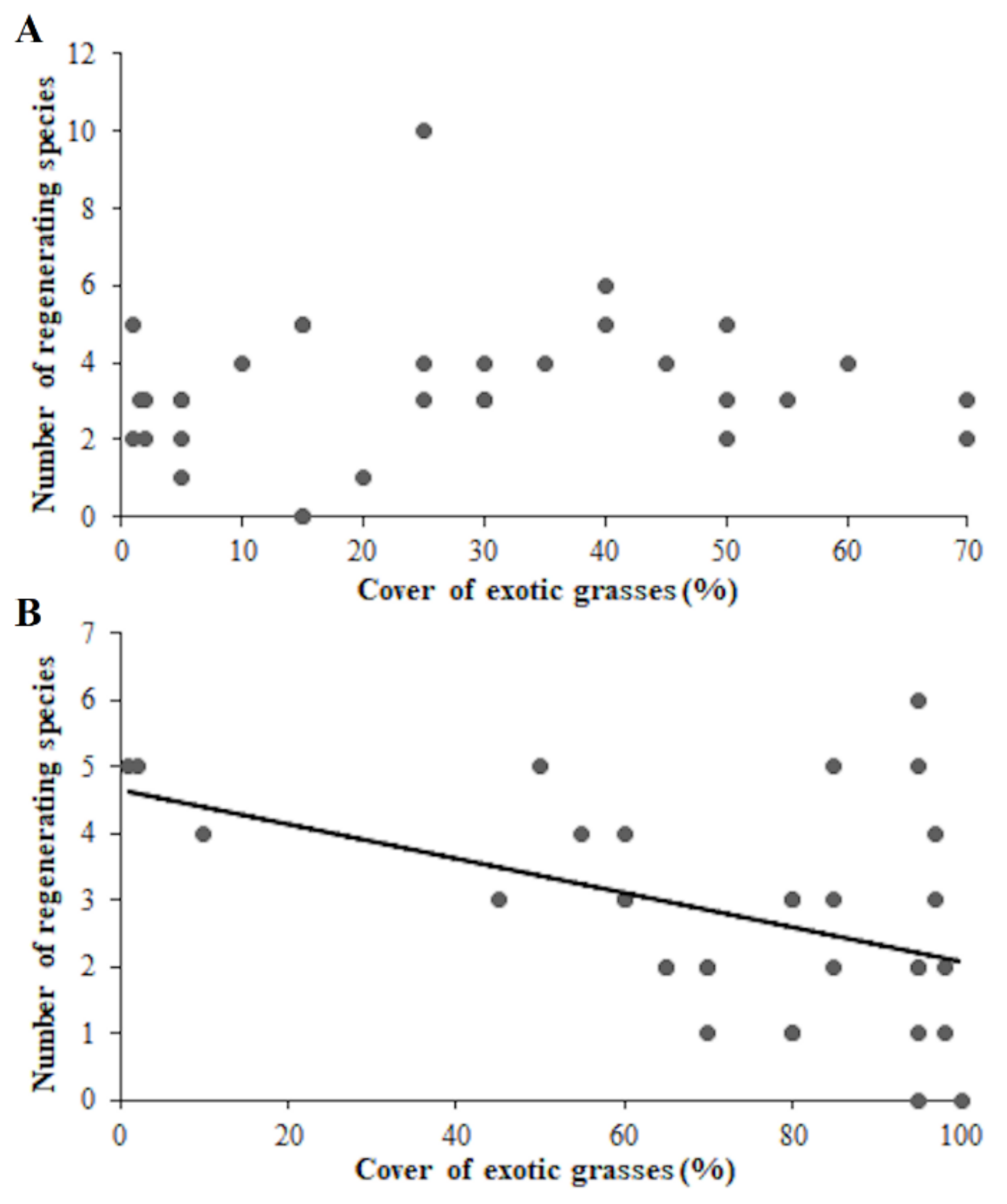
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Fundação SOS Mata Atlântica; INPE. Atlas Dos Remanescentes Florestais da Mata Atlântica, Período 2015–2016; Fundação SOS Mata Atlântica e Inpe: São Paulo, Brazil, 2017. [Google Scholar]
- Dean, W. A Ferro e Fogo: A História e a Devastação da Mata Atlântica Brasileira; Companhia das Letras: São Paulo, Brazil, 1996. [Google Scholar]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Silva, R.F.B.D.; Batistella, M.; Moran, E.F.; Lu, D. Land changes fostering Atlantic Forest transition in Brazil: Evidences from the Paraíba Valley. Prof. Geographer. 2017, 69, 80–93. [Google Scholar] [CrossRef]
- Brancalion, P.H.S.; Niamir, A.; Broadbent, E.; Crouzeilles, R.; Barros, F.S.M.; Zambrano, A.M.A.; Baccini, A.; Aronson, J.; Goetz, S.; Reid, J.L.; et al. Global restoration opportunities in tropical rainforest landscapes. Sci. Adv. 2019, 5, eaav3223. [Google Scholar] [CrossRef] [PubMed]
- WRI (World Resources Institute). Restoration Commitments. Initiative 20 × 20. 2018. Available online: https://www.wri.org/our-work/project/initiative-20x20/ restoration-commitments (accessed on 11 July 2019).
