Abstract
During the 1980s, reforestation programs using exotic species (Pinus spp.) were established in the páramo ecosystem of Ecuador. The aims of this study were: (1) to compare the natural regeneration between pine plantations (Pi) and natural grassland (NG) across an elevational gradient and (2) to identify the attributes of Pi and soil properties that were influencing herbaceous and woody plant composition and their plant cover. In total, six independent Pinus patula (Schltdl. & Cham. plantations (two per each elevation) were selected and distributed in an elevational range (3200–3400, 3400–3600, 3600–3800 m a.s.l.). Adjacent to Pi, plots in NG were established for recording natural regeneration. Both, namely the attributes and the soil samples, were measured in Pi. The results showed that natural regeneration differs significantly between both types of vegetation. As expected, NG holds more plant diversity than Pi; the elevational range showed a clear tendency that there was more herbaceous richness when elevation range increases, while the opposite was found for woody species. Moreover, attributes of Pi influenced herbaceous and woody vegetation, when saturated hydraulic conductivity (Ksat) in the soil, basal area (BA) and canopy density (CD) increased, herbaceous species richness and its cover decreseased; and when Ksat and the acidity in the soil increased, woody plants richness and its cover decreased. The plantations have facilitated the establishment of shade tolerant species. More studies are needed to evaluate if removal with adequate management of pine plantations can improve the restoration and conservation of the native vegetation of the páramo ecosystem.
1. Introduction
The Neotropical alpine ecosystem of the “páramo” provides several ecosystem services like water regulation and supply, carbon storage and biodiversity conservation [1,2]. Furthermore, the páramo ecosystem hosts the richest high mountain flora in the world [3], and the fastest average net diversification rates of all ‘hotspots’ or areas featuring exceptional concentrations of endemic species that are experiencing exceptional loss of habitat [4,5]. According to Hofstede et al. [2], 1,524 species of vascular plants have been registered in the páramo of Ecuador, from which approximately 628 are endemic (15% of Ecuadorian endemic plants). This great biodiversity of this ecosystem is related to the diversity of the ecological conditions linked to the glacial geomorphology that has resulted in a large number of different plant associations, each one with their typical species [6].
Elevation is an important factor that shapes plant diversity in the páramo. The elevational gradients combine sets of environmental conditions such as: temperature, wind velocity, atmospheric gas composition, water availability, nutrient deposition and cycling, soil weathering and solar radiation, all of which determine the composition and structure of vegetation [7]. Based on the influence of these factors and vegetation structure, the páramo has been divided into three zones, from lowest to highest: subpáramo, páramo (páramo grassland) and superpáramo [8]. The subpáramo, also called páramo forest, shrubby páramo, subpáramo woodland and subpáramo elfin forest [9], is the transition zone (ecotone) between the forest (upper montane cloud forest) and the páramo grassland [8,9,10,11]. The subpáramo is usually an entangle of shrubs and small dispersed trees, gradually reduced in size, that gives way to grasses and herbs [9]. The páramo vegetation zone, also called grass páramo or páramo grassland, is characterized by tussock grasses dominated by species of Calamagrostis and/or Festuca. Finally, above the páramo, there is the superpáramo, which is the zone located between the páramo and the permanent snow. In some cases, small isolated woodlands of Polylepis could be found above the subpáramo zone [9,10,11].
Unfortunately, human activities can significantly alter páramo biodiversity [12], associated with land use change and climate change, which are promoting loss of native grassland cover [13]. It is estimated that 40% of the original Ecuadorian páramo has been transformed into agroecosystems and that 30% is used for extensive livestock grazing [2]. Livestock has a negative effect on the vegetation structure by making it more open and less tall, and also on its composition by reducing shrubs and endemic plants [14,15]. Cattle raising is usually combined with burning of natural grassland to provide the cattle with fresh and more tender grasses [12,16]. The impacts of burning are a decrease in the productivity of the vegetation and a drastic change in its composition, depending on the frequency and intensity of the fires [2]. Woody species are the least resistant to burning, and the greater frequency and intensity of burning favors the establishment of exotic weed species [17]. Another activity that alters biodiversity is afforestation, which in the last decades has been promoted in the páramos of Ecuador for timber production and carbon sequestration with exotic species such as P. patula and Pinus radiata D. Don. Pine species have been selected because of their fast growth which make them more appreciated by local people also due to the limited forestry knowledge of native species [18,19,20].
In the scientific community, the debate of the impact of afforestation on biodiversity, specifically on the floristic composition due to the conversion of grassland into forest plantations, is still going on [21]. In the region of the study, the impact of these plantations on ecosystem services has generated disputed perceptions among their stakeholders [22], as most of them have been established on non-forest vegetation that alters the hydrology [23,24,25] and soil characteristics [18,19,26,27]. In terms of plant diversity, Ohep and Herrera [28] found that in the páramo of Venezuela not much understory vegetation was growing under dense pine plantations due to the lack of light passing through the canopies. In the highlands of Colombia, Van Wesenbeeck et al. [29] found that species diversity of native vegetation decreased when pine plantations coverage increased. Also, Cavalier and Santos [30] found few species growing under pine plantations because of the accumulation of needles and high biomass of fine roots. Nevertheless, in the páramo of Ecuador, Hofstede et al. [18] observed that in some cases the vegetation growing in some pine plantations was similar to the natural grassland; and Bremer [31] found that in one area, plant species richness was lower in pine plantations than in natural grasslands, but higher in another plantation area that was adjacent to a native forest.
In other regions of the world, there is enough evidence that plantations can provide protective functions and have a nurse effect for the natural forest regeneration by modifying both the physical and biological site conditions [32,33,34]. The importance of nurse plants lies in that they facilitate the growth and development of other plant species, offering a microhabitat with optimal conditions for seed germination and/or seedling recruitment, Ren et al. [34]. Therefore, plantations with exotic species could provide complementary conservation services [35].
Afforestation with pines reduces soil organic matter contents as a result of a faster decomposition due to a lower soil water content [1], however there is a lack of information of how soil properties under pine plantations impact the natural regeneration of both herbaceous and woody species. Several studies have shown changes in soil properties after the establishment of plantations on grasslands [18,19,36,37,38,39]. However, little is known about the effects on herbaceous and woody plant richness and composition. Besides, several authors agree that, in mountain regions, the elevational gradient explains the variation in soil properties [40,41].
Our study addressed the following questions: (1) Are there differences in herbaceous and woody floristic composition in an elevation range (3200–3400, 3400–3600, 3600–3800 m above sea level (a.s.l.) and in different types of vegetation (pine plantation and natural grassland) in the páramo ecosystem of Southern Ecuador? and (2) What are the effects of soil properties and plantation attributes on herbaceous and woody plant composition under pine plantations among different elevational ranges?
2. Materials and methods
The study area is located in the Azuay Province in Southern Ecuador. In total, six pine plantations of Pinus patula were chosen for the study in three different elevational ranges, and two different sites were selected in each of these ranges: La Paz and Nero from 3200 to 3400 m a.s.l., Tutupali Chico and Tutupali Grande from 3400 to 3600 m a.s.l. and Quimsacocha and Soldados from 3600 to 3800 m a.s.l. Additionally, natural grassland sites adjacent to these plantations were also selected for recording natural regeneration information (Figure 1).
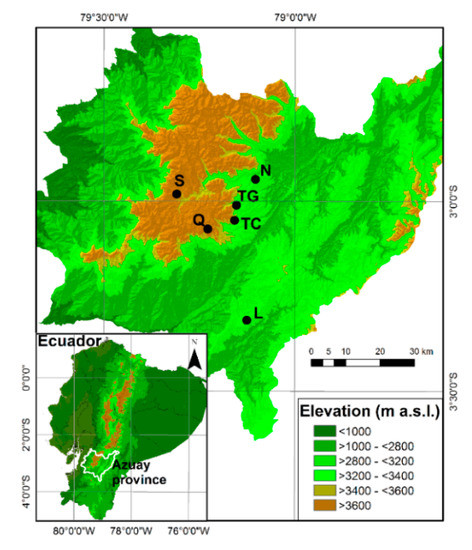
Figure 1.
Location map of the study area showing the sites that correspond to natural grassland and pine plantations in three different elevational ranges: N (Nero) and L (La Paz) from 3200 to 3400 m a.s.l., TC (Tutupali Chico) and TG (Tutupali Grande) from 3400 to 3600 m a.s.l. and Q (Quimsacocha) and S (Soldados) from 3600 to 3800 m a.s.l.
In regard to climate conditions, the páramo ecosystem in the Azuay province is characterized by high differences in temperature during the day and night [9,25]. Rainfall presents a high spatial variability, it is well distributed year round, and seasonality is less pronounced at higher elevations; the mean annual precipitation ranges from 660 to 3400 mm [42]. The high variability depends on the geographic location with a high precipitation increment from west to east influenced by the Pacific regimen and air masses from the Atlantic [43]. Table 1 shows information of meteorological characteristics according to each elevational range in the study area.

Table 1.
Characteristics of pine plantations across the elevational range in the study area. Except for temperature and precipitation, all variables include the median and, between parentheses, the quartiles Q1 and Q3. Bi = pine biomass, TD = tree density, DBH = diameter at breast height, TH = tree height, BA = basal area, CD = canopy density.
In the páramo of Southern Ecuador, soils are classified as Aluandic or Silandic Andosols presenting Hydric and Histic properties with low volcanic glass content [46]. These soils are dark, humid and have excellent water infiltration and retention; a high organic carbon content between 10 and 40%, and water storage capacities could be more than 0.4 cm3/cm3 [47].
2.1. Description of Natural Grassland and Pine Plantations
In general, the natural grassland (NG) is found between 3200 and 3800 m a.s.l. [48], dominated by tussock grasses, mainly Calamagrostis spp. and Festuca spp. A great diversity of herbs, sub-shrubs and shrubs grows under or between the tussocks. The presence of woody species was very low above 3600 m a.s.l. The only forest able to grow at such high elevation is the one formed by Polylepis spp. However, in our study area, we did not include this genus because they form specific patches mostly in concave sites in very protected places and distant from the pine plantations. We identified six NG sites situated near each plantation site.
The plantations of the study have been established for the purpose of timber production (its wood is used in plywood, chopsticks, and in the form of densified wood). Five of the plantations are part of a program of carbon sequestration through afforestation. Because the growth of P. patula in the highlands decreases at 25, harvesting is generally done between 20 and 25 years. The selected plantations were between 16 and 22 years old (in 2015) according to personal communication with the landowners. Most of the plantations were established on grazed páramo, all of them are first rotation with 3 × 3 m spacing, and they have been protected from grazing since their establishment. At each elevational range, the average biomass of the pines varied, showing a clear tendency of decreasing biomass with increasing elevation. Table 1 shows the characteristics of the pine plantations distributed in the elevational range.
2.2. Experimental Design and Data Collection
Fieldwork was carried out from July to November 2015. For recording natural regeneration in both types of vegetation (Pi and NG), 20 independent plots of 576 m2 (24 × 24 m) were randomly located and established in each elevation range (total 60 plots for herbaceous and 40 plots for woody plants). In each plot, subplots were established to record different types of understory vegetation: (i) two subplots of 100 m2 (10 × 10 m) located in each corner of the diagonal of the plot, each for woody species including non-prostrate shrubs, treelet and trees only; (ii) three subplots of 25 m2 (5 × 5 m) located in each corner and in the center of the diagonal of the plot, each for herbaceous species including prostrate shrubs-sub shrubs and vines. The subplot size of 25 m2 was based on the method used by Sklenar and Ramsay [49]. For the purposes of our study, we did not differentiate the type of natural regeneration (from self-sown seed, coppice shoots or root suckers).
In our study area above 3600 m a.s.l., woody plant composition was not registered because of the low abundance of this type of vegetation. Additionally, cover vegetation for all species was estimated using the Braun-Blanquet scale [50], (r = 0.01%, + = 0.1%, 1 = 1–5%, 2 = 5–25%, 3 = 25–50%, 4 = 50–75%, 5 = 75–100%) subsequently converted into percentage coverage for the respective analysis using their midpoint values. The plant identification was done at species level, but in some cases it was only possible to identify plants at the genus or family level.
