Sampling and Detection Strategies for the Pine Pitch Canker (PPC) Disease Pathogen Fusarium circinatum in Europe
Abstract
1. Introduction
2. Survey Programs in Different Geographical Regions
2.1. European Countries with Existing or Eradicated F. circinatum Epidemics
2.2. European Countries without F. circinatum Outbreaks
3. Protocols Used for Sampling in Nurseries
4. Protocols Used for Sampling in Forests and Plantations
4.1. Soil Samplings
4.2. Cone and Seed Samplings
4.3. Twig and Branch Samplings
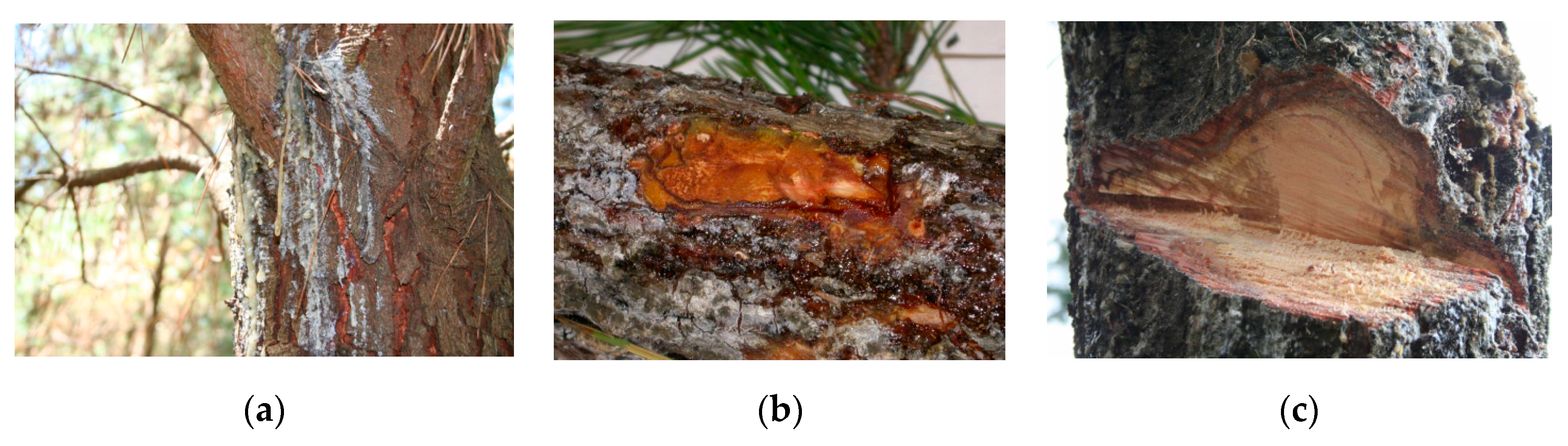
4.4. Canker Samplings
4.5. Root Samplings
4.6. Spore Trapping
4.7. Sampling of Insect Vectors of F. circinatum
5. Recommendations for Sample Processing in the Laboratory
5.1. Media for the Isolation of F. circinatum
5.2. Samples from Branches, Twigs, Cankers, Roots and Seedlings
5.3. Seed Samples
5.4. Soil Samples
5.5. Insect Samples
6. F. circinatum Morphological Identification
6.1. Culture Media for the Identification of F. circinatum
6.2. Characters Associated with the Sexual Stage of F. circinatum
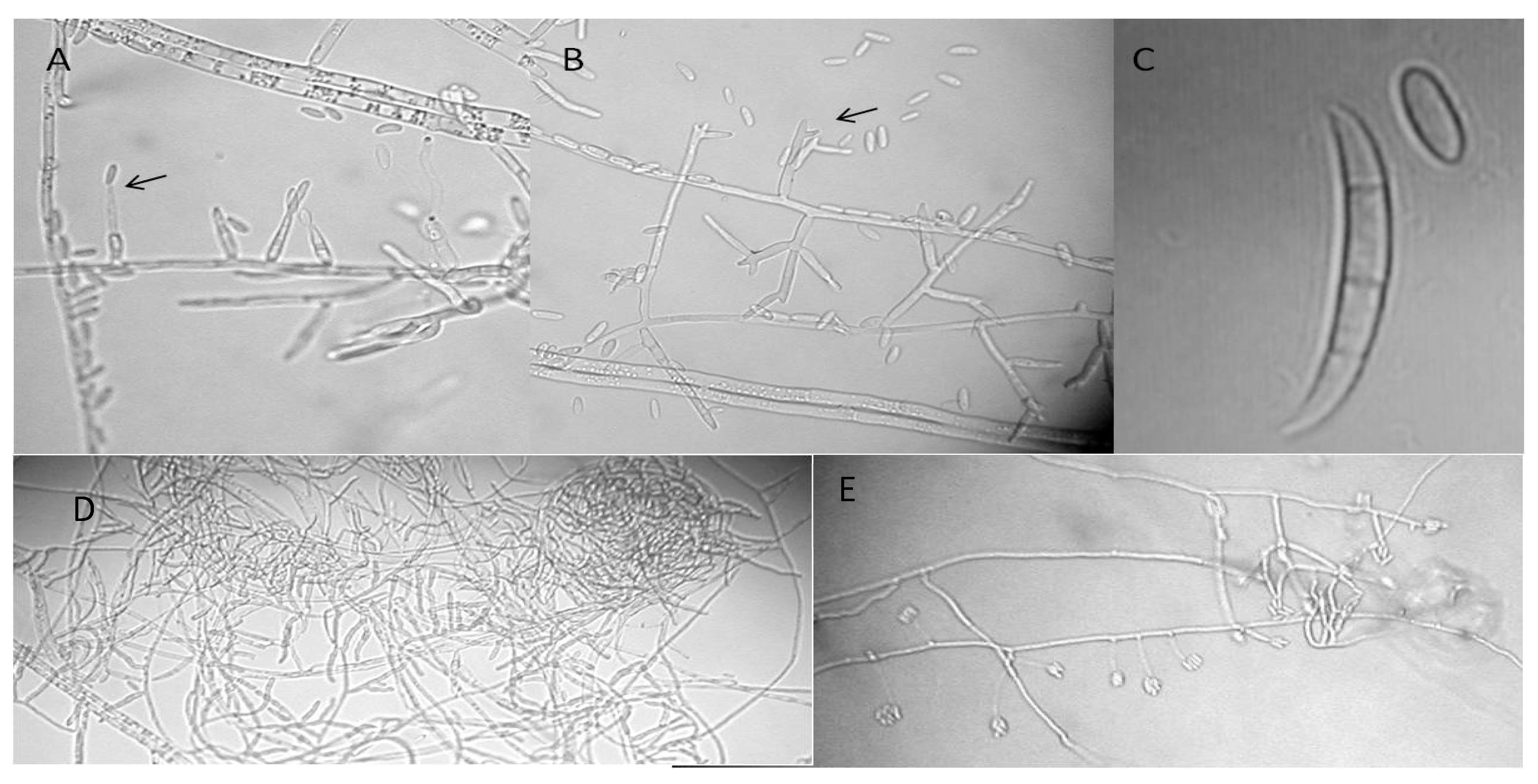
6.3. Characters Associated with the Asexual Stage of F. circinatum
6.4. Maintenance and Conservation of F. circinatum Isolates
6.5. Molecular Methods for Pathogen Detection
6.6. Diagnosis Using Conventional PCR
6.7. Diagnosis Using qPCR
6.8. Specificity of DNA-Based Tests
7. Global Situation
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alonso, R.; Bettucci, L. First report of the pitch canker fungus Fusarium circinatum affecting Pinus taeda seedlings in Uruguay. Australas. Plant Dis. Notes 2009, 4, 91–92. [Google Scholar]
- Wingfield, M.J.; Jacobs, A.; Coutinho, T.A.; Ahumada, R.; Wingfield, B.D. First report of the pitch canker fungus, Fusarium circinatum, on pines in Columbia. Australas. Plant Pathol. 2002, 41, 481–491. [Google Scholar]
- Steenkamp, E.T.; Rodas, C.A.; Kvas, M.; Wingfield, M.J. Fusarium circinatum and pitch canker of Pinus in Columbia. Australas. Plant Pathol. 2012, 41, 483–491. [Google Scholar] [CrossRef]
- Pfenning, L.H.; Da Sliva Costa, S.; Pereira De Melo, M. First report and characterization of Fusarium circinatum, the causal agent of pitch canker in Brazil. Trop. Plant Pathol. 2014, 39, 201–216. [Google Scholar] [CrossRef]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.O. First report of Fusarium subglutinans f. sp. pini on pine seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Kobayashi, T.; Muramoto, M. Pitch canker of Pinus luchuensis, a new disease of Japanese forests. Forest Pest. 1989, 40, 169–173. [Google Scholar]