- Brasil. Lei no 12.651 of 2012b. Available online: http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/L12651compilado.htm (accessed on 10 March 2019).
- Brasil. Decreto no 7.830 of 2012a. Available online: http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/decreto/d7830.htm (accessed on 10 March 2019).
- Brasil. Decreto no 8.972 of 2017. Available online: http://www.planalto.gov.br/ccivil_03/_ato2015-2018/2017/decreto/D8972.htm (accessed on 10 March 2019).
- Soares-Filho, B.; Rajão, R.; Macedo, M.; Carneiro, A.; Costa, W.; Coe, M.; Rodrigues, H.; Alencar, A. Cracking Brazil’s Forest Code. Science 2014, 344, 363–364. [Google Scholar] [CrossRef]
- Pinto, S.; Melo, F.; Tabarelli, M.; Padovesi, A.; Mesquita, A.; Scaramuzza, C.A.; Castro, P.; Carrascosa, H.; Calmon, M.; Rodrigues, R.R.; et al. Governing and delivering a biome-wide restoration initiative: The case of Atlantic Forest Restoration Pact in Brazil. Forests 2014, 5, 2212–2229. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Leitão-Filho, H.F. Matas Ciliares: Conservação e Recuperação; Edusp: São Paulo, Brazil, 2004. [Google Scholar]
- Morrison, E.B.; Lindell, C.A. Active or Passive Forest Restoration? Assessing Restoration Alternatives with Avian Foraging Behavior. Restor. Ecol. 2010, 19, 170–177. [Google Scholar] [CrossRef]
- Norden, N.; Chazdon, R.L.; Chao, A.; Jiang, Y.J.; Vilchez-Alvarado, B. Resilience of tropical rain forests: Tree community reassem- bly in secondary forests. Ecol. Lett. 2009, 12, 385–394. [Google Scholar] [CrossRef]
- Gómez-Pompa, A.; Vazquez-Yanes, C.; Guevara, S. The Tropical Rain Forest: A Nonrenewable Resource. Science 1972, 177, 762–765. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Aide, T.M. Restoration success: How is it being measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of Degraded Tropical Forest Landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Brancalion, P.H.S.; Schweizer, D.; Gaudare, U.; Mangueira, J.R.; Lamonato, F.; Farah, F.T.; Nave, A.G.; Rodrigues, R.R. Balancing economic costs and ecological outcomes of passive and active restoration in agricultural landscapes: The case of Brazil. Biotropica 2016, 48, 856–867. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Brancalion, P.H.S.; Isernhagen, I. Pacto Para a Restauração Ecológica da Mata Atlântica: Referencial dos Conceitos e Ações de Restauração Florestal; Instituto BioAtlântica: São Paulo, Brazil, 2009. [Google Scholar]
- Souza, F.M.; Batista, J.L.F. Restoration of seasonal semideciduous forests in Brazil: Influence of age and restoration design on forest structure. For. Ecol. Manag. 2004, 191, 185–200. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Lima, R.A.; Gandolfi, S.; Nave, A.G. On the restoration of high diversity forests: 30 years of experience in the Brazilian Atlantic Forest. Biol. Conserv. 2009, 142, 1242–1251. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Gandolfi, S.; Nave, A.G.; Aronson, J.; Barreto, T.E.; Vidal, C.Y.; Brancalion, P.H. Large-scale ecological restoration of high-diversity tropical forests in SE Brazil. For. Ecol. Manag. 2011, 261, 1605–1613. [Google Scholar] [CrossRef]
- Garcia, L.C.; Hobbs, R.J.; Ribeiro, D.B.; Tamashiro, J.Y.; Santos, F.A.M.; Rodriues, R.R. Restoration over time: Is it possible to restore trees and non-trees in high-diversity for- ests? Appl. Veg. Sci. 2016, 19, 655–666. [Google Scholar] [CrossRef]
- Chaves, R.B.; Durigan, G.; Brancalion, P.H.S.; Aronson, J. On the need of legal frameworks for assessing restoration projects success: New perspectives from So Paulo state (Brazil). Restor. Ecol. 2015, 23, 754–759. [Google Scholar] [CrossRef]