In each plot of 24 × 24 m at Pi, five points were selected (four in the corners and one in the center) for measuring canopy density (CD) using a convex spherical densitometer [51]. The average of all the points per plot was calculated for the respective data analysis. The basal area (BA) was calculated based on all tree measurements using diameter at breast height (DBH) and the average of data per plot was calculated. The slope and the aspect were measured from the center of the plot using a Suunto compass. In order to avoid the influence of the slope aspect on the analysis, 90% of the plots were located facing East.
2.3. Soil Sampling
In Pi, the soil sampling was carried out between 0–10 cm of depth in three different subplots located randomly in each plot of 24 × 24 m. In each subplot, the soil samples were taken at a distance of 75 cm from the tree, one sample of 1 kg of disturbed soil and two samples with rings of 100 cm3, each of undisturbed soil, were taken. The disturbed sample was used for analyzing the chemical properties of the soil, and the undisturbed samples were used for analyzing the physical properties.
Additionally, saturated hydraulic conductivity was determined in the field using three replicates through inversed auger-hole method [52]. All samples for physical analysis were carried to the soil laboratory at the University of Cuenca, and for chemical analysis to the soil laboratory of the Institute of Silviculture at the Technical University of Munich, Germany.
2.4. Soil Analysis
The disturbed soil samples were air-dried at room temperature and passed through a 2-mm sieve. The carbon-nitrogen ratio was calculated by determining the organic carbon and nitrogen with the wet combustion method using an elemental analyzer (Vario EL III, Elementar Analysensysteme, Hanau, Germany). The pH was analyzed using a potentiometer with a soil-water ratio of 1:2.5. The undisturbed soil samples were used to determine water content at saturation point (StC) (pressure 1 cm H2O) and water content at field capacity (FC) (pressure 330 cm H2O) through pressure chambers. To determine the wilting point (WP), a saturated soil paste was made with disturbed soil, and later placed in a high pressure chamber at 15,300 cm H2O [53]. The gravitational water (GW) was obtained as the difference between water content at saturation point and water content at field capacity, while the water availability (AW) was obtained as the difference between water content at field capacity and wilting point. Bulk density (BD) was determined with dried undisturbed samples at 105 °C for 24 h.
2.5. Data Analysis
In order to detect the effects of elevational range and type of vegetation on species richness and plant cover of herbaceous and woody species, a linear mixed model (LMM) was carried out. We used as fixed factors, the elevational range and type of vegetation, and as random factor, each site nested within the elevation. This model was selected based on previous running models with different combinations of fixed and random factors. Therefore, the best model with goodness of fit was chosen according to information criteria such as the widely used Akaike Information Criteria (AIC) and the Bayesian Information Criteria (BIC). This analysis was performed using R package nlme [54].
For evaluating the composition and floristic assembly of plant communities, rank species abundance curves were used. In both Pi and NG at each elevational range, the abundance value of each species was calculated at plot level using the average of the plant cover among subplots.
A Canonical Correspondence Analysis (CCA) was performed to evaluate the relationship between the attributes of Pi and soil properties (physical and chemical) and herbaceous and woody species richness and their cover, in three different elevational ranges. Box-Cox transformations were used due to the lack of normality according to the Shapiro test (p < 0.05). For this analysis, the vegan package [55] from R software was used. All statistical analyses were executed in the R Project program version 3.2.3 [56].
3. Results
3.1. Effects of Elevational Range and Type of Vegetation on Hebaceous and Woody Vegetation
Herbaceous vegetation: The results showed a clear tendency that species richness increases with elevational range (Table 2, Figure 2a) (p < 0.0001). As expected, NG had more species richness than Pi cover, showing a high statistical significance for both factors (elevation range and type of vegetation) (p < 0.0001) (Table 2, Figure 2a). However, the interaction of both factors did not show a high statistical significance (p = 0.2304), indicating that their combination did not contribute to the performance of natural regeneration. The percentage of plant cover differed significantly among the three elevational ranges (p < 0.0001) (Table 2), with a marked difference between 3200–3400 and 3400–3600 m a.s.l, and between NG and Pi (Figure 2b) which was highly significant (p < 0.0001). However, herbaceous vegetation cover under NG was reduced in the highest elevational range compared to the mid elevational range and it was similar to the herbaceous vegetation cover under Pi (Figure 2b). A list of herbaceous species is presented in Appendix A.

Table 2.
Influence of elevational range and type of vegetation on species richness and percentage of plant cover of herbaceous vegetation according to the ANOVA analysis obtained from the linear mixed model (LMM).
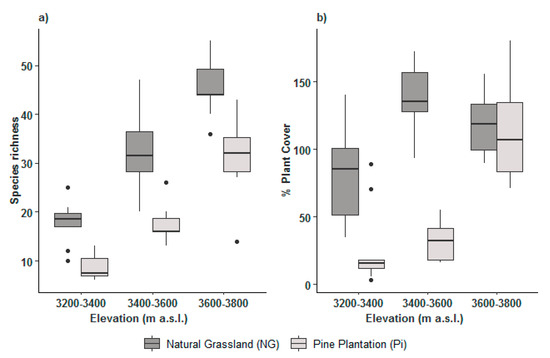
Figure 2.
Box plots for the effects of elevational range (3200–3400, 3400–3600, and 3600–3800 m a.s.l.) and vegetation (Pi, NG) on (a) herbaceous species richness and (b) percentage of herbaceous vegetation cover.
Woody vegetation: In contrast to the herbaceous vegetation, woody species richness and their plant cover had the tendency to decrease with elevational range (the effect was not statistically significant, p > 0.05) (Table 3, Figure 3a,b); however, the interaction between elevational range and type of vegetation for species richness and plant cover was statistically significant (p < 0.005) (Table 3), indicating that the interaction of both factors plays an important role on evaluating the variables of species richness and plant cover. Besides species richness and plant cover were also higher at NG than Pi, showing high statistical significance (p < 0.001, Figure 3a,b). Appendix A presents a list of woody species.

Table 3.
Influence of elevational range and type of vegetation on species richness and plant cover of woody vegetation according to an ANOVA analysis obtained from the linear mixed model (LMM).
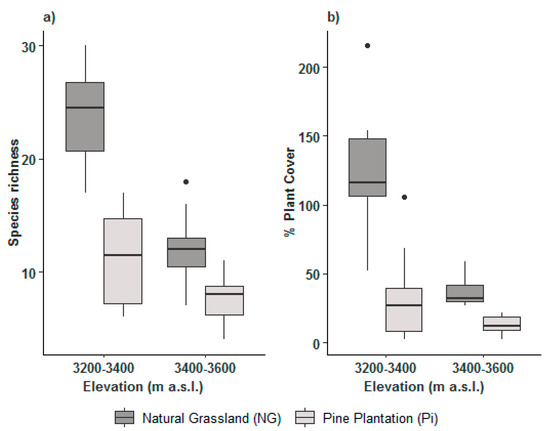
Figure 3.
Box plots for the effects of elevational range (3200–3400 and 3400–3600 m a.s.l.) and type of vegetation (Pi and NG) on (a) woody species richness and (b) woody plant cover.
Endemic species: In total, thirteen endemic species were recorded in our observational plots, eight species under Pi cover and eleven species in the NG cover across all elevational ranges. From the endemic species registered eleven species are included in the International union for Conservation of Nature and Natural Resources (IUCN) Red List of Threatened Species [57]. Five species occurred exclusively in NG, from which Lysipomia vitreola McVaugh [58] and Brachyotum jamesonii Triana [59] are considered an endangered and a vulnerable species respectively; and Gynoxys miniphylla Cuatrec [60] and Miconia pernettifolia Triana [61] found only under Pi sites are considered vulnerable species according to the IUCN (Table 4).

Table 4.
List of endemic species with their percentage of occurrence in the plots at natural grassland (NG) and pine plantation (Pi) sites in three different elevational ranges in m a.s.l. (Total 30 plots for herbaceous plants for each vegetation cover, and 20 plots for woody plants for each vegetation cover). Lf = life form, Cs = conservation status according to the IUCN Red List of Threatened Species [57], H = herbaceous plant, W = woody plant. LC = least concern, NT = near threatened, VU = vulnerable, Ni = not included in the Red List, EN = endangered.
3.2. Vegetation Assemblages along Elevational Ranges and Type of Vegetation Cover
Herbaceous vegetation: According to rank-abundance curves, a marked difference of dominant species was found between NG and Pi, mainly at the lower and middle elevational ranges; all three dominant species do not coincide in both type of vegetation. For instance, at 3200–3400 m a.s.l. under NG Calamagrostis intermedia (J. Presl) Steud, Austrolycopodium magellanicum (P. Beauv) Holub, and Paspalum bonplandianum Flüggé had the highest abundance (Figure 4a), while under Pi it was Triniochloa stipoides (Kunth) Hitchc, Peperomia sp, and Pecluna sp. (Figure 4b). At 3400–3600 m a.s.l, C. intermedia, Festuca subulifolia Benth., and Polystichum orbiculatum (Desv) (Figure 4c), were the dominant species, while in Pi, there were Cerastium danguyi J.F. Macbr. and Muehlenbeckia tamnifolia (Kunth) Meisn. (Figure 4d). At 3600–3800 m a.s.l., the species, C. intermedia and F. subulifolia were presented in both types of vegetation (Figure 4e,f), while Lachemilla orbiculata (Ruiz & Pav.) Rydb. was observed with high dominance only under Pi (Figure 4f). Interestingly, C. intermedia was the dominant species present in all three elevational ranges at NG (Figure 4a,c,e).
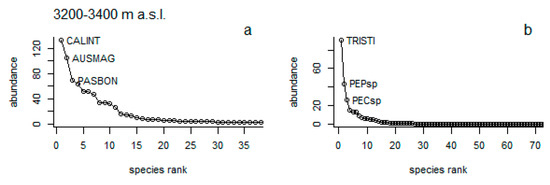
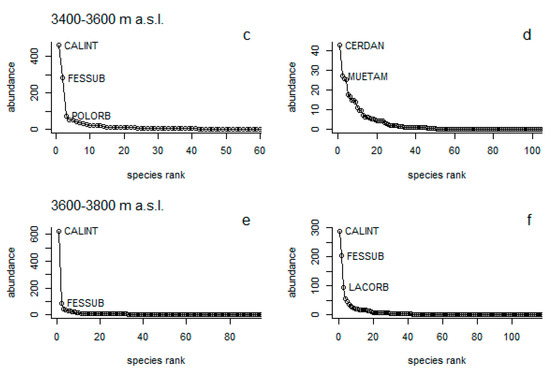
Figure 4.
Herbaceous species abundance rank at natural grassland (NG) cover (a,c,e) and pine plantations (Pi) cover (b,d,f) across three different elevational gradients (3200–3400, 3400–3600, and 3600–3800 m a.s.l.).CALINT = Calamagrostis intermedia, AUSMAG = Austrolycopodium magellanicum, PASBON = Paspalum bonplandianum, TRISTI = Triniochloa stipoides, PEPsp = Peperomia sp, PECsp = Pecluna sp, FESSUB = Festuca subulifoli, POLORB = Polystichum orbiculatum, CERDAN = Cerastium danguyi, MUETAM = Muehlenbeckia tamnifolia, LACORB = Lachemilla orbiculate.
Woody vegetation: The results showed that within the lower elevational range, species such as Morella parvifolia (Benth.) Parra-Os. and Myrsine dependens (Ruiz & Pav.) Spreng. were dominant under NG (Figure 5a), while Miconia theaezans (Bonpl.) Cogn. and M. dependens, dominated in Pi (Figure 5b). In the higher elevational range, these species were not present in both types of vegetation cover. Here, the dominant species were Valeriana hirtella Kunth and M. parvifolia in the NG (Figure 5c), and Miconia crocea (Desr.) Naudin and Gynoxys sp. under Pi (Figure 5d).
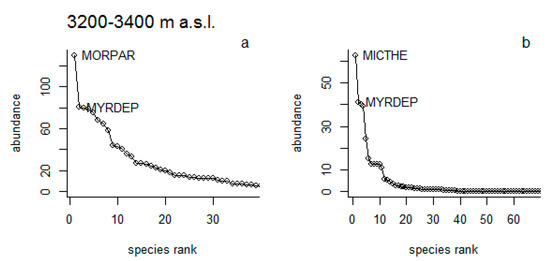
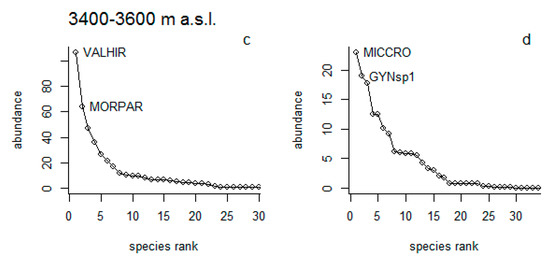
Figure 5.