- Lee, J.K.; Lee, S.H.; Yang, S.I.; Lee, Y.W. First report of pitch canker disease on Pinus rigida in Korea. Plant Pathol. 2000, 16, 52–54. [Google Scholar]
- Bragança, H.; Diogo, E.; Moniz, F.; Amaro, P. First report of pitch canker on pines caused by Fusarium circinatum in Portugal. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First report of pitch canker caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef] [PubMed]
- EPPO. First Report of Gibberella Circinata in France, EPPO Reporting Service; EPPO Global Database: Paris, France, 2006; Volume 5, Num. article: 104. [Google Scholar]
- Landeras, E.; García, P.; Fernández, Y.; Braña, M.; Fernández-Alonso, O.; Méndez-Lodos, S.; Armengol, J. Outbreak of Pitch Canker Caused by Fusarium circinatum on Pinus spp. in Northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ganley, R.J.; Watt, M.S.; Manning, L.; Iturritxa, E. A global climatic risk assessment of pitch canker disease. Can. J. For. Res. 2009, 39, 2246–2256. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority) Panel on Plant Health (PLH). Risk assessment of Gibberella circinata for the EU territory and identification and evaluation of risk management options. EFSA J. 2010, 8, 1620. [Google Scholar] [CrossRef]
- Dwinell, L.D.; Barrows-Broaddus, J.B.; Kuhlman, E.G. Pitch canker: A disease complex. Plant Dis. 1985, 69, 270–276. [Google Scholar] [CrossRef]
- Gordon, T.R.; Storer, A.J.; Wood, D.L. The pitch canker epidemics in California. Plant Dis. 2001, 85, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Iturritxa, E.; Mesanza, N.; Elvira-Recuenco, M.; Serrano, Y.; Quintana, E.; Raposo, R. Evaluation of genetic resistance in Pinus to pitch canker in Spain. Australas. Plant Pathol. 2012, 41, 601–607. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Raposo, R.; García-Serna, I.; Mesanza, N.; Kirkpatrick, S.C.; Gordon, T.R. Resistance levels of Spanish conifers against Fusarium circinatum and Diplodia pinea. For. Pathol. 2013, 43, 488–495. [Google Scholar] [CrossRef]
- Martínez-Alvarez, P.; Pando, V.; Diez, J.J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant Pathol. 2014, 63, 1086–1094. [Google Scholar] [CrossRef]
- Martín-Garcia, J.; Lukacevicová, A.; Flores-Pacheco, J.A.; Diez, J.J.; Dovrák, M. Evaluation of the susceptibility of several Czech conifer provenances to Fusarium circinatum. Forests 2018, 9, 72. [Google Scholar] [CrossRef]
- French Ministry of Agriculture. Analyse du Risque Phytosanitaire Fusarium Circinatum, Internal Document updated in 2005; Alimagri: Paris, France, 1999. [Google Scholar]