- Pontes, D.M.F.; Engel, V.L.; Parrotta, J.A. Forest Structure, Wood Standing Stock, and Tree Biomass in Diferent Restoration Systems in the Brazilian Atlantic Forest. Forests 2019, 10, 588. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Lima, R.A.F.; Gandolfi, S.; Brancalion, P.H.S. Restauração Florestal; Oficina de Textos: São Paulo, Brazil, 2015. [Google Scholar]
- Melo, F.P.; Tabarelli, M.; Rodrigues, R.R.; Brancalion, P.H.S. Biodiversity Persistence in Highly Human-Modified Tropical Landscapes Depends on Ecological Restoration. Trop. Conserv. Sci. 2013, 6, 705–710. [Google Scholar]
- Brancalion, P.H.S.; Viani, R.A.G.; Calmon, M.; Carrascosa, H.; Rodrigues, R.R. How to Organize a Large-Scale Ecological Restoration Program? The Framework Developed by the Atlantic Forest Restoration Pact in Brazil. J. Sustain. For. 2013, 32, 728–744. [Google Scholar] [CrossRef]
- Viani, R.A.G.; Barreto, T.E.; Farah, F.T.; Rodrigues, R.R.; Brancalion, P.H.S. Monitoring Young Tropical Forest Restoration Sites: How Much to Measure? Trop. Conserv. Sci. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Viani, R.A.G.; Holl, K.D.; Padovezi, A.; Strassburg, B.B.N.; Farah, F.T.; Garcia, L.C.; Chaves, R.B.; Rodrigues, R.R.; Brancalion, P.H.S. Protocol for Monitoring Tropical Forest Restoration: Perspectives from the Atlantic Forest Restoration Pact in Brazil. Trop. Conserv. Sci. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Suganuma, M.S.; Durigan, G. Indicators of restoration success in riparian tropical forests using multiple reference ecosystems. Restor. Ecol. 2015, 23, 238–251. [Google Scholar] [CrossRef]
- Mantoani, M.C.; Torezan, J.M.D. Regeneration response of Brazilian Atlantic Forest woody species to four years of Megathyrsus maximus removal. For. Ecol. Manag. 2016, 359, 141–146. [Google Scholar] [CrossRef]
- Embrapa (Empresa Brasileira de Pesquisa Agropecuária). Súmula da X Reunião Técnica de Levantamento de Solos; Serviço Nacional de Levantamento e Conservação dos Solos: Rio de Janeiro, Brazil, 1979. [Google Scholar]
- Brasil Ministério da Agricultura. Levantamento de Reconhecimento dos Solos do Estado de São Paulo; Serviço Nacional de Pesquisas Agronomicas: Rio de Janeiro, Brazil, 1960.
- Köppen, W. Climatologia: Con un Estudio de los Climas de la Tierra; Fundo de Cultura Econômica: Mexico City, Mexico, 1948. [Google Scholar]
- Urbanetz, C.; Tamashiro, J.Y.; Kinoshita, L.S. Chave de identificação de espécies lenhosas de um trecho de Floresta Ombrófila Densa Atlântica, no Sudeste do Brasil, baseada em caracteres vegetativos. Biota Neotropica 2010, 10, 349–398. [Google Scholar] [CrossRef]
- Eltink, M.; Ramos, E.; Torres, R.B.; Tamashiro, J.Y.; Galembeck, E.; Kimura, E. Chave de identificação de espécies do estrato arbóreo da Mata Atlântica em Ubatuba (SP), com base em caracteres vegetativos. Biota Neotropica 2011, 11, 393–405. [Google Scholar] [CrossRef]
- APG IV (Angiosperm Phylogeny Group IV). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Flora do Brasil 2020 em construção. Jardim Botânico do Rio de Janeiro. Available online: http://www.floradobrasil.jbrj.gov.br (accessed on 03 March 2019).
- São Paulo (Estado). Listagem das Espécies Arbóreas e Indicação de sua Ocorrência Natural nos Biomas/Ecossistemas e Regiões Ecológicas do Estado de São Paulo, com a Classificação Sucessional e a Categoria de Ameaça de Extinção; Secretaria do Meio Ambiente: São Paulo, Brazil, 2008.