Woody species abundance rank at natural grassland (NG) cover (a,c) and pine plantation (Pi) cover (b,d) across three different elevational gradients (3200–3400 and 3400–3600 m a.s.l.). MORPAR = Morella parvifolia, MYRDEP = Myrsine dependens, MICTHE = Miconia theaezans, VALHIR = Valeriana hirtella, MICCRO = Miconia crocea, GYNsp1= Gynoxys sp.
3.3. Relationship between Herbaceous Species Richness and Its Vegetation Cover with Edaphic Properties and Attributes of Plantations
Herbaceous vegetation: In the CCA 40.89% of the variance was explained in the two axes. In the CCA1, the variables related to the attributes of Pi and soil characteristics with highest contribution were elevation (Ele), basal area (BA), saturated hydraulic conductivity (Ksat) and canopy density (CD), while in CCA2 pH was the variable with the highest contribution (Figure 6). According to CCA, herbaceous species richness and its cover showed that Ele was positively correlated (p < 0.001); therefore, herbaceous species richness increased with higher elevation. Moreover, there was a negative correlation between the herbaceous species richness and its cover with CD (p < 0.001), BA (p < 0.001) and Ksat (p < 0.001). On the other hand, the herbaceous species richness was lower in those plots where the pH was more acid (p < 0.001) (Figure 6).
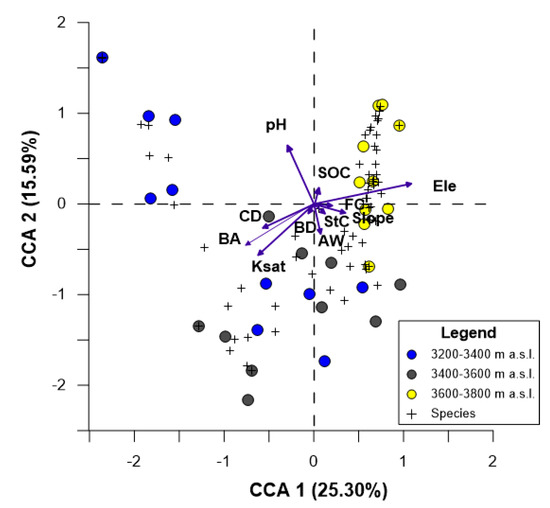
Figure 6.
Canonical Correspondence Analysis (CCA) showing ordination of herbaceous species richness and their plant cover (+), plot (circles), and attributes of pine plantation and their physical and chemical soil characteristics across an elevational range (arrows). Abbreviations are as follows: CD = canopy density, BA = basal area, Ksat = saturated hydraulic conductivity, SOC = soil organic carbon, StC = water content at saturation point, FC = water content at field capacity, AW = available water capacity, pH = potential hydrogen, Ele = elevation, BD = bulk density.
Woody species: In the CCA, 57.30% of the variance was explained in the two axes. In the CCA1, the most relevant variables were Ele and pH in soil while in CCA2 the Ksat and slope had the highest contribution (Figure 7). The CCA also explained that, the woody species richness and its cover was negatively correlated to Ele (p < 0.001); indicating that number of these were lower at the highest elevational range. The Ksat variable showed the same tendency as well as Ele. The pH variable showed a positive relation with the woody species and its cover (p < 0.01) while the plots with steep slope showed a low presence of woody species (p < 0.01) (Figure 7). The soil properties of all pine plantations sites (Pi) are shown in Appendix B.
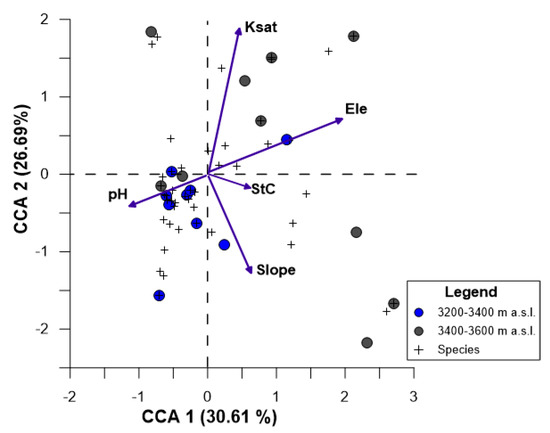
Figure 7.
Canonical Correspondence Analysis (CCA), showing ordination of woody species richness and their plant cover (+), plot (circles) and attributes of pine plantation and their physical and chemical soil characteristics across an elevational range (arrows). Abbreviations are as follows: CD = canopy density, BA = basal area, Ksat = saturated hydraulic conductivity, SOC = soil organic carbon, StC = water content at saturation point, FC = water content at field capacity, AW = available water capacity, pH = potential hydrogen, Ele = elevation, BD = bulk density. The other variables that contributed little to the analysis are not visible here.
4. Discussion
4.1. Natural Regeneration under the Influence of Pine Plantations in an Elevational Gradient
Our results demonstrate that species richness and its cover were lower under Pi than NG across the elevational gradient and thus, pines have a negative impact on natural regeneration. Several authors found similar results with the establishment of pine plantations in the páramo ecosystem of Ecuador [18,31] and Colombia [29]. On a larger scale, Bremer and Farley [69] evaluated plant biodiversity on 11 afforested grasslands of different location around the world, and also found a reduction in plant species richness. On the other hand, we found that herbaceous and woody native and endemic species of plants were existing in the understory of Pi, taking advantage of the dense canopy of the pines that blocks solar radiation and creates an adequate microclimate for their development [32,69,70]. Nevertheless, these native species are shade tolerant with high physiological adaptation to the new conditions offered by Pi. In the same way, Hofstede et al. [18] and Bremer [31] found understories of native vegetation in several pine plantation plots which coincides with our results.
In our study, there was a significant influence of the elevation on herbaceous species richness and its cover, which increased at higher elevation while the opposite result was found for woody species richness and cover, even though it was not statistically significant for woody species. Several studies describe that above the tree line (below the subpáramo), the vegetation becomes smaller and scattered as the elevation increases, and shrubs become even more dispersed at the highest elevations [9,10,71]. Among the responsible factors that determine the marked distribution between woody and herbaceous species in an elevational gradient in the páramo are lower temperatures in the upper zones, especially frost which can occur year-round at night [72,73], strong solar radiation due to the combination of low latitude and high elevation [72], and variation of soil conditions (i.e., bulk density and water availability for plants) [74]. These factors may be responsible for the lower productivity of the pine plantations (smaller trees and less dense plantations) at the higher elevational range. Therefore, these plantations have more open areas with enough available light for the establishment of natural regeneration [75,76,77]. Probably, this is why we found similar herbaceous coverage between NG and Pi at the highest elevational range.
Regarding the composition of the species, the most important families in our study were Asteraceae containing 17% of the species, and Poaceae containing 9% of the species. These results are similar to the ones obtained by Ramsay [10] (20% of the species belonged to Asteraceae and 14% to Poaceae) in the research that covered most of the páramos of Ecuador. With regard to the herbaceous vegetation assemblage across the elevational gradient in the NG, it was observed that tussock grasses represented by C. intermedia were the most dominating species. In the two lower elevational ranges, F. subulifolia was one of the species also dominating the plant community. These two species are very typical in the páramo ecosystem [8,9,10,78]. Most likely, these species evolved to survive at the highest elevation, thereby demonstrating physiological mechanisms of adaptation. For example, due to the fact that in the higher elevations of the páramo, water is available only for few hours of the day, tussock grasses have developed long and thin leaves to avoid water loss by transpiration [79]. In addition, dead leaves are maintained and decay on the external part of the plant providing good insulation from cold temperatures and high heat, as well as protection from radiation, for the young leaves located in the inside of the plant [10,16,80]. Also these dead leaves retain nutrients that are used for the growth of the plants [10,81].
The shift in species composition that we found between NG and Pi at the two lower elevational ranges could be related once again to the amount of light that reaches the understory; in this case, the larger canopies block more light and facilitate the establishment of shade-tolerant species. There was limited information about the ecology of the dominant species found in the understory of the plantations. However, at the lower elevational range, we found that one of the dominant species, T. stipoides, has also been described as a common herbaceous species in the understory of Mexican pine forests [82,83]. In the case of the woody vegetation, it is known that M. theaezans, a dominant species in the understory of our study, is highly capable of natural regeneration and is a common species in secondary succession [84]. In the mid-elevational range, from the herbs that we registered, M. tamnifolia, one of our dominant species, has also been listed in most of the plant communities in a research carried out in the Colombian subpáramo [29], and it was one of the dominant species in an Andean forest of the same country [85]. Finally, in the higher elevational range, there were no important changes in species composition between NG and Pi.
The majority of the species was registered in NG (85%) of which 31.9% were registered only in NG, and 68% of the species were registered in Pi, of which 14.8% were registered only in Pi. In comparison to the studies of van Wesenbeeck et al. [29] and Bremer [31], the number of species that we found in Pi only is much higher, probably because our study covered a wider elevational range, which therefore included more species. In relation to endemic species, we found a 23% decrease of species between NG and Pi, which is less compared to what Bremer and Farley´s [69] found in their study. Among the endemic species registered, because of their status of conservation, L. vitreola [58] and B. jamesonii, [61] found only under NG, and G. miniphylla [62] and M. pernettifolia [61] found only under Pi, special consideration should be given to protect these natural grasslands and to manage the plantations in a way that will guarantee the conservation of these spp. Concerning introduced spp, we found five adventive herbs, Anthoxanthum odoratum L., Holcus lanatus L., Rumex acetosella L., Euphorbia peplus L. and Taraxacum officinale F.W. Wigg. (the last two species were found only inside the plantations). However, all the introduced species that we found in the study are considered indicators of human and grazing disturbances, and nowadays most parts of the Andean páramos are affected by these introduced plants from Europe [9,86]. It should be noted that we did not find any pine seedling in any of the two types of vegetation cover, so we do not consider this species as an invasive one.
4.2. Natural Regeneration Influenced by Pine Plantation Attributes and Soil Properties
Our results showed that herbaceous species richness and cover are influenced by the characteristics of pine plantations, finding a higher herbaceous species richness and cover in pine plantations with lower canopy density and basal area, which is consistent with the results reported in several studies [18,76,77]. With less CD and BA there is more availability of light and water for the development of herbaceous plants within Pi. According to Brockerhoff et al. [75], the characteristics of the plantations directly affect the availability of light, which is necessary for the development of understory vegetation within the plantations. In addition, due to high water requirements and the interception of rainfall by plantations [1], there is less water available in the soil for the germination, growth and establishment of herbaceous vegetation within the plantations. Also, the Ksat of pine plantation soils showed a negative relationship with the herbaceous species richness and its cover. This relationship is due to the fact that plantations with a high Ksat show a high speed of water movement in the soil, causing fast drying [74] and loss of SOM [87], limiting the development of herbaceous plants. Therefore, we can conclude that besides elevation, herbaceous species richness and its cover within plantations depend substantially on the attributes of the plantations as well as on the properties of the soils.
Woody species richness and its cover decreased when the Ksat of the soil increased and the pH was more acidic, which agrees with Riesch et al. [88], who found that one of the main properties of soils that control the composition and richness of woody plants is the pH. In addition, soils with very acidic pH show a lower availability of nutrients [89] with toxicity problems for plants [90] that directly affect species richness. Several studies from different parts of the world show that generally, afforestation of grasslands with pines leads to moderate soil acidification, on average 0.3 units [36,38]. According to Jobbágy et al. [91], the forestation of grasslands which generates higher rates of primary production, involves a greater sequestration of soil nutrients by the pines. This transference of nutrients and of other cations from the páramo soil towards the pine biomass would be accompanied by a release of acidity from the pines towards the soil to balance the charges [92]. This is consistent with our results, in which a lower woody species rischness and its cover were observed in plantations with very acidic soils (pH < 4.4). This highlights that certain plantations with soil acidification processes would cause a negative effect on the regeneration of woody plants.