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Informe de la Reunión Del Grupo de Trabajo de Laboratorio de Diagnóstico Y Prospecciones Fitosanitarias; MAPA: Madrid, Spain, 1996. [Google Scholar]
- Dwinell, L.D.; Adams, D.; Guerra-Santos, J.J.; Aguirre, J.R.M. Pitch canker disease of Pinus radiata. In Proceedings of the 7th International Congress of Plant Pathology: Abstracts, Edinburgh, UK, 9–16 August 1998; British Society for Plant Pathology: London, UK, 1998; pp. 9–16. [Google Scholar]
- EFSA (European Food Safety Authority); Ciubotaru, R.M.; Cortiñas Abrahantes, J.; Oyedele, J.; Parnell, S.; Schrader, G.; Zancanaro, G.; Vos, S. Technical report of the methodology and Work-plan for developing plant pest survey guidelines. EFSA Supporting Publ. 2018, 15, 3. [Google Scholar]
- Bezos, D.; Martínez-Alvarez, P.; Fernández, M.; Diez, J.J. Epidemiology and management of pine pitch canker disease in Europe—A review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Sousa, E.; Rodrigues, J.; Bonifácio, L.; Naves, P.; Rodrigues, A. Chapter 6—Management and control of the Pine Wood Nematode, Bursaphelenchus xylophilus in Portugal. In Nematodes: Morphology, Functions and Management Strategies; Boeri, F., Chung, J., Hauppauge, J., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; pp. 157–178. ISBN 13 978-1614707844. [Google Scholar]
- MIPAAF. Monitoraggio nazionale degli organismi nocivi dei vegetali e prodotti vegetali anno. In The Italian Ministry of Agriculture and Forestry; MIPAAF: Rome, Italy, 2017; Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/11365 (accessed on 21 June 2019).
- MIPAAF. Monitoraggio nazionale degli organismi nocivi dei vegetali e prodotti vegetali anno. In The Italian Ministry of Agriculture and Forestry; MIPAAF: Rome, Italy, 2018; Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/12457 (accessed on 22 June 2019).
- Möykkynen, T.; Capretti, P.; Pukkala, T. Modelling the potential spread of Fusarium circinatum, the causal agent of pitch canker in Europe. Ann. For. Sci. 2015, 72, 169–181. [Google Scholar] [CrossRef]
- EPPO. Gibberella circinata. EPPO Bull. 2009, 39, 298–309. [Google Scholar] [CrossRef]
- IPPC (International Plant Protection Convention), ISPM 27 Annex 22 (International Standards for Phytosanitary Measures). Diagnostic Protocols for Regulated Pests 22: Fusarium Circinatum; IPPC: Rome, Italy, 2017; Available online: https://www.ippc.int/static/media/files/publication/en/2017/04/DP_22_2017_En_2017-04-12.pdf (accessed on 30 June 2019).