- Barbosa, L.M.; Shirasuna, R.T.; Lima, F.C.; De Ortiz, P.R.T. Lista de Espécies Indicadas Para Restauração Ecológica Para Diversas Regiões do Estado de São Paulo. Instituto de Botânica: São Paulo; Instituto de Botânica: São Paulo, Brazil, 2016. Available online: http://botanica.sp.gov.br/files/2016/01/Lista_de_especies_de_SP_CERAD-IBTSMA_2015.pdf (accessed on 10 March 2019).
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 03 March 2019).
- Lima, H.C.; Guedes-Bruni, R.R. Serra de Macaé de Cima: Diversidade florística e conservação em Mata Atlântica; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Marques, M.C.M.; Silva, S.M.; Liebsch, D. Coastal plain forests in southern and southeastern Brazil: Ecological drivers, floristic patterns and conservation status. Braz. J. Bot. 2015, 38, 1–18. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Machado, J.N.M. Composição florística de uma floresta semidecídua montana, na Serra de São José, Tiradentes, MG. Acta Bot. Bras. 1993, 7, 71–88. [Google Scholar] [CrossRef]
- Sartori, R.A.; Carvalho, D.A.; Berg, E.V.D.; Marques, J.J.; Dos Santos, R.M. Variações florísticas e estruturais do componente arbóreo de uma floresta estacional semidecidual montana em Socorro, SP. Rodriguésia 2015, 66, 33–49. [Google Scholar] [CrossRef]
- Stopes, C.; Millington, S.; Woodward, L. Dry matter and nitrogen accumulation by three leguminous green manure species and the yield of a following wheat crop in an organic production system. Agric. Ecosyst. Environ. 1996, 57, 189–196. [Google Scholar] [CrossRef]
- Sultani, M.I.; Gill, M.A.; Anwar, M.M.; Athar, M. Evaluation of soil physical properties as influenced by various green manuring legumes and phosphorus fertilization under rain fed conditions. Int. J. Environ. Sci. Technol. 2007, 4, 109–118. [Google Scholar] [CrossRef][Green Version]
- Engel, V.L.; Parrotta, J.A.; Lamb, D.; Nardoto, G.B.; Ometto, J.P.H.B.; Martinelli, L.A.; Schmidt, S.; Siddique, I.; Siddique, I. Dominance of legume trees alters nutrient relations in mixed species forest restoration plantings within seven years. Biogeochemistry 2008, 88, 89–101. [Google Scholar]
- Howe, H.F.; Smallwood, J. Ecology of Seed Dispersal. Annu. Rev. Ecol. Evol. S 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Da Silva, F.R.; Montoya, D.; Furtado, R.; Memmott, J.; Pizo, M.A.; Rodrigues, R.R. The restoration of tropical seed dispersal networks. Restor. Ecol. 2015, 23, 852–860. [Google Scholar] [CrossRef]
- Carson, W.P.; Schnitzer, S.A. Tropical Forest Community Ecology; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Janzen, D.H. Synchronization of sexual reproduction of trees within the dry season in central America. Evolution 1967, 21, 620–637. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Ecology of tropical dry forest. Annu. Rev. Ecol. Evol. S 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Derroire, G.; Tigabu, M.; Odén, P.C.; Healey, J.R. The Effects of Established Trees on Woody Regeneration during Secondary Succession in Tropical Dry Forests. Biotropica 2016, 48, 290–300. [Google Scholar] [CrossRef]
- Vieira, D.L.M.; Scariot, A. Principles of Natural Regeneration of Tropical Dry Forests for Restoration. Restor. Ecol. 2006, 14, 11–20. [Google Scholar] [CrossRef]
- Moraes, V.H.F. Periodicidade de crescimento do tronco em árvores da floresta amazônica. Pesqui. Agropecu. Bras. 1970, 5, 315–320. [Google Scholar]