4.3. Recommendations for Pine Plantation Management
Based on the differences of herbaceous and woody plant richness and its cover between páramo grassland and pine plantations, we suggest that these plantations should be gradually harvested. According to the understory biodiversity that we have found, these plantations could be managed for ecological restoration purposes. Some of the species registered in the plantations are being used in ecological restoration projects such as: M. tamnifolia [93], M. theaezans [94,95], Lupinus spp. [96], Solanum spp, [97]. However, the biodiversity that has been developed inside these plantations is threatened by the future harvesting of the plantation. Due to profitability reasons, the type of harvest practiced in the country is clear-cutting, which has negative consequences such as a very erosive effect on the soil [98,99,100]. In addition, the regeneration that has taken place will surely be destroyed with this type of harvesting [99]. Although the understory developed in the plantations is not the ideal model for conservation management with a proper silvicultural treatment that could support the restoration of the structural and functional attributes of the páramo. Future work should therefore include different silvicultural treatments in these plantations to develop the most appropriate management, thereby ensuring the conservation of the páramo biodiversity.
5. Conclusions
Afforested páramo grassland with P. patula showed a decrease in species richness and cover and a different composition of herbaceous and woody species compared to the natural páramo grassland. Nevertheless, in the plantations, which were established on natural grassland or grazed páramo and had none or very limited silvicultural management and have not been grazed since its establishment, native vegetation, including even endemic and endangered species was maintained. In addition, the presence of these species within the plantations has surely taken place because they have not been exposed to lifestock and fire since the establishement of the plantations. The impacts of these activities on the native vegetation will vary depending on the intensity of the grazing and the frequency of the burning. This highlights the importance of controlling these activities that are commonly practiced along the Andean páramo. Therefore, from this research we conclude that under suitable conditions these plantations in the páramos could also contribute to the ecological restoration programs of this ecosystem. This in no way implies that we are promoting any kind of afforestation in the páramo ecosystem. In order to conserve the native vegetation found within the plantations, we suggest that the plantations should be managed in a way that considers the factors that we found having a great influence on the richness, cover and composition of vegetation such as: basal area, canopy density and saturated hydraulic conductivity.
Author Contributions
Conceptualization, C.Q.D., P.C. and X.P.; data curation, C.Q.D., R.A. and F.M.; methodology, C.Q.D., R.A. and F.M.; formal analysis, F.M., R.A. and X.P.; writing of the original draft and preparation, X.P., C.Q.D. and F.M. Supervision, M.W. and X.P.; project administration P.C. and M.W.; writing of the paper, C.Q.D.; all authors contributed to the paper´s structure and provided extensive revision. All authors read and approved the final manuscript.
Funding
This research was funded by Empresa Pública Municipal de Telecomunicaciones, Agua Potable, Alcantarillado y Saneamiento (ETAPA), DIUC (the Research Office of the University of Cuenca via the research project: “Mejoramiento de las estrategias de manejo forestal en los páramos del sur del Ecuador: Una contribución a la conservación y sostenibilidad del uso de la tierra”) and the DFG project PAK 824/B3. Carlos Quiroz Dahik was funded via a scholarship of the Secretaría de Educación Superior, Ciencia, Tecnología e Innovación (SENESCYT). This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) within the framework of the Open Access Publishing Program.
Acknowledgments
The authors would like to thank all plantation’s landowners for their collaboration in the survey. We thank Annalisa Maschi for her writing assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Inventory of species classified by type of vegetation, natural grassland (NG) and pine plantation (Pi), and elevational range in m a.s.l. S = biogeographic current condition of the species in Ecuador (N = native, E = endemic, I = introduced), Lf = life form (H = herbaceous, H* = prostrate shrubs-sub shrubs and vines, W = woody plant). “X” represents the presence of the species.
Table A1.
Inventory of species classified by type of vegetation, natural grassland (NG) and pine plantation (Pi), and elevational range in m a.s.l. S = biogeographic current condition of the species in Ecuador (N = native, E = endemic, I = introduced), Lf = life form (H = herbaceous, H* = prostrate shrubs-sub shrubs and vines, W = woody plant). “X” represents the presence of the species.
| Family | Specie | S | Lf | NG | Pi | NG | Pi | NG | Pi |
|---|---|---|---|---|---|---|---|---|---|
| 3200–3400 | 3400–3600 | 3600–3800 | |||||||
| ADOXACEAE | Viburnum triphyllum Benth. | N | W | X | X | X | |||
| ALSTROMERIACEA | Bomarea sp. | N | H | X | X | X | X | X | |
| APIACEAE | Azorella biloba (Schltdl.) Wedd. | N | H | X | X | X | X | ||
| APIACEAE | Azorella sp. 1 | N | H | X | X | X | X | ||
| APIACEAE | Eryngium humile Cav. | N | H | X | X | X | X | ||
| APIACEAE | Oreomyrrhis andicola (Kunth) Endl. ex Hook. f. | N | H | X | X | ||||
| APOCYNACEAE | Matalea sp. | N | H* | X | X | ||||
| ARALIACEAE | Hydrocotyle sp. 1 | N | H | X | X | X | X | X | |
| ARALIACEAE | Hydrocotyle sp. 2 | N | H | X | X | ||||
| ARALIACEAE | Hydrocotyle sp. 3 | N | H | X | |||||
| ARALIACEAE | Hydrocotyle sp. 4 | N | H | X | |||||
| ARALIACEAE | Oreopanax andreanus Marchal | E | W | X | |||||
| ARALIACEAE | Oreopanax avicenniifolius (Kunth) Decne. & Planch. | E | W | X | X | X | X | ||
| ARALIACEAE | Oreopanax sp. 3 | N | W | X | |||||
| ARALIACEAE | Oreopanax sp. 4 | N | W | X | |||||
| ASPLENIACEAEA | Asplenium sp. 1 | N | H | X | X | ||||
| ASPLENIACEAEA | Asplenium sp. 2 | N | H | X | X | ||||
| ASPLENIACEAEA | Asplenium cf | N | H | X | |||||
| ASTERACEAE | Achyrocline alata (Kunth) DC. | N | H | X | X | ||||
| ASTERACEAE | Ageratina sp | N | W | X | X | X | X | ||
| ASTERACEAE | Ageratina sp. 2 | N | W | X | |||||
| ASTERACEAE | Aphanactis jamesoniana Wedd. | E | H | X | X | X | |||
| ASTERACEAE | Aristeguietia cacalioides (Kunth) R.M. King & H. Rob. | N | W | X | X | X | |||
| ASTERACEAE | Asteraceae sp. 2 | N | H | X | X | ||||
| ASTERACEAE | Asteraceae sp. 3 | N | H | X | |||||
| ASTERACEAE | Asteraceae sp. 4 | N | W | X | X | ||||
| ASTERACEAE | Baccharis caespitosa (Ruiz & Pav.) Pers. | N | H* | X | X | ||||
| ASTERACEAE | Baccharis genistelloides (Lam.) Pers. | N | H* | X | X | X | X | ||
| ASTERACEAE | Baccharis sp. 2 | N | W | X | |||||
| ASTERACEAE | Baccharis sp. 3 | N | W | X | |||||
| ASTERACEAE | Baccharis sp. 4 | N | W | X | |||||
| ASTERACEAE | Baccharis tricuneata (L. f.) Pers. | N | W | X | |||||
| ASTERACEAE | Barnadesia arborea Kunth | N | W | X | X | ||||
| ASTERACEAE | Bidens andicola Kunth | N | H | X | X | X | X | X | |
| ASTERACEAE | Chaptalia cordata Hieron. | N | H | X | X | X | X | ||
| ASTERACEAE | Chrysactinium acaule (Kunth) Wedd. | N | H | X | X | X | X | ||
| ASTERACEAE | Chrysactinium sp. | N | H | X | |||||
| ASTERACEAE | Chuquiraga jussieui J.F. Gmel. | N | W | X | X | ||||
| ASTERACEAE | Cotula mexicana (DC.) Cabrera | N | H | X | X | ||||
| ASTERACEAE | Diplostephium glandulosum Hieron. | N | H | X | X | ||||
| ASTERACEAE | Dorobaea pimpinellifolia (Kunth) B. Nord. | N | H | X | X | X | |||
| ASTERACEAE | Erato sodiroi (Hieron.) H. Rob. | N | W | X | X | ||||
| ASTERACEAE | Galinsoga cf. quadriradiata Ruiz & Pav. | N | H | X | |||||
| ASTERACEAE | Gamochaeta americana (Mill.) Wedd. | N | H | X | X | X | X | ||
| ASTERACEAE | Gamochaeta purpurea (L.) Cabrera | N | H | X | X | ||||
| ASTERACEAE | Gnaphalium sp. | N | H | X | X | X | |||
| ASTERACEAE | Guevaria sodiroi (Hieron.) R.M. King & H. Rob. | N | H | X | |||||
| ASTERACEAE | Gynoxys miniphylla Cuatrec. | E | W | X | |||||
| ASTERACEAE | Gynoxys sp. 1 | N | W | X | X | X | X | ||
| ASTERACEAE | Gynoxys sp. 2 | N | W | X | X | ||||
| ASTERACEAE | Gynoxys sp. 3 | N | W | X | X | ||||
| ASTERACEAE | Gynoxys sp. 4 | N | W | X | X | ||||
| ASTERACEAE | Hieracium sp. 1 | N | H | X | X | X | |||