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006; pp. 150–151. [Google Scholar]
- Serrano, Y.; Iturritxa, E.; Elvira-Recuenco, M.; Raposo, R. Survival of Fusarium circinatum in soil and Pinus radiata needle and branch segments. Plant Pathol. 2016, 66, 934–940. [Google Scholar] [CrossRef]
- Dwinell, L.D. Contamination of Pinus radiata seeds in California by Fusarium circinatum. In Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, States Department of Agriculture (USDA) Forest Service, San Diego, CA, USA, 1–4 November 1999. [Google Scholar]
- Storer, A.J.; Gordon, T.R.; Clark, S.L. Association of the pitch canker fungus, Fusarium subglutinans f. sp. pini, with Monterey pine seeds and seedlings in California. Plant Pathol. 1998, 47, 649–656. [Google Scholar] [CrossRef]
- ISTA, International Rules for Testing. 7–009: Detection of Fusarium Moniliforme var. Subglutinans Wollenw & Reinke on Pinus Taeda and P. Elliotii (Pine); International Seed Testing Association (ISTA): Basseldorf, Switzerland, 2002. [Google Scholar]
- González-Peñalta, S.; Pintos-Varela, C.; Mansilla-Vázquez, J.P.; Aguín-Casal, O.; Pérez-Otero, R. Presencia de especies de Fusarium sobre semillas de Pinus spp. en Galicia. Cuad. Soc. Esp. Cienc. For. 2008, 26, 149–154. [Google Scholar]
- Pintos, C.; Pérez-Otero, R.; Aguín, O.; Mansilla, J.P.; González-Penalta, B.; Rial, C. Presencia de Fusarium circinatum en estructuras reproductivas de Pinus pinaster en Galicia. In XIV Congress of the Spanish Phytopathological Association; Congress of the Spanish Society of Phytopathology: Lugo, Spain, 2006. [Google Scholar]
- Hernandez-Escribano, L.; Iturritxa, E.; Aragonés, A.; Mesanza, N.; Berbegal, M.; Raposo, R.; Elvira-Recuenco, M. Root infection of canker pathogens, Fusarium circinatum and Diplodia sapinea, in asymptomatic trees in Pinus radiata and Pinus pinaster plantations. Forests 2018, 9, 128. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Alves-Santos, F.M.; Diez, J.J. In vitro and in vivo interactions between Trichoderma viride and Fusarium circinatum. Silva Fenn. 2012, 46, 303–316. [Google Scholar] [CrossRef]
- Chehri, K.; Salleh, B.; Soleimani, M.J.; Reddy, K.R.N.; Zakari, L. Occurrence of Fusarium spp. associated with root tissues and rhizosphere soils of forest trees and assessment of their pathogenicity on Prunus amygdalus seedlings. Aus. J. Bot. 2010, 58, 679–686. [Google Scholar] [CrossRef]
- Gordon, T.R. Pitch canker disease of pines. Phytopathology 2006, 96, 657–659. [Google Scholar] [CrossRef]
- Garbelotto, M.; Smith, T.; Schweigkofler, W. Variation in rates of spore deposition of Fusarium circinatum, the causal agent of pine pitch canker over a 12-month-period at two locations in northern California. Phytopathology 2008, 98, 137–143. [Google Scholar] [CrossRef]
- Schweigkofler, W.; O’Donnell, K.; Garbelotto, M. Detection and quantification of airborne conidia of Fusarium circinatum, the causal agent of pine pitch canker, from two California sites by using a real-time PCR approach combined with a simple spore trapping method. Appl. Environ. Microbiol. 2004, 70, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Fourie, G.; Wingfield, M.J.; Wingfield, B.D.; Jones, N.B.; Morris, A.R.; Steenkamp, E.T. Culture-independent detection and quantification of Fusarium circinatum in a pine-producing seedling nursery. SF JSF 2014, 76, 137–143. [Google Scholar]
- Quesada, T.; Hughes, J.; Smith, K.; Shin, K.; James, P.; Smith, J. Low-cost spore trap allows collection and real-time PCR quantification of airborne Fusarium circinatum spores. Forests 2018, 9, 586. [Google Scholar] [CrossRef]
- Dvořák, M.; Janoš, P.; Botella, L.; Rotková, G.; Zas, R. Spore dispersal patterns of Fusarium circinatum on an infested Monterey pine forest in North-Western Spain. Forests 2017, 8, 432. [Google Scholar] [CrossRef]
- Ioos, R.; Fourrier, C.; Iancu, G.; Gordon, T.R. Sensitive detection of Fusarium circinatum in pine seed by combining an enrichment procedure with a real-time PCR using dual-labeled probe chemistry. Phytopathology 2009, 99, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, E.G.; Dick, M.; Ganley, R.; Roques, A.; Storer, A.J. Role of insect vectors in epidemiology and invasion risk of Fusarium circinatum, and risk assessment of biological control of invasive Pinus contorta. Biol. Invasions 2006, 18, 1177–1190. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Diez, J.J.; Fernández, M.M. The pine shoot beetle Tomicus piniperda as a plausible vector of Fusarium circinatum in northern Spain. Ann. For. Sci. 2015, 72, 1079–1088. [Google Scholar] [CrossRef]