- Melotto, A.; Nicodemo, M.L.; Laura, V.A.; Schleder, D.D.; Pott, A.; Da Silva, V.P.; Bocchese, R.A.; Neto, M.M.G. Sobrevivência e crescimento inicial em campo de espécies florestais nativas do Brasil Central indicadas para sistemas silvipastoris. Revista Árvore 2009, 33, 425–432. [Google Scholar] [CrossRef]
- Bray, E.A. Encyclopedia of Life Sciences; John Wiley & Sons: New York, NY, USA, 2007; pp. 1–7. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth and Yield; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–39. [Google Scholar]
- Shimamoto, C.Y.; Botosso, P.C.; Marques, M.C. How much carbon is sequestered during the restoration of tropical forests? Estimates from tree species in the Brazilian Atlantic forest. For. Ecol. Manag. 2014, 329, 1–9. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [PubMed]
- Campoe, O.C.; Stape, J.L.; Mendes, J.C.T. Can intensive management accelerate the restoration of Brazil’s Atlantic forests? For. Ecol. Manag. 2010, 259, 1808–1814. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Vitousek, P.M. Biological Invasions by Exotic Grasses, the Grass/Fire Cycle, and Global Change. Annu. Rev. Ecol. Syst. 1992, 23, 63–87. [Google Scholar] [CrossRef]
- Leger, E.A.; Espeland, E.K. Coevolution between native and invasive plant competitors: Implications for invasive species management. Evol. Appl. 2010, 3, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ferez, A.P.C.; Campoe, O.C.; Mendes, J.C.T.; Stape, J.L. Silvicultural opportunities for increasing carbon stock in restoration of Atlantic forests in Brazil. For. Ecol. Manag. 2015, 350, 40–45. [Google Scholar] [CrossRef]
- Elgar, A.T.; Freebody, K.; Pohlman, C.L.; Shoo, L.P.; Catterall, C.P. Overcoming barriers to seedling regeneration during forest restoration on tropical pasture land and the potential value of woody weeds. Front. Plant Sci. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Brancalion, P.H.S.; Campoe, O.; Mendes, J.C.T.; Noel, C.; Moreira, G.G.; Van Melis, J.; Stape, J.L.; Guillemot, J. Intensive silviculture enhances biomass accumulation and tree diversity recovery in tropical forest restoration. Ecol. Appl. 2019, 29, e01847. [Google Scholar] [CrossRef]
| Family | Species | N | EG | SD | LD |
|---|---|---|---|---|---|
| Anacardiaceae | Schinus molle L. | 1 | P | ZOO | E |
| Schinus terebinthifolia Raddi | 4 | P | ZOO | E | |
| Bignoniaceae | Handroanthus chrysotrichus (Mart. ex D.C.) Mattos | 3 | NP | ANE | D |
| Handroanthus impetiginosus (Mart. ex D.C). Mattos | 2 | NP | ANE | D | |
| Handroanthus sp. | 1 | - | ANE | - | |
| Handroanthus umbellatus (Sond.) Mattos | 1 | NP | ANE | D | |
| Connaraceae | Connarus regnellii G. Schellenb. | 2 | NP | ZOO | E |
| Euphorbiaceae | Croton sp. | 1 | - | AUTO | - |
| Fabaceae | Anadenanthera colubrina (Vell.) Brenan | 8 | NP | AUTO | D |
| Dalbergia nigra (Vell.) Allemão ex Benth. | 2 | P | AUTO | D | |
| Inga laurina (Sw.) Willd. | 1 | NP | ZOO | E | |
| Inga marginata Willd. | 1 | NP | ZOO | S | |
| Inga sp. | 7 | - | ZOO | - | |
| Inga vera Willd. subsp. affinis (DC.) T. D. Penn. | 15 | P | ZOO | S | |
| Piptadenia gonoacantha (Mart.) J.F. Macbr. | 5 | P | AUTO | S | |
| Poincianella pluviosa (DC.) L.P. Queiroz | 1 | NP | AUTO | S | |
| Schizolobium parahyba (Vell.) Blake | 10 | P | AUTO | D | |
| Lauraceae | Ocotea sp. | 1 | - | ZOO | - |