| ASTERACEAE | Hieracium sp. 2 | N | H | X | |||||
| ASTERACEAE | Hypochaeris sessiliflora Kunth | N | H | X | X | ||||
| ASTERACEAE | Jungia sp. | N | W | X | X | ||||
| ASTERACEAE | Lasiocephalus lingulatus Schltdl. | E | H | X | X | ||||
| ASTERACEAE | Loricaria sp. | N | W | X | |||||
| ASTERACEAE | Monticalia empetroides (Cuatrec.) C. Jeffrey | N | W | X | |||||
| ASTERACEAE | Munnozia senecionidis Benth. | N | W | X | X | X | |||
| ASTERACEAE | Oligactis coriacea (Hieron.) H. Rob. & Brettell | N | W | X | X | ||||
| ASTERACEAE | Oritrophium crocifolium (Kunth) Cuatrec. | N | H | X | X | ||||
| ASTERACEAE | Senecio cf | N | H | X | |||||
| ASTERACEAE | Senecio cf chionogeton Wedd. | N | H | X | X | X | X | ||
| ASTERACEAE | Senecio sp. 1 | N | H | X | X | ||||
| ASTERACEAE | Taraxacum officinale F.H. Wigg. | I | H | X | |||||
| ASTERACEAE | Werneria nubigena Kunth | N | H | X | X | ||||
| ASTERACEAE | Werneria pygmaea Gillies ex Hook. & Arn. | N | H | X | |||||
| ASTERACEAE | Xenophyllum humile (Kunth) V.A. Funk | N | H | X | X | ||||
| BERBERIDACEAEA | Berberis cf lutea Ruiz & Pav. | N | W | X | |||||
| BERBERIDACEAEA | Berberis sp. 1 | N | W | X | |||||
| BERBERIDACEAEA | Berberis sp. 2 | N | W | X | |||||
| BERBERIDACEAEA | Berberis sp. 3 | N | W | X | |||||
| BERBERIDACEAEA | Berberis sp. 4 | N | W | X | X | ||||
| BLECHNACEAE | Blechnum sp. | N | H | X | X | X | |||
| BRASSICACEAE | Draba sp. | N | H | X | |||||
| BROMELIACEAE | Bromeliaceae 1 | N | H | X | |||||
| BROMELIACEAE | Bromeliaceae 2 | N | H | X | |||||
| BROMELIACEAE | Guzmania sp | N | H | X | |||||
| BROMELIACEAE | Puya sp. 1 | N | H | X | X | X | X | ||
| BROMELIACEAE | Puya sp. 2 | N | H | X | X | ||||
| BROMELIACEAE | Puya sp. 3 | N | H | X | X | ||||
| BROMELIACEAE | Tillandsia sp | N | H | X | |||||
| CAMPANULACEAE | Campanulacea cf | N | W | X | |||||
| CAMPANULACEAE | Centropogon sp. | N | W | X | |||||
| CAMPANULACEAE | Lysipomia sphagnophila Griseb. ex Wedd. | N | H | X | X | ||||
| CAMPANULACEAE | Lysipomia vitreola McVaugh | E | H | X | |||||
| CAMPANULACEAE | Siphocampylus giganteus (Cav.) G. Don | N | W | X | |||||
| CAMPANULACEAE | Lobelia tenera Kunth | N | H | X | |||||
| CAPRIFOLIACEAE | Valeriana hirtella Kunth | N | W | X | X | X | |||
| CAPRIFOLIACEAE | Valeriana microphylla Kunth | N | H | X | X | X | X | ||
| CAPRIFOLIACEAE | Valeriana niphobia Briq. | N | H | X | X | ||||
| CAPRIFOLIACEAE | Valeriana pyramidalis Kunth | N | H | X | X | ||||
| CAPRIFOLIACEAE | Valeriana rigida Ruiz & Pav. | N | H | X | |||||
| CARYOPHYLLACEAE | Arenaria cf. | N | H | X | |||||
| CARYOPHYLLACEAE | Cerastium cf | N | H | X | |||||
| CARYOPHYLLACEAE | Cerastium danguyi J.F. Macbr. | N | H | X | X | X | X | ||
| CARYOPHYLLACEAE | Stellaria recurvata Willd. ex D.F.K. Schltdl. | N | H | X | X | ||||
| CELASTRACEAEA | Maytenus cf verticillata (Ruiz & Pav.) DC. | N | W | X | X | ||||
| CHLORANTHACEAE | Hedyosmum luteynii Todzia | N | W | X | |||||
| CLETHRACEAE | Clethra sp. | N | W | X | |||||
| CONVOLVULACEA | Dichondra aff microcalyx (Hallier f.) Fabris | N | H | X | X | ||||
| CORNACEAEA | Cornus peruviana J.F. Macbr. | N | W | X | X | ||||
| CUNONIACEAE | Weinmannia fagaroides Kunth | N | W | X | X | X | X | ||
| CYPERACEAE | Carex crinalis Boott | N | H | X | X | X | X | X | |
| CYPERACEAE | Carex ecuadorica Kük. | N | H | X | X | ||||
| CYPERACEAE | Carex jamesonii Boott | N | H | X | X | X | X | X | |
| CYPERACEAE | Carex pichinchensis Kunth | N | H | X | X | ||||
| CYPERACEAE | Carex sp. 3 | N | H | X | X | ||||
| CYPERACEAE | Carex sp. 4 | N | H | X | X | X | X | ||
| CYPERACEAE | Carex sp. 5 | N | H | X | |||||
| CYPERACEAE | Carex tamana Steyerm. | N | H | X | X | X | |||
| CYPERACEAE | Carex tristicha Spruce ex Boott | N | H | X | X | X | X | ||
| CYPERACEAE | Eleocharis acicularis (L.) Roem. & Schult. | N | H | X | |||||
| CYPERACEAE | Oreobolopsis inversa Dhooge & Goetgh. | N | H | X | X | X | |||
| CYPERACEAE | Oreobolus ecuadorensis T. Koyama | N | H | X | |||||
| CYPERACEAE | Oreobolus goeppingeri Suess. | N | H | X | X | X | |||
| CYPERACEAE | Rhynchospora sp. 1 | N | H | X | X | X | X | ||
| CYPERACEAE | Rhynchospora sp. 2 | N | H | X | |||||
| CYPERACEAE | Rhynchospora vulcani Boeckeler | N | H | X | X | X | X | X | X |
| CYPERACEAE | Uncinia tenuis Poepp. ex Kunth Search in The Plant List | N | H | X | X | X | |||
| DENNSTAEDTIACEAE | Pteridium arachnoideum (Kaulf.) Maxon | N | H | X | |||||
| DIOSCOREACEAE | Dioscorea cf choriandra Uline ex R. Knuth | E | H | X | X | ||||
| DRYOPTERIDACEAEA | Elaphoglossum sp. 1 | N | H | X | |||||
| DRYOPTERIDACEAEA | Elaphoglossum sp. 2 | N | H | X | |||||
| DRYOPTERIDACEAEA | Elaphoglossum sp. 3 | N | H | X | |||||
| DRYOPTERIDACEAEA | Elaphoglossum sp. 4 | N | H | X | |||||
| DRYOPTERIDACEAEA | Elaphoglossum sp. 5 | N | H | X | |||||
| DRYOPTERIDACEAEA | Elaphoglossum sp. 6 | N | H | X | |||||
| DRYOPTERIDACEAEA | Polystichum orbiculatum (Desv.) J. Rémy & Fée | N | H | X | X | X | X | ||
| ELAEOCARPACEAE | Vallea stipularis L. f. | N | W | X | X | X | |||
| EQUISETACEAE | Equisetum myriochaetum Schltdl. & Cham. | N | H | X | X | X | |||
| ERICACEAE | Bejaria resinosa Mutis ex L. f. | N | W | X | |||||
| ERICACEAE | Cavendishia bracteata (Ruiz & Pav. ex J. St.-Hil.) Hoerold | N | W | X | X | ||||
| ERICACEAE | Disterigma empetrifolium (Kunth) Drude | N | H | X | X | X | |||
| ERICACEAE | Gaultheria amoena A.C. Sm. | N | H | X | X | ||||
| ERICACEAE | Gaultheria erecta Vent. | N | W | X | |||||
| ERICACEAE | Gaultheria glomerata (Cav.) Sleumer | N | W | X | |||||
| ERICACEAE | Gaultheria reticulata Kunth | N | W | X | |||||
| ERICACEAE | Gaultheria sp | N | W | X | X | X | X | ||
| ERICACEAE | Gaultheria tomentosa Kunth | N | W | X | X | X | |||
| ERICACEAE | Macleania rupestris (Kunth) A.C. Sm. | N | W | X | X | ||||
| ERICACEAE | Pernettya prostrata (Cav.) DC. | N | H* | X | X | X | |||
| ERICACEAE | Pernettya sp. | N | W | X | |||||
| ERICACEAE | Vaccinium floribundum Kunth | N | H* | X | X | X | X | X | X |
| ERIOCAULACEAE | Paepalanthus sp. | N | H | X | X | ||||
| ESCALLONIACEAE | Escallonia myrtilloides L. f. | N | W | X | |||||
| EUPHORBIACEA | Euphorbia peplus L. | I | H | X | |||||
| FABACEAE | Lupinus tauris Benth. | N | H | X | X | X | X | ||
| GENTIANACEAE | Gentianella cerastioides (Kunth) Fabris | N | H | X | X | X | |||
| GENTIANACEAE | Gentianella rapunculoides (Willd. ex Schult.) J.S. Pringle | N | H | X | X | ||||
| GENTIANACEAE | Halenia taruga-gasso Gilg | E | H | X | X | X | X | ||
| GERANIACEAE | Geranium diffusum Kunth | N | H | X | X | X | |||
| GERANIACEAE | Geranium maniculatum H.E. Moore | N | H | X | X | X | |||
| GERANIACEAE | Geranium multipartitum Benth. | N | H | X | X | ||||
| GERANIACEAE | Geranium sibbaldioides Benth. | N | H | X | X | X | X | ||
| GROSSULARIACEAE | Ribes cf. | N | W | X | X | ||||
| GROSSULARIACEAE | Ribes lehmannii Jancz. | E | W | X | X | ||||
| HYPERICACEAE | Hypericum aciculare Kunth | N | W | X | |||||
| HYPERICACEAE | Hypericum decandrum Turcz. | N | H* | X | X | X | X | ||
| HYPERICACEAE | Hypericum laricifolium Juss. | N | W | X | X | ||||
| HYPERICACEAE | Hypericum quitense R. Keller | E | W | X | |||||
| IRIDACEAE | Orthrosanthus chimboracensis (Kunth) Baker | N | H | X | X | X | X | X | X |
| IRIDACEAE | Sisyrinchum sp.1 | N | H | X | X | X | |||
| JUNCACEAE | Juncus sp. | N | H | X | |||||
| JUNCACEAE | Luzula sp. | N | H | X | X | ||||
| LAMIACEAE | Clinopodium nubigenum (Kunth) Kuntze | N | H | X | X | ||||
| LAMIACEAE | Lepechinia rufocampii Epling & Mathias | N | H | X | |||||
| LAMIACEAE | Salvia corrugata Vahl | N | W | X | |||||
| LAMIACEAE | Stachys cf elliptica Kunth | N | H | X | X | X | |||
| LAURACEAE | Ocotea heterochroma Mez & Sodiro | N | W | X | X | ||||
| LORANTHACEAE | Gaiadendron punctatum (Ruiz & Pav.) G. Don | N | W | X | |||||
| LYCOPODIACEAE | Austrolycopodium magellanicum (P. Beauv.) Holub | N | H | X | X | X | X | X | X |
| LYCOPODIACEAE | Huperzia crassa (Humb. & Bonpl. ex Willd.) Rothm. | N | H | X | |||||
| LYCOPODIACEAE | Huperzia sp. 1 | N | H | X | X | X | |||
| LYCOPODIACEAE | Huperzia sp. 2 | N | H | X | X | ||||
| LYCOPODIACEAE | Lycopodium clavatum L. | N | H | X | X | X | X | X | |
| LYCOPODIACEAE | Lycopodium magellanicum (P. Beauv.) Sw. | N | H | X | X | X | |||
| MELASTOMATACEAE | Miconia aspergillaris (Bonpl.) Naudin | N | W | X | |||||
| MELASTOMATACEAE | Miconia chionophila Naudin | N | H | X | X | X | X | ||
| MELASTOMATACEAE | Miconia crocea (Desr.) Naudin | N | W | X | X | ||||