- Romón, P.; Troya, M.; Fernández de Gamarra, M.E.; Eguzkitza, A.; Iturrondobeitia, J.C.; Goldarazena, A. Fungal communities associated with pitch canker disease of Pinus radiata caused by Fusarium circinatum in northern Spain: Association with insects and pathogen-saprophyte antagonistic interactions. Can. J. Plant Pathol. 2008, 30, 241–253. [Google Scholar] [CrossRef]
- Romón, P.; Iturrondobeitia, J.C.; Gibson, K.; Lindgren, S.B.; Goldarazena, A. Quantitative association of bark beetles with pitch canker fungus and effects of verbenone on their semiochemical communication in Monterey Pine Forests in Northern Spain. Environ. Entomol. 2007, 36, 743–750. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Diez, J.J.; Fernández, M.M. Association levels between Pityophthorus pubescens and Fusarium circinatum in pitch canker disease affected plantations in northern Spain. Entomol. Gen. 2016, 36, 43–54. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Sanz-Ros, V.A.; Martín-García, J.; Fernandez, M.M.; Diez, J.J. Fungal communities associated with bark beetles in Pinus radiata plantations in Northern Spain affected by Pine Pitch Canker, with special focus on Fusarium species. Forests 2018, 9, 698. [Google Scholar] [CrossRef]
- Fourrier, C.; Antoine, S.; Piou, D.; Ioos, R. Rapid detection of Fusarium circinatum propagules on trapped pine beetles. For. Pathol. 2015, 45, 324–330. [Google Scholar] [CrossRef]
- Erbilgin, N.; Ritokova, G.; Gordon, T.R.; Wood, D.L.; Storer, A.J. Temporal variation in contamination of pine engraver beetles with Fusarium circinatum in native Monterey pine forests in California. Plant Pathol. 2008, 57, 1103–1108. [Google Scholar] [CrossRef]
- Storer, A.J.; Wood, D.L.; Gordon, T.R. Twig beetles, Pityophthorus spp. (Coleoptera: Scolytidae), as vectors of the pitch canker pathogen in California. Can. Entomol. 2004, 136, 685–693. [Google Scholar] [CrossRef]
- Andrews, S.; Pitt, J.I. Selective medium for the isolation of Fusarium species and dematiaceous Hypomycetes from cereals. Appl. Environ. Microbiol. 1986, 51, 1235–1238. [Google Scholar]
- Ioos, R.; Belhadj, A.; Menez, M. Occurrence and distribution of Microdochium nivale and Fusarium species isolated from barley, durum and soft wheat grains in France from 2000 to 2002. Mycopathologia 2004, 58, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Komada, H. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev. Plant Prot. Res. 1975, 8, 114–124. [Google Scholar]
- Aegerter, B.J.; Gordon, T.R. Rates of pitch canker induced seedling mortality among Pinus radiata families varying in levels of genetic resistance to Gibberella circinata (anamorph Fusarium circinatum). For. Ecol. Manag. 2006, 235, 14–17. [Google Scholar] [CrossRef]
- IPPC. International standards for phytosanitary measures. In Complement to the 2006 edition: ISPM No. 31 “Methodologies for Sampling of Consignments”; IPPC: Rome, Italy, 2008. [Google Scholar]
- ISTA. International Rules for Seed Testing, Chapter 2: Sampling. Int. Seed Test. Assoc. 2018, 1, i-2-44. [Google Scholar] [CrossRef]
- Nirenberg, H.I.; O’Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Geiser, D.M.; Aoki, T.; Bacon, C.W.; Bhattacharyya, M.K.; Brandt, M.E.; Brown, D.W.; Burgess, L.W.; Chulze, S.; Coleman, J.J.; Correll, J.C.; et al. One fungus, one name: Defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 2004, 103, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, W.; Nirenberg, H. The genus Fusarium—A pictorial atlas. Mitt. Aus Der Biol Bundesanstalt Fur Land-Und Forstwirtsch. 1982, 209, 406. [Google Scholar] [CrossRef]
- Fisher, N.L.; Burgess, W.; Toussoun, A.; Nelson, P.E. Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 1982, 72, 151. [Google Scholar] [CrossRef]
- Snyder, W.C.; Hansen, H.N. Advantages of natural media and environments in the culture of fungi. Phytopathology 1947, 37, 420–421. [Google Scholar] [PubMed]
- Martin, S.H.; Wingfield, B.D.; Wingfield, M.J.; Steenkamp, E.T. Structure and evolution of the Fusarium mating type locus: New insights from the Gibberella fujikuroi complex. Fungal Genet. Biol. 2011, 48, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, E.T.; Wingfield, B.D.; Coutinho, T.A.; Zeller, K.A.; Wingfield, M.J.; Marasas, W.F.O.; Leslie, J.F. PCR-based identification of MAT-1 and MAT-2 in the Gibberella fujikuroi species complex. Appl. Environ. Microbiol. 2000, 66, 4378–4382. [Google Scholar] [CrossRef] [PubMed]