| Malpighiaceae | Byrsonima crassifolia (L.) Kunth | 1 | P | ZOO | S |
| Malvaceae | Ceiba speciosa (A.St.-Hil.) Ravenna | 1 | NP | ANE | D |
| Melastomataceae | Tibouchina granulosa (Desr.) Cogn. | 4 | P | ANE | S |
| Myrtaceae | Campomanesia guazumifolia (Cambess.) O. Berg | 1 | P | ZOO | D |
| Eugenia stipitata Mc Vaugh | 1 | NP | ZOO | E | |
| Plinia edulis (Vell.) Sobral | 4 | NP | ZOO | E | |
| Psidium guajava L. | 6 | NP | ZOO | S | |
| Psidium guineense Sw. | 2 | NP | ZOO | E | |
| Primulaceae | Myrsine sp. | 1 | - | ZOO | - |
| Sapindaceae | Cupania vernalis Cambess. | 1 | NP | ZOO | S |
| Urticaceae | Cecropia hololeuca Miq. | 13 | P | ZOO | E |
| Verbenaceae | Citharexylum myrianthum Cham. | 10 | P | ZOO | D |
| Unknown | Unknown 1 | 1 | - | - | - |
| Unknown 2 | 1 | - | - | - | |
| Unknown 3 | 1 | - | - | - | |
| Unknown 4 | 1 | - | - | - | |
| Unknown 5 | 4 | - | - | - | |
| 119 |
| Interval | Parameters | Legumes | Non-Legumes | p-Value | Test |
|---|---|---|---|---|---|
| S1–S2 | DGL (cm) | 1.6 (0.8; 2.5) | 1.1 (0.5; 1.6) | 0.005 | W = 1780.5 |
| H (m) | 0.6 (0.4; 1.0) | 0.4 (0.2; 0.8) | 0.024 | W = 1695.5 | |
| CD (m) | 0.6 (0.1; 0.9) | 0.2 (0; 0.5) | 0.028 | W = 1685.5 | |
| S2–S3 | DGL (cm) | 0.4 (0; 1.0) | 0.3 (0; 0.6) | 0.421 | W = 1416.0 |
| H (m) | 0.1 (0; 0.4) | 0.1 (0; 0.4) | 0.321 | W = 1147.5 | |
| CD (m) | 0.1 (0; 0.7) | 0 (0; 0.1) | 0.122 | W = 1527.0 | |
| DGL (cm) | 2.3 (1.4; 3.3) | 1.6 (0.6; 2.3) | 0.002 | W = 1972.5 | |
| S1–S3 | H (m) | 0.8 (0.4; 1.1) | 0.6 (0. 3;1) | 0.178 | W = 1697.0 |
| CD (m) | 0.7 (0.4; 1.2) | 0.3 (0; 0.5) | <0.001 | W = 2114.0 |
| Interval | Parameters | Pioneers | Non-Pioneers | p-Value | Test |
|---|---|---|---|---|---|
| S1–S2 | DGL (cm) | 1.4 (0.8; 2.2) | 1.2 (0.6; 1.8) | 0.206 | W = 846.5 |
| H (m) | 0.7 (0.3; 1) | 0.5 (0.2; 0.7) | 0.015 | W = 698.0 | |
| CD (m) | 0.4 (0; 0.7) | 0.2 (0; 0.9) | 0.843 | W = 981.0 | |
| S2–S3 | DGL (cm) | 0.5 (0; 0.9) | 0.3 (0; 0.6) | 0.481 | W = 887.0 |
| H (m) | 0.2 (0; 0.4) | 0.1 (0; 0.4) | 0.295 | W = 1102.5 | |
| CD (m) | 0 (0; 0.5) | 0 (0; 0.2) | 0.435 | W = 877.0 | |
| DGL (cm) | 1.9 (1.3; 2.9) | 1.6 (0.6; 2.7) | 0.107 | W = 871.5 | |
| S1–S3 | H (m) | 0.8 (0.4; 1.2) | 0.6 (0.4; 1) | 0.433 | W = 982.5 |
| CD (m) | 0.5 (0.1; 0.9) | 0.4 (0; 0.6) | 0.238 | W = 929.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, N.V.; Ferreira, C.C.; Dzedzej, M.; Massi, K.G. Monitoring of a Seedling Planting Restoration in a Permanent Preservation Area of the Southeast Atlantic Forest Biome, Brazil. Forests 2019, 10, 768. https://doi.org/10.3390/f10090768
Fiore NV, Ferreira CC, Dzedzej M, Massi KG. Monitoring of a Seedling Planting Restoration in a Permanent Preservation Area of the Southeast Atlantic Forest Biome, Brazil. Forests. 2019; 10(9):768. https://doi.org/10.3390/f10090768
Chicago/Turabian StyleFiore, Nathalia V., Carolina C. Ferreira, Maíra Dzedzej, and Klécia G. Massi. 2019. "Monitoring of a Seedling Planting Restoration in a Permanent Preservation Area of the Southeast Atlantic Forest Biome, Brazil" Forests 10, no. 9: 768. https://doi.org/10.3390/f10090768
APA StyleFiore, N. V., Ferreira, C. C., Dzedzej, M., & Massi, K. G. (2019). Monitoring of a Seedling Planting Restoration in a Permanent Preservation Area of the Southeast Atlantic Forest Biome, Brazil. Forests, 10(9), 768. https://doi.org/10.3390/f10090768