| MELASTOMATACEAE | Miconia pernettifolia Triana | E | H | X | |||||
| MELASTOMATACEAE | Miconia salicifolia Naudin | N | W | X | X | ||||
| MELASTOMATACEAE | Miconia sp. 1 | N | W | X | X | ||||
| MELASTOMATACEAE | Miconia sp. 3 | N | W | X | |||||
| MELASTOMATACEAE | Miconia sp. 4 | N | W | X | X | ||||
| MELASTOMATACEAE | Miconia sp. 6 | N | W | X | |||||
| MELASTOMATACEAE | Miconia theaezans (Bonpl.) Cogn. | N | W | X | X | X | |||
| MELASTOMATACEAE | Brachyotum confertum (Bonpl.) Triana | E | W | X | X | X | X | ||
| MELASTOMATACEAE | Brachyotum jamesonii Triana | E | W | X | |||||
| MONNIMIACEAE | Monnina ligustrifolia Kunth | N | W | X | |||||
| MONNIMIACEAE | Monnina sp. | N | W | X | X | X | |||
| MONOCOTILEDONEA | Monocotiledonea | N | H | X | |||||
| MYRICACEAE | Morella parvifolia (Benth.) Parra-Os. | N | W | X | X | X | X | ||
| PRIMULACEAE | Myrsine andina (Mez) Pipoly | N | W | X | X | X | |||
| PRIMULACEAE | Myrsine dependens (Ruiz & Pav.) Spreng. | N | W | X | X | X | X | ||
| MYRTACEAE | Myrtaceae sp. | N | W | X | |||||
| ONAGRACEAE | Fuchsia sp. | N | W | X | |||||
| OPHIOGLOSSACEAE | Ophioglossum cf crotalophoroides Walter | N | H | X | |||||
| ORCHIDACEAE | Aa sp. | N | H | X | |||||
| ORCHIDACEAE | Epidendrum sp. | N | H | X | X | ||||
| ORCHIDACEAE | Maxilaria sp. | N | H | X | X | ||||
| ORCHIDACEAE | Orchidaceae | N | H | X | X | X | |||
| ORCHIDACEAE | Stellis sp. | N | H | X | |||||
| OROBANCHACEAEA | Bartsia laticrenata Benth. | N | H | X | X | ||||
| OROBANCHACEAEA | Bartsia sp. 1 | N | H | X | X | X | |||
| OROBANCHACEAEA | Bartsia sp. 2 | N | H | X | |||||
| OROBANCHACEAEA | Castilleja fissifolia L. f. | N | H | X | X | ||||
| OXALIDACEAE | Oxalis sp. 1 | N | H | X | X | X | X | ||
| OXALIDACEAE | Oxalis sp. 2 | N | H | X | |||||
| OXALIDACEAE | Oxalis sp. 3 | N | H | X | X | ||||
| OXALIDACEAE | Oxalis sp. 4 | N | H | X | |||||
| OXALIDACEAE | Oxalis sp. 5 | N | H | X | |||||
| PASSIFLORACEAE | Passiflora sp. | N | H* | X | |||||
| PINGUICULACEAE | Pinguicula calyptrata Kunth | N | H | X | |||||
| PIPERACEAE | Peperomia sp. 1 | N | H | X | X | X | X | ||
| PIPERACEAE | Peperomia sp. 2 | N | H | X | |||||
| PIPERACEAE | Peperomia sp. 3 | N | H | X | X | ||||
| PIPERACEAE | Peperomia sp. 4 | N | H | X | X | ||||
| PIPERACEAE | Peperomia sp. 5 | N | H | X | |||||
| PIPERACEAE | Piper sp. | N | W | X | |||||
| PLANTAGINACEAE | Plantago cf tubulosa Decne. | N | H | X | |||||
| PLANTAGINACEAE | Plantago australis Lam. | N | H | X | |||||
| PLANTAGINACEAE | Plantago linearis Kunth | N | H | X | X | ||||
| PLANTAGINACEAE | Plantago rigida Kunth | N | H | X | |||||
| PLANTAGINACEAE | Plantago sericea Ruiz & Pav. | N | H | X | |||||
| POACEAE | Aciachne acicularis Lægaard | N | H | X | X | ||||
| POACEAE | Agrostis breviculmis Hitchc. | N | H | X | |||||
| POACEAE | Agrostis perennans (Walter) Tuck. | N | H | X | X | X | X | X | X |
| POACEAE | Agrostis sp. 1 | N | H | X | X | X | |||
| POACEAE | Agrostis tolucensis Kunth | N | H | X | X | X | |||
| POACEAE | Anthoxanthum odoratum L. | I | H | X | X | X | X | X | |
| POACEAE | Bromus lanatus Kunth | N | H | X | X | ||||
| POACEAE | Bromus pitensis Kunth | N | H | X | |||||
| POACEAE | Calamagrostis aff. recta (Kunth) Trin. ex Steud. | N | H | X | X | ||||
| POACEAE | Calamagrostis intermedia (J. Presl) Steud. | N | H | X | X | X | X | X | X |
| POACEAE | Calamagrostis bogotensis (Pilg.) Pilg. | N | H | X | X | ||||
| POACEAE | Calamagrostis sp. | N | H | X | X | X | |||
| POACEAE | Cortaderia hapalotricha (Pilg.) Conert | N | H | X | X | X | |||
| POACEAE | Cortaderia jubata (Lemoine) Stapf | N | H | X | |||||
| POACEAE | Cortaderia nitida (Kunth) Pilg. | N | H | X | |||||
| POACEAE | Cortaderia sericantha (Steud.) Hitchc. | N | H | X | X | ||||
| POACEAE | Elymus cordilleranus Davidse & R.W. Pohl | N | H | X | X | ||||
| POACEAE | Festuca subulifolia Benth. | N | H | X | X | X | X | X | X |
| POACEAE | Holcus lanatus L. | I | H | X | X | X | |||
| POACEAE | Paspalum bonplandianum Flüggé | N | H | X | X | X | X | X | X |
| POACEAE | Poa annua L. | N | H | X | |||||
| POACEAE | Poa pauciflora Roem. & Schult. | N | H | X | X | X | |||
| POACEAE | Poaceae sp. 1 | N | H | X | |||||
| POACEAE | Poaceae sp. 2 | N | H | X | |||||
| POACEAE | Triniochloa stipoides (Kunth) Hitchc. | N | H | X | X | X | X | ||
| POACEAE | Stipa rosea Hitchc. | N | H | X | X | X | X | X | |
| POLYGONACEAE | Muehlenbeckia tamnifolia (Kunth) Meisn. | N | H* | X | X | X | X | ||
| POLYGONACEAE | Rumex acetosella L. | I | H | X | X | X | X | ||
| POLYGONACEAE | Rumex sp. 2 | N | H | X | |||||
| POLYPODIACEAE | Melpomene moniliformis (Lag. ex Sw.) A.R. Sm. & R.C. Moran | N | H | X | X | X | X | ||
| POLYPODIACEAE | Niphidium sp. | N | H | X | X | X | |||
| POLYPODIACEAE | Pecluma sp. 1 | N | H | X | X | ||||
| POLYPODIACEAE | Pecluma sp. 2 | N | H | X | X | ||||
| POLYPODIACEAE | Pecluma sp. 3 | N | H | X | |||||
| POLYPODIACEAE | Polypodium sp. | N | H | X | X | ||||
| PROTEACEAE | Lomatia hirsuta (Lam.) Diels | N | W | X | X | X | X | ||
| PROTEACEAE | Oreocallis grandiflora (Lam.) R. Br. | N | W | X | X | X | X | ||
| PTERIDACEAE | Eriosorus sp. | N | H | X | X | ||||
| PTERIDACEAE | Jamesonia sp. 1 | N | H | X | X | X | X | X | |
| PTERIDACEAE | Jamesonia sp. 2 | N | H | X | |||||
| PTERIDACEAE | Pteridacea sp. | N | H | X | |||||
| PTERIDOPHYTA | Pteridophyta | N | H | X | |||||
| RANUNCULACEAE | Ranunculus peruvianus Pers. | N | H | X | |||||
| ROSACEAE | Hesperomeles ferruginea (Pers.) Benth. | N | W | X | |||||
| ROSACEAE | Hesperomeles obtusifolia (Pers.) Lindl. | N | W | X | X | X | |||
| ROSACEAE | Lachemilla hispidula (L.M. Perry) Rothm. | N | H | X | X | X | |||
| ROSACEAE | Lachemilla orbiculata (Ruiz & Pav.) Rydb. | N | H | X | X | X | X | X | |
| ROSACEAE | Lachemilla sp. 1 | N | H | X | |||||
| ROSACEAE | Lachemilla sp. 2 | N | H | X | X | X | X | ||
| ROSACEAE | Lachemilla vulcanica (Schltdl. & Cham.) Rydb. | N | H | X | X | ||||
| ROSACEAE | Potentilla dombeyi Nestl. | N | H | X | |||||
| ROSACEAE | Rubus coriaceus Poir. | N | H | X | X | X | X | X | |
| ROSACEAE | Rubus sp. 1 | N | W | X | X | ||||
| ROSACEAE | Rubus sp. 2 | N | W | X | |||||
| ROSACEAE | Rubus sp. 3 | N | W | X | X | ||||
| ROSACEAE | Rubus sp. 4 | N | W | X | |||||
| RUBIACEAE | Arcytophyllum filiforme (Ruiz & Pav.) Standl. | N | H* | X | X | X | X | X | |
| RUBIACEAE | Arcytophyllum sp. 2 | N | H* | X | X | X | |||
| RUBIACEAE | Galium hypocarpium (L.) Endl. ex Griseb. | N | H | X | X | X | X | X | |
| RUBIACEAE | Nertera granadensis (Mutis ex L. f.) Druce | N | H | X | |||||
| RUBIACEAE | Palicourea sp. 1 | N | W | X | |||||
| RUBIACEAE | Palicourea weberbaueri K. Krause | N | W | X | X | ||||
| SCROPHULARIACEAE | Sibthorpia repens (L.) Kuntze | N | H | X | X | X | X | ||
| SOLANACEAEA | Iochroma cyaneum (Lindl.) M.L. Green ex G.H.M. Lawr. & J.M. Tucker | N | W | X | |||||
| SOLANACEAEA | Solanum sp. 1 | N | W | X | X | ||||
| SOLANACEAEA | Solanum sp. 2 | N | W | X | |||||
| SYMPLOCACEAE | Symplocos sp. 1 | N | W | X | X | ||||
| URTICACEAE | Pilea sp.1 | N | H | X | |||||
| VERBENACEAE | Citharexylum ilicifolium Kunth | N | W | X | |||||
| VIOLACEAE | Viola arguta Willd. ex Roem. & Schult. | N | H | X | |||||
| VIOLACEAE | Viola dombeyana DC. | N | H | X | X | ||||
| XYRIDACEAE | Xyris subulata Ruiz & Pav. | N | H | X | |||||
Appendix B

Table A2.
Species richness and coverage, and soil properties of pine plantations (Pi) sites across the elevational range. The data indicate the median and between parentheses quartiles (Q1 and Q3). HR = herbaceous richness, HC = herbaceous cover, WR = woody plant richness, WC = woody plant coverage, Ksat = saturated hydraulic conductivity, BD = bulk density, StC = water content at saturation point, FC = water content at field capacity, WP = wilting point, GW = gravitational water, AW = available water capacity, N = nitrogen, SOC = soil organic carbon, pH = potential of hydrogen, CN = carbon-nitrogen ratio.
Table A2.
Species richness and coverage, and soil properties of pine plantations (Pi) sites across the elevational range. The data indicate the median and between parentheses quartiles (Q1 and Q3). HR = herbaceous richness, HC = herbaceous cover, WR = woody plant richness, WC = woody plant coverage, Ksat = saturated hydraulic conductivity, BD = bulk density, StC = water content at saturation point, FC = water content at field capacity, WP = wilting point, GW = gravitational water, AW = available water capacity, N = nitrogen, SOC = soil organic carbon, pH = potential of hydrogen, CN = carbon-nitrogen ratio.