- Kerényi, Z.; Moretti, A.; Waalwijk, C.; Oláh, B.; Hornok, L. Mating type sequences in asexually reproducing Fusarium species. Appl. Environ. Microbiol. 2004, 70, 4419–4423. [Google Scholar]
- Britz, H.; Coutinho, T.A.; Wingfield, M.J.; Marasas, W.F.O. Validation of the description of Gibberella circinata and morphological differentiation of the anamorph Fusarium circinatum. Sydowia 2002, 54, 922. [Google Scholar]
- Pérez-Sierra, A.; Landeras, E.; León, M.; Berbegal, M.; García-Jiménez, J.; Armengol, J. Characterization of Fusarium circinatum from Pinus spp. in northern Spain. Mycol. Res. 2007, 111, 832–839. [Google Scholar] [CrossRef]
- Inman, A.R.; Kirkpatrick, S.C.; Gordon, T.R.; Shaw, D.V. Limiting effects of low temperature on growth and spore germination in Gibberella circinata, the cause of pitch canker in pine species. Plant Dis. 2008, 92, 542–545. [Google Scholar] [CrossRef]
- Mullett, M.; Pérez-Sierra, A.; Armengol, J.; Berbegal, M. Phenotypical and molecular characterisation of Fusarium circinatum: Correlation with virulence and fungicide sensitivity. Forests 2017, 8, 458. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Kirk, P.M.; Sutton, B.C.; Pegler, D.N. Ainsworth & Bisby’s Dictionary of the Fungi; International Mycologyical Institute: Richmond, UK, 1995. [Google Scholar]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Ichikawa, K. Fusarium fractiflexum sp. nov. and two other species within the Gibberella fujikuroi species complex recently discovered in Japan that form aerial conidia in false heads. Mycoscience 2001, 42, 461–478. [Google Scholar] [CrossRef]
- Humber, R.A. Fungi: Preservation of Cultures. In Manual of Techniques in Insect Pathology; Academic Press: London, UK, 1997; pp. 269–280. [Google Scholar]
- Kitamoto, Y.; Suzuki, A.; Yamanaka, S.S.K. A new method for the preservation of fungus stock cultures by deep-freezing. Mycoscience 2002, 43, 143–149. [Google Scholar] [CrossRef]
- McGinnis, M.R.; Padhye, A.A.; Ajello, L. Storage of stock cultures of filamentous fungi, yeasts, and some aerobic actinomycetes in sterile distilled water. J. Appl. Microbiol. 1974, 28, 218–222. [Google Scholar]
- Espinel-Ingroff, A.; Montero, D.; Martin-Mazuelos, E. Long-term preservation of fungal isolates in commercially prepared cryogenic microbank vials. J. Clin. Microbiol. 2004, 42, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Onions, A.H. A comparison of some preservation techniques for fungi. Trans. Br. Mycol. Soc. 1983, 81, 535–540. [Google Scholar] [CrossRef]
- Windels, C.E.; Burnes, M.; Kommedahl, T. Five-Year preservation of Fusarium species on silica gel and soil. Phytopathology 1988, 78, 107–109. [Google Scholar] [CrossRef]
- Windels, C.E.; Bumes, P.M.; Kommendahl, T. Fusarium species stored on silica gel and soil for ten years. Mycologia 1993, 85, 21–23. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Barton, C.A. Vitrification and devitrification in cryopreservation. In Advances in Low-Temperature Biology; Steponkus, E.L., Ed.; JAI Press: London, UK, 1992; Volume 1, pp. 221–278. [Google Scholar]
- Fong, Y.K.; Anuar, S.; Lim, H.P.; Tham, F.Y.; Sanderson, F.R. A modified filter paper technique for long-term preservation of some fungal cultures. Mycologist 2000, 14, 127–130. [Google Scholar] [CrossRef]
- Ramsfield, T.D.; Dobbie, K.; Dick, M.; Ball, R.D. Polymerase chain reaction-based detection of Fusarium circiantum, the causal agent of pitch canker disease. Mol. Ecol. Res. 2008, 8, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar]
- Ioos, R.; Aloi, F.; Piškur, B.; Guinet, C.; Mullett, M.; Berbegal, M.; Bragança, H.; Cacciola, S.O.; Oskay, F.; Cornejo, C.; et al. Transferability of PCR-based diagnostic protocols: An international collaborative case study assessing protocols targeting the quarantine pine pathogen Fusarium circinatum. Sci. Rep. 2019, 9, 8195. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, T.J.; Smith, J.A.; Barnard, E.L.; Blakeslee, G. Development and evaluation of a real-time PCR seed lot screening method for Fusarium circinatum, causal agent of pitch canker disease. For. Pathol. 2012, 42, 405–411. [Google Scholar] [CrossRef]
- Lamarche, J.; Potvin, A.; Pelletier, G.; Stewart, D.; Feau, N.; Alayon, D.I.O.; Dale, A.L.; Coelho, A.; Uzunovic, A.; Bilodeau, G.J.; et al. Molecular detection of 10 of the most unwanted alien forest pathogens in Canada using real-time PCR. PLoS ONE 2015, 10, e0134265. [Google Scholar] [CrossRef]
- Luchi, N.; Pepori, A.; Bartolini, P.; Ioos, R.; Santini, A. Duplex real-time PCR assay for the simultaneous detection of Caliciopsis pinea and Fusarium circinatum in pine samples. Appl. Microbiol. Biotechnol. 2018, 102, 7135–7146. [Google Scholar] [CrossRef]
- Herron, D.A.; Wingfield, M.J.; Wingfield, B.D.; Rodas, C.A.; Marincowitz, S.; Steenkamp, E.T. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud. Mycol. 2015, 80, 131–150. [Google Scholar] [CrossRef]
- Maphosa, M.N.; Steenkamp, E.T.; Wingfield, B.D. Genome-based selection and characterization of Fusarium circinatum-specific sequences. G3 Genes Genom. Genet. 2016, 6, 631–639. [Google Scholar] [CrossRef]
- EPPO. Gibberella circinata detected again in France. In EPPO Reporting Service; EPPO: Paris, France, 2010; Volume 2, Num. article: 034. [Google Scholar]
- EPPO. Situation of Gibberella circinata in France. In EPPO Reporting Service; EPPO: Paris, France, 2009; Volume 5, Num. article: 093. [Google Scholar]
- Chapin, E.; Chauvel, G. Phytosanitary overview for 2006 of tree plantations, shrubs and clumps in green spaces. PHM Rev. Hortic. 2007, 490, 40–44. [Google Scholar]
- Britz, H.; Coutinho, T.A.; Wingfield, B.D.; Marasas, W.F.O.; Wingfield, M.J. Diversity and differentiation in two populations of Gibberella circinata in South Africa. Plant Pathol. 2005, 54, 46–52. [Google Scholar] [CrossRef]
- Steenkamp, E.T.; Makhari, O.; Coutinho, T.A.; Wingfield, B.D.; Wingfield, M.J. Evidence for a new introduction of the pitch canker fungus Fusarium circinatum in South Africa. Plant Pathol. 2014, 63, 530–538. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Steenkamp, E.T.; Coutinho, T.A.; Wingfield, M.J. The pitch canker fungus, Fusarium circinatum: Implications for South African forestry. South For. 2011, 73, 1–13. [Google Scholar] [CrossRef]
- Coutinho, T.A.; Steenkamp, E.T.; Mongwaketsi, K.; Wilmot, M.; Wingfield, M.J. First outbreak of pitch canker in a South African Plantation. Australas. Plant Pathol. 2007, 36, 256. [Google Scholar] [CrossRef]
- Fru, F.F.; Steenkamp, E.T.; Wingfield, M.J.; Santana, Q.C.; Roux, J. Unique clones of the pitch canker fungus, Fusarium circinatum, associated with a disease outbreak in a new region of South Africa. Eur. J. Plant Pathol. 2017, 148, 97–107. [Google Scholar] [CrossRef]
- Santana, Q.; Coetzee, M.; Wingfield, B.; Wingfield, M.J.; Steenkamp, E.T. Nursery-linked plantation outbreaks and evidence for multiple introductions of the pitch canker pathogen Fusarium circinatum into South Africa. Plant Pathol. 2016, 65, 357–368. [Google Scholar] [CrossRef]
- Fru, F.F.; Steenkamp, E.T.; Wingfield, M.J.; Santana, Q.C.; Roux, J. High genetic diversity of Fusarium circinatum associated with the first outbreak of pitch canker on Pinus patula in South Africa. SF JFS 2019, 81, 69–78. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted forest health: The need for a global strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef]
| Country and Years | Number in the Member State (Total Hectares) | Visually Surveyed Locations (Number/Hectares) | Number of Laboratory Samples Taken | Results for Visually Surveyed Locations +/− | Results for Laboratory Analyses+/− | In case of Positive Findings Size (ha) and Location of Demarcated Area (Infected Zone + Buffer Zone) * |
|---|---|---|---|---|---|---|
| Spain, Castilla y León, 2009–2017 | 13–121 nurseries of forest reproductive material (Pinus and/or P. menziesii) per year (658 in total) | 21–52 per year (304 in total) | 106–822 seedlings per year (3499 in total), and 1–165 seed lots per year (371 in total) | 1 without symptoms | 3 positive/3496 negative | Data not available |
| Spain, Cantabria 2009–2017 | 1–3 nurseries of forest reproductive material (Pinus and P. menziesii) per year | 1–3 nurseries per year (17 in total) | 102 samples in total | all negative | 4 positive in total | n.a. |
| Portugal, 2009–2017 | 100–224 nurseries of forest reproductive material (Pinus and P. menziesii) per year (1593 in total) | 64–209 nurseries of forest reproductive material (Pinus and P. menziesii) per year (1245 in total) | 154–259 per year (1908 in total) | 4–17 with symptoms per year (79 in total) | 0–13 positive, 154–257 negative per year (in total 40 positive, 1869 negative) | 500–6269 ha located in 1–3 different regions per year (25,935 ha in total) |
| Italy, 2010–2017 | 509–966 nurseries and garden centers of forest and ornamental trees per year | 309–481 nurseries and garden centers of forest and ornamental trees per year (3240 in total) | 43–309 per year (1111 in total) | all negative | all negative | n.a. |
| France, 2013–2017 | 1650–2554 nurseries per year (8187 in total; some of them are inspected every year) | 1062–1331 per year (4868 in total) | 17–47 per year (0–9 plants, 14–47 seed lots; 152 in total) | all negative | one positive seedlot from USA in 2016; all other years negative | n.a. |
| Bulgaria, 2015–2016 | 109 registered nurseries/1583.58 ha (authorized to issue plant passports) | 84–89 nurseries (plants for planting) 226–334 ha | 3–9 | all negative | all negative | n.a. |
| Northern Macedonia, 2007–2017 | 25–26 nurseries (total 80 ha) of forest propagation material (Pinus spp. and P. menziesii) + 5 ha of nurseries of ornamental shrubs and trees | 25–26 nurseries; 50–52 checks per year (Pinus spp. 30,337,150 seedlings and P. menziesii 1,612,400 seedlings in total) | 58–80 | all negative | all negative or n.a. | n.a. |
| Slovenia, 2007–2017 | 19–45 places of production of plants for planting (per year) | 19–45 visually surveyed locations (per year) | 0–10 (per year) | all negative | all negative | n.a. |
| Great Britain, 2007–2017 | 206–313 Nurseries registered to issue plant passports where plants were inspected | 163–323 sites inspected each year | 0–84 (per year) | all negative | all negative or n.a. | n.a. |
| Sweden, 2014–2017 | 98 registered nurseries for forest propagation material | 26–90 nurseries of forest reproductive material + 100 garden centers | 0 | no occurrence detected in surveys; no further sampling | n.a. | n.a. |
| Finland, 2007–2017 | 33–76 nurseries of forest propagation material (Pinus and P. menziesii) + ca. 400 ha of nurseries of ornamental shrubs and trees | 0–21 nurseries of forest reproductive material (up to ca. 22 million plants) + 9–77 nurseries of ornamental shrubs and trees | 0–11 per year | samples taken from up to 4 locations; all negative | all negative or n.a. | n.a. |
| Country and Years | Total Hectares/Number in the Member State | Visually Surveyed Locations Hectares/Number | Number of Laboratory Samples Taken | Results for Visually Surveyed Locations +/− | Results for Laboratory Analyses+/− | In case of Positive Findings Size (Ha) and Location of Demarcated Area (Infected Zone + Buffer Zone) |
|---|---|---|---|---|---|---|
| Spain, Castilla y León, 2009–2017 | 3–10 sites per year | 50 sites per year (129 sites in total) | 23–2329 samples per year (3560 in total) | 1–8 sites with symptoms | 11 positives (between 2009 and 2011) | 2535.7 ha in total |
| Spain, Cantabria, 2009–2017 | data not available | data not available | data not available | data not available | data not available | data not available |
| Portugal, 2009–2017 | 183 sites; about 12,000 ha (per year) | 161 sites; about 201,802 ha (per year) | 1–30 per year (110 in total) | 1–26 sites with symptoms per year (79 in total) | 2 positive (2016); other years negative | 600 ha located in 1 region (2016); other years n.a. |
| Italy, 2010–2017 | 17,920–25,279 ha + 0–4 sites (2011–2017) 68 sites (2010) per year | 27–1693 + 0–4 sites (2011–2017) 41 sites (2010) per year (total 5120 ha + 53 sites) | 0–46 per year (108 in total) | all negative | all negative | n.a. |
| France, 2013–2017 | 21,894 ha per year (109,470 ha in total) | included in forest survey | see nurseries and forests | see nurseries and forests | see nurseries and forests | see nurseries and forests |
| Bulgaria, 2015–2016 | 3134.52 ha Pinus spp. and P. menziesii forestry stand and seed orchard | 6.05–6.50 ha | 0–11 (seed samples) | negative | all negative | not applicable |
| Northern Macedonia, 2007–2017 | not available | Pinus spp. 3 ha and P. menziesii 0.5 ha | 0 | n.a. | all negative | n.a. |
| Slovenia, 2007–2017 | not available | included in forest surveys | included in forest surveys | all negative | all negative | n.a. |
| Great Britain, 2007–2017 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Sweden, 2014–2017 | for Pinus spp. a 628 ha forestry stand and 1080 ha seed orchard; and