| Elevational Range (m a.s.l.) | 3200–3400 | 3400–3600 | 3600–3800 | |||
|---|---|---|---|---|---|---|
| Plantations (Pi) | Nero | La Paz | Tutupali Chico | Tutupali Grande | Quimsacocha | Soldados |
| HR (%) | 11 (9–12) | 7 (7–7) | 16 (16–20) | 16 (16–18) | 33 (32–33) | 33 (27–36) |
| HC (%) | 17.84 (17.68–17.84) | 11.84 (5.68–15.17) | 39.67 (17.35–41.84) | 29.17 (19.85–35.18) | 110.00 (101.36–130.18) | 105.86 (73.84–136.03) |
| WR (%) | 15(14–16) | 7(6–8) | 9(8–10) | 7(6–8) | ||
| WC (%) | 40.84 (34.67–68.84) | 8.17 (4.50–10.01) | 12.17 (11.68–19.34) | 8.84 (5.67–16.67) | ||
| Ksat (cm/h) | 3.61 (3.48–3.84) | 3.77 (3.46–3.84) | 6.55 (6.45–7.47) | 4.71 (3.64–5.16) | 2.11 (2.01–2.17) | 2.20 (2.13–2.45) |
| BD (g/cm3) | 0.46 (0.45–0.47) | 0.87 (0.86–0.90) | 0.52 (0.52–0.65) | 0.65 (0.48–0.76) | 0.33 (0.33–0.36) | 0.66 (0.57–0.66) |
| StC (cm3/cm3) | 0.75 (0.74–0.76) | 0.63 (0.62–0.64) | 0.76 (0.70–0.77) | 0.74 (0.67–0.78) | 0.85 (0.84–0.85) | 0.71 (0.69–0.72) |
| FC (cm3/cm3) | 0.54 (0.51–0.55) | 0.41 (0.39–0.41) | 0.54 (0.51–0.55) | 0.61 (0.55–0.64) | 0.62 (0.6–0.63) | 0.52 (0.50–0.55) |
| WP (cm3/cm3) | 0.39 (0.38–0.41) | 0.32 (0.32–0.33) | 0.38 (0.35–0.38) | 0.41 (0.41–0.42) | 0.39 (0.38–0.40) | 0.42 (0.41–0.45) |
| GW (cm3/cm3) | 0.21 (0.19–0.21) | 0.24 (0.23–0.26) | 0.21 (0.19–0.21) | 0.13 (0.12–0.14) | 0.23 (0.21–0.25) | 0.17 (0.16–0.20) |
| AW (cm3/cm3) | 0.14 (0.10–0.15) | 0.06 (0.06–0.08) | 0.16 (0.16–0.18) | 0.18 (0.18–0.22) | 0.23 (0.21–0.24) | 0.10 (0.10–0.10) |
| N (%) | 0.87 (0.78–0.99) | 0.34 (0.29–0.43) | 1.12 (0.91–1.16) | 0.66 (0.62–0.73) | 1.25 (1.12–1.28) | 0.89 (0.76–0.91) |
| SOC (%) | 14.72 (13.87–17.23) | 6.33 (4.82–7.45) | 15.99 (14.84–16.86) | 9.64 (9.26–12.77) | 20.12 (18.17–20.39) | 12.41 (11.79–16.14) |
| pH | 4.52 (4.52–4.88) | 4.14 (4.11–4.14) | 4.40 (4.30–4.45) | 4.10 (4.06–4.16) | 4.15 (4.09–4.17) | 4.77 (4.63–4.81) |
| CN | 17.47 (14.29–17.88) | 17.70 (16.48–18.15) | 14.52 (14.23–16.06) | 15.02 (14.71–15.6) | 16.07 (15.9–16.29) | 16.55 (16.33–17.47) |
References
- Buytaert, W.; Iñiguez, V.; De Bièvre, B. The effects of afforestation and cultivation on water yield in the Andean páramo. For. Ecol. Manag. 2007, 251, 22–30. [Google Scholar] [CrossRef]
- Hofstede, R.; Calles, J.; López, V.; Polanco, R.; Torres, F.; Ulloa, J.; Vásquez, A.; Cerra, M. Los Páramos Andinos ¿Qué sabemos? Estado de Conocimiento Sobre el Impacto del Cambio Climático en el Ecosistema Páramo; UICN: Quito, Ecuador, 2014; ISBN 978-9978-9932-9-3. [Google Scholar]
- Smith, J.M.B.; Cleef, A.M. Composition and Origins of the World’s Tropicalpine Floras. J. Biogeogr. 1988, 15, 631. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Madriñán, S.; Cortés, A.J.; Richardson, J.E. Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Front. Genet. 2013, 4, 192. [Google Scholar] [CrossRef] [PubMed]
- Rangel, O. Colombia diversidad biótica. La región paramuna. In Colombia diversidad biotica; Universidad Nacional de Colombia: Bogotá, Colombia, 2000. [Google Scholar]
- Alonso-Amelot, M. High altitude plants, chemistry of acclimation and adaptation. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; Volume 34, pp. 883–982. ISBN 9780444531803. [Google Scholar]
- Cuatrecasas, J. Paramo vegetation and its life forms. In Geo-Ecology of the Mountainous Regions of the Tropical Americas; Troll, C., Ed.; Dümmler in Kommission: Bonn, Germany, 1968; pp. 163–186. [Google Scholar]
- Luteyn Luteyn, J.L.; Churchill, S.P.; Griffin, D., III; Gradstein, S.R.; Sipman, H.J.M.; Gavilanes, A. Páramos. A Checklist of Plant Diversity, Geographical Distribution, and Botanical Literature; New York Botanical Garden: The Bronx, NY, USA, 1999; ISBN 0893274275. [Google Scholar]
- Ramsay, P.M. The Paramo Vegetation of Ecuador: The Community Ecology, Dynamics and Productivity of Tropical Grasslands in the Andes. Ph.D. Thesis, Prifysgol Bangor University, Bangor, Wales, 1992. [Google Scholar]
- Llambí, L.D. Estructura, Diversidad Y Dinámica De La Vegetación En El Ecotono Bosque-Páramo: Revisión De La Evidencia En La Cordillera De Mérida. Acta Biol. Colomb. 2015, 20, 5–19. [Google Scholar] [CrossRef]
- Luteyn, J.L.; Balslev, H. Paramos: Why Study Them? In Paramo: An Andean Ecosystem under Human Influence; Balslev, H., Luteyn, J.L., Eds.; Academic Press: London, UK, 1992; pp. 1–14. [Google Scholar]
- López, S.; Wright, C.; Costanza, P. Environmental change in the equatorial Andes: Linking climate, land use, and land cover transformations. Remote Sens. Appl. Soc. Environ. 2017, 8, 291–303. [Google Scholar] [CrossRef]
- Verweij, P.A. Spatial and Temporal Modelling of Vegetation Patterns—Burning and Grazing in the Paramo of Los Nevados National Park. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1995. [Google Scholar]
- Suarez, G.; Medina, E. Vegetation Structure and Soil Properties in Ecuadorian Páramo Grasslands with Different Histories of Burning and Grazing. Arct. Antarct. Alp. Res. 2001, 33, 158–164. [Google Scholar] [CrossRef]
- Hofstede, R. The Effects of Grazing and Burning on Soil and Plant Nutrient Concentrations in Colombian Paramo Grasslands. Plant Soil 1995, 173, 111–132. [Google Scholar] [CrossRef]
- Laegaard, S. Influence of fire in the grass páramo vegetation of Ecuador. In Páramo: An Andean Ecosystem under Human Influence; Academic Press: London, UK, 1992; pp. 151–170. [Google Scholar]
- Hofstede, R.G.M.; Groenendijk, J.P.; Coppus, R.; Fehse, J.C.; Sevink, J. Impact of Pine Plantations on Soils and Vegetation in the Ecuadorian High Andes. Mt. Res. Dev. 2002, 22, 159–167. [Google Scholar] [CrossRef]
- Farley, K.A.; Kelly, E.F.; Hofstede, R.G.M. Soil Organic Carbon and Water Retention after Conversion of Grasslands to Pine Plantations in the Ecuadorian Andes. Ecosystems 2004, 7, 729–739. [Google Scholar] [CrossRef]
- Chacón, G.; Gagnon, D.; Paré, D. Comparison of soil properties of native forests, Pinus patula plantations and adjacent pastures in the Andean highlands of southern Ecuador: Land use history or recent vegetation effects? Soil Use Manag. 2009, 25, 427–433. [Google Scholar] [CrossRef]
- Holmes, G.; Sandbrook, C.; Fisher, J.A. Understanding conservationists’ perspectives on the new-conservation debate. Conserv. Biol. 2017, 31, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Quiroz Dahik, C.; Crespo, P.; Stimm, B.; Murtinho, F.; Weber, M.; Hildebrandt, P. Contrasting Stakeholders’ Perceptions of Pine Plantations in the Páramo Ecosystem of Ecuador. Sustainability 2018, 10, 1707. [Google Scholar] [CrossRef]
- Bosch, J.M.; Hewlett, J.D.D.; Bosch, J.M.; Hewlett, J.D.; Bosch, J.M.; Hewlett, J.D.D. A review of catchment experiments to determine the effect of vegetation changes on water yield and evapotranspiration. J. Hydrol. 1982, 55, 3–23. [Google Scholar] [CrossRef]
- Farley, K.A.; Jobbagy, E.G.; Jackson, R.B.; Jobbágy, E.G.; Jackson, R.B. Effects of afforestation on water yield: A global synthesis with implications for policy. Glob. Chang. Biol. 2005, 11, 1565–1576. [Google Scholar] [CrossRef]
- Buytaert, W.; Célleri, R.; De Bièvre, B.; Cisneros, F.; Wyseure, G.; Deckers, J.; Hofstede, R. Human impact on the hydrology of the Andean páramos. Earth-Sci. Rev. 2006, 79, 53–72. [Google Scholar] [CrossRef]
- Farley, K.A.; Bremer, L.L.; Harden, C.P.; Hartsig, J. Changes in carbon storage under alternative land uses in biodiverse Andean grasslands: Implications for payment for ecosystem services. Conserv. Lett. 2013, 6, 21–27. [Google Scholar] [CrossRef]
- Bremer, L.L.; Farley, K.A.; Chadwick, O.A.; Harden, C.P. Changes in carbon storage with land management promoted by payment for ecosystem services. Environ. Conserv. 2016, 43, 397–406. [Google Scholar] [CrossRef]
- Ohep, N.; Herrera, L. Impacto de Las Plantaciones de Coníferas Sobre la Vegetación Originaria del Páramo de Mucubají; Universidad de Los Andes, Facultad de Ciencias Forestales: Mérida, Mexico, 1985. [Google Scholar]
- Van Wesenbeeck, B.K.; van Mourik, T.; Duivenvoorden, J.F.; Cleef, A.M. Strong effects of a plantation with Pinus patula on Andean subpáramo vegetation: A case study from Colombia. Biol. Conserv. 2003, 114, 207–218. [Google Scholar] [CrossRef]
- Cavelier, J.; Santos, C. Efectos de plantaciones abandonadas de especies exóticas y nativas sobre la regeneración natural de un bosque montano en Colombia. Rev. Biol. Trop. 1999, 47, 775–784. [Google Scholar]
- Bremer, L.L. Land-Use Change, Ecosystem Services, and Local Livelihoods: Ecological and Socioeconomic Outcomes of Payment for Ecosystem Services in Ecuadorian Páramo Grasslands. Ph.D. Thesis, University of California, Santa Barbara and San Diego State University, Santa Barbara, CA, USA, 2012. [Google Scholar]
- Parrotta, J.A.; Turnbull, J.W.; Jones, N. Catalyzing native forest regeneration on degraded tropical lands. For. Ecol. Manag. 1997, 99, 1–7. [Google Scholar] [CrossRef]
- Feyera, S.; Beck, E.; Lüttge, U. Exotic trees as nurse-trees for the regeneration of natural tropical forests. Trees 2002, 16, 245–249. [Google Scholar] [CrossRef]
- Ren, H.; Yang, L.; Liu, N. Nurse plant theory and its application in ecological restoration in lower subtropics of China. Prog. Natl. Sci. USA 2008, 18, 137–142. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Avila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Jobbágy, E.; Avissar, R.; Baidya Roy, S.; Barrett, D.; Cook, C.; Farley, K.; Le Maitre, D.; McCarl, B.; Murray, B. Trading Water for Carbon with Biological Carbon Sequestration. Science 2005, 310, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Homeier, J.; Werner, F.A.; Gradstein, S.R.; Breckle, S.W.; Richter, M. Flora and Fungi: Composition and Function. In Gradients in a Tropical Mountain Ecosystem of Ecuador. Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; pp. 87–100. ISBN 978-3-540-73526-7. [Google Scholar]
- Berthrong, S.T.; Jobbágy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Harden, C.P.; Hartsig, J.; Farley, K.A.; Lee, J.; Bremer, L.L. Effects of Land-Use Change on Water in Andean Páramo Grassland Soils. Ann. Assoc. Am. Geogr. 2013, 103, 375–384. [Google Scholar] [CrossRef]
- Zehetner, F.; Miller, W.P. Soil variations along a climatic gradient in an Andean agro-ecosystem. Geoderma 2006, 137, 126–134. [Google Scholar] [CrossRef]
- Soethe, N.; Wilcke, W.; Homeier, J.; Lehmann, J.; Engels, C. Plant Growth along the Altitudinal Gradient —Role of Plant Nutritional Status, Fine Root Activity, and Soil Properties. In Gradients in a Tropical Mountain Ecosystem of Ecuador; Springer: Berlin/Heidelberg, Germany, 2008; pp. 259–266. [Google Scholar]
- Celleri, R.; Willems, P.; Buytaert, W.; Feyen, J. Space–time rainfall variability in the Paute basin, Ecuadorian Andes. Hydrol. Process. 2007, 21, 3316–3327. [Google Scholar] [CrossRef]
- Uytaert, W.; Celleri, R.; Willems, P.; De Bievre, B.; Wyseure, G. Spatial and temporal rainfall variability in mountainous areas: A case study from the south Ecuadorian Andes. J. Hydrol. 2006, 329, 413–421. [Google Scholar] [CrossRef]
- Córdova, M.; Célleri, R.; Shellito, C.J.; Orellana-Alvear, J.; Abril, A.; Carrillo-Rojas, G. Near-Surface Air Temperature Lapse Rate Over Complex Terrain in the Southern Ecuadorian Andes: Implications for Temperature Mapping. Arct. Antarct. Alp. Res. 2016, 48, 673–684. [Google Scholar] [CrossRef]
- Quiroz Dahik, C.; Crespo, P.; Stimm, B.; Mosandl, R.; Cueva, J.; Weber, M.; Patrick, H. Carbon Stocks in Pine Plantations on páramo Sites. Unpublished manuscript. 2019. [Google Scholar]
- Buytaert, W.; Deckers, J.; Wyseure, G. Description and classification of nonallophanic Andosols in south Ecuadorian alpine grasslands (páramo). Geomorphology 2006, 73, 207–221. [Google Scholar] [CrossRef]
- Buytaert, W.; Wyseure, G.; De Bièvre, B.; Deckers, J. The effect of land-use changes on the hydrological behaviour of Histic Andosols in south Ecuador. Hydrol. Process. 2005, 19, 3985–3997. [Google Scholar] [CrossRef]
- Farley, K.A. Grasslands to tree plantations: Forest transition in the Andes of Ecuador. Ann. Assoc. Am. Geogr. 2007, 97, 755–771. [Google Scholar] [CrossRef]
- Sklenar, P.; Ramsay, P.M. Diversity of zonal paramo plant communities in Ecuador. Divers. Distrib. 2001, 7, 113–124. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Fitosociología, Bases Para el Estudio de Las Comunidades Vegetales; Edición en; Blume: Madrid, Spain, 1979. [Google Scholar]
- Lemmon, P.E. A Spherical Densiometer for Estimating Forest Overstory Density. For. Sci. 1956, 2, 314–320. [Google Scholar] [CrossRef]
- Oosterbaan, R.; Nijland, H. Determining the Saturated Hydraulic Conductivity. In Drainagem Principles and Applications; Alterra-ILRI: Wageninge, The Netherlands, 1994; p. 37. ISBN 90-70754-3-39. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Center (ISRIC): Wageningen, The Netherlands, 2002. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. NLME: Linear and Nolinear Mixed Effects Models; R Package Version 3.1-141; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O´Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R package version 2.5-5; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2019-2. 2019. Available online: http://WWW.iucnredlist.org (accessed on 22 August 2019).
- Moreno, P.; Pitman, N. The IUCN Red List of Threatened Species 2003: e.T43552A10811358. Available online: http://dx.doi.org/10.2305/IUCN.UK.2003.RLTS.T43552A10811358 (accessed on 27 August 2019).
- Cotton, E.; Pitman, N. Brachyotum jamesonii. The IUCN Red List of Threatened Species 2004: e.T45691A11007884. Available online: http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T45691A11007884 (accessed on 27 August 2019).
- Montúfar, R.; Pitman, N. The IUCN Red List of Threatened Species 2003: e.T43435A10804316. Available online: http://dx.doi.org/10.2305/IUCN.UK.2003.RLTS.T43435A10804316 (accessed on 27 August 2019).
- Cotton, E.; Pitman, N. The IUCN Red List of Threatened Species 2004: e.T46046A11031781. Available online: http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T46046A11031781 (accessed on 27 August 2019).
- Montúfar, R.; Pitman, N. Oreopanax andreanus. The IUCN Red List of Threatened Species 2003: e.T43024A10770931. Available online: http://dx.doi.org/10.2305/IUCN.UK.2003.RLTS.T43024A10770931.en (accessed on 27 August 2019).
- Montúfar, R.; Pitman, N. Oreopanax avicenniifolius. The IUCN Red List of Threatened Species 2003: e.T43025A10771054. Available online: http://dx.doi.org/10.2305/IUCN.UK.2003.RLTS.T43025A10771054.en (accessed on 27 August 2019).
- Montúfar, R.; Pitman, N. Aphanactis jamesoniana. The IUCN Red List of Threatened Species 2003: e.T43122A10778814. Available online: http://dx.doi.org/10.2305/IUCN.UK.2003.RLTS.T43122A10778814.en (accessed on 27 August 2019).
- Montúfar, R.; Pitman, N. Halenia taruga-gasso. The IUCN Red List of Threatened Species 2004: e.T45284A10986246. Available online: http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T45284A10986246.en (accessed on 27 August 2019).
- Freire-Fierro, A.; Pitman, N. Ribes lehmannii. The IUCN Red List of Threatened Species 2004: e.T45376A10990002. Available online: http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T45376A10990002.en (accessed on 27 August 2019).
- Nicolalde, F.; Pitman, N. Hypericum quitense. The IUCN Red List of Threatened Species 2004: e.T45116A10981458. Available online: http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T45116A10981458.en (accessed on 27 August 2019).
- Cotton, E.; Pitman, N. Brachyotum confertum. The IUCN Red List of Threatened Species 2004: e.T45684A11007210. Available online: http://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T45684A11007210.en (accessed on 27 August 2019).
- Bremer, L.L.; Farley, K.A. Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers. Conserv. 2010, 19, 3893–3915. [Google Scholar] [CrossRef]
- Carnus, J.M.; Parrotta, J.; Brockerhoff, E.; Arbez, M.; Jactel, H.; Kremer, A.; Lamb, D.; O’Hara, K.; Walters, B. Planted forests and biodiversity. J. For. 2006, 104, 65–77. [Google Scholar]
- Bader, M.Y. Tropical Alpine Treelines: How Ecological Processes Control Vegetation Patterning and Dynamics; Wageningen University: Wageningen, The Netherlands, 2007; ISBN 9085045959. [Google Scholar]
- Bader, M.Y.; van Geloof, I.; Rietkerk, M. High solar radiation hinders tree regeneration above the alpine treeline in northern Ecuador. Plant Ecol. 2007, 191, 33–45. [Google Scholar] [CrossRef]
- Rada, F.; García-Núñez, C.; Rangel, S. Low temperature resistance in saplings and ramets of Polylepis sericea in the Venezuelan Andes. Acta Oecol. 2009, 35, 610–613. [Google Scholar] [CrossRef]
- Marín, F.; Quiroz Dahik, C.; Mosquera, G.; Feyen, J.; Cisneros, P.; Crespo, P. Changes in Soil Hydro-Physical Properties and SOM Due to Pine Afforestation and Grazing in Andean Environments Cannot Be Generalized. Forests 2018, 10, 17. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Ecroyd, C.E.; Leckie, A.C.; Kimberley, M.O. Diversity and succession of adventive and indigenous vascular understorey plants in Pinus radiata plantation forests in New Zealand. For. Ecol. Manag. 2003, 185, 307–326. [Google Scholar] [CrossRef]
- Lemenih, M.; Gidyelew, T.; Teketay, D. Effects of canopy cover and understory environment of tree plantations on richness, density and size of colonizing woody species in southern Ethiopia. For. Ecol. Manag. 2004, 194, 1–10. [Google Scholar] [CrossRef]
- Corredor-Velandia, S.; Vargas Ríos, O. Efectos de la creación de claros experimentales con diferentes densidades, sobre los patrones iniciales de sucesión vegetal en plantaciones de Pinus patula. In Restauración Ecológica del Bosque Altoandino. Estudios Diagnósticos y Experimentales en Los Alrededores del Embalse de Chisacá (Localidad de Usme, Bogotá D.C.); Vargas, O., Ed.; Universidad nacional de Colombia: Bogotá, Colombia, 2007; p. 517. [Google Scholar]
- Acosta Solís, M. Divisiones Fitogeográficas y Formaciones Geobotánicas del Ecuador; Casa de la Cultura Ecuatoriana: Quito, Ecuador, 1968. [Google Scholar]
- Ramsay, P.M.; Oxley, E.R.B. The growth form composition of plant communities in the ecuadorian páramos. Plant Ecol. 1997, 131, 173–192. [Google Scholar] [CrossRef]
- Hedberg, I.; Hedberg, O. Tropical-Alpine Life-Forms of Vascular Plants. Oikos 1979, 33, 297. [Google Scholar] [CrossRef]
- Chapin, F.S., III; van Cleve, K.; Chapin, M.C. Soil Temperature and Nutrient Cycling in the Tussock Growth Form of Eriophorum Vaginatum. J. Ecol. 1979, 67, 169–189. [Google Scholar] [CrossRef]
- González-Espinosa, M.; Quintana-Ascencio, P.F.; Ramírez-Marcial, N.; Gaytán-Guzmán, P. Secondary succession in disturbed Pinus-Quercus forests in the highlands of Chiapas, Mexico. J. Veg. Sci. 1991, 2, 351–360. [Google Scholar] [CrossRef]
- Fuentes-Moreno, H.; Trejo-Ortíz, A.; Cervantes, F.A. Los mamíferos del Área Reservada para la Recreación y Educación Ecológica San Juan del Monte, Las Vigas de Ramírez, Veracruz, México. Rev. Mex. Biodivers. 2017, 88, 978–984. [Google Scholar] [CrossRef]
- Minga Ochoa, D.; Verdugo Navas, A. Árboles y Arbustos de Los Ríos de Cuenca; Serie Textos Apoyo a la Docencia Universitaria del Azuay; Imprenta Don Bosco: Cuenca, Spain, 2016; ISBN 9978325425. [Google Scholar]
- Villate Suárez, C.A. Las Perchas Para aves como Estrategia de Restauración Ecológica, su Influencia Sobre la Dispersión de Semillas y Reclutamiento de Plántulas en la Microcuenca del río La Vega, Tunja—Boyacá; Universidad Pedagógica y Tecnológica de Colombia: Tunja, Colombia, 2017. [Google Scholar]
- Sandoya, V.; Pauchard, A.; Cavieres, L.A. Natives and non-natives plants show different responses to elevation and disturbance on the tropical high Andes of Ecuador. Ecol. Evol. 2017, 7, 7909–7919. [Google Scholar] [CrossRef]
- Pesántez, J.; Mosquera, G.M.; Crespo, P.; Breuer, L.; Windhorst, D. Effect of land cover and hydro-meteorological controls on soil water DOC concentrations in a high-elevation tropical environment. Hydrol. Process. 2018, 32, 2624–2635. [Google Scholar] [CrossRef]
- Riesch, F.; Stroh, H.G.; Tonn, B.; Isselstein, J. Soil pH and phosphorus drive species composition and richness in semi-natural heathlands and grasslands unaffected by twentieth-century agricultural intensification. Plant Ecol. Divers. 2018, 11, 239–253. [Google Scholar] [CrossRef]
- Roem, W.; Berendse, F. Soil acidity and nutrient supply ratio as possible factors determining changes in plant species diversity in grassland and heathland communities. Biol. Conserv. 2000, 92, 151–161. [Google Scholar] [CrossRef]
- Van den Berg, L.; Dorland, E.; Vergeer, P.; Hart, M.A.; Bobbink, R.; Roelofs, J. Decline of acid-sensitive plant species in heathland can be attributed to ammonium toxicity in combination with low pH. New Phytol. 2005, 166, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Jobbágy, E.; Vasallo, M.; Farley, K.; Piñeiro, G.; Garbulsky, M.; Nosetto, M.; B Jackson, R.; Paruelo, J. Forestación en pastizales: Hacia una visión integral de sus oportunidades y costos ecológicos. Agrociencia 2006, 10, 109–124. [Google Scholar]
- Marschner, H.; Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123849052. [Google Scholar]
- Rodríguez-Sánchez, C.A.; Vargas Ríos, O. Sucesiones experimentales en claros de plantaciones de Pinus patula y Cupressus lusitanica en los alrededores del embalse de Chisacá. In Restauración Ecológica en Zonas Invadidas por Retamo Espinoso y Plantaciones Forestales de Especies Exóticas; Vargas, O., Léon, O., Díaz Espinosa, A., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2009; ISBN 978-958-719-314-5. [Google Scholar]
- Cantillo Higuera, E.E.; Lozada Silva, A.; Pinzon Gonzalez, J. Successional study for the restoration at Carpatos forest reserve in Guasca, Cundinamarca. Colomb. For. 2009, 12, 103–118. [Google Scholar] [CrossRef]
- Albuquerque, L.B.; Aquino, F.G.; Costa, L.C.; Miranda, Z.J.G.; Sousa, S.R. Especies de Melastomataceae Juss. con potencial para la restauración ecológica de la vegetación riparia del cerrado/savana. Polibotánica 2013, 35, 1–19. [Google Scholar]
- Gómez-Ruiz, P.A.; Lindig-Cisneros, R.; Vargas-Ríos, O. Facilitation among plants: A strategy for the ecological restoration of the high-andean forest (Bogotá, D.C.—Colombia). Ecol. Eng. 2013, 57, 267–275. [Google Scholar] [CrossRef]
- Mora, J.; Figueroa, Y.; Vivas, T. Análisis multi-escala de la vegetación de los alrededores del embalse de Chisacá (Cundinamarca, Colombia). Implicaciones para la formulación de proyectos de restauración ecológica a nivel local. In Restauración Ecológica del Bosque Altoandino. Estudios Diagnósticos y Experimentales en Los Alrededores del Embalse de Chisacá (Localidad de Usme, Bogotá D.C.); Vargas, O., Grupo de Restauración, E., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2007; pp. 16–103. ISBN 978-958-701-848-6. [Google Scholar]
- Gayoso, J. Costos ambientales en plantaciones de Pinus radiata D. Don. Bosque (Valdivia) 1996, 17, 15–26. [Google Scholar] [CrossRef]
- Hofstede, R.G. Aspectos técnicos ambientales de la forestación en los Páramos. In Forestación en los Páramos. Serie Páramo 6; Abya, Y., Ed.; GTP/Abya Yala: Quito, Ecuador, 2000; ISBN 9978-04-632-1. [Google Scholar]
- Keenan, R.J.; (Hamish) Kimmins, J.P. The ecological effects of clear-cutting. Environ. Rev. 1993, 1, 121–144. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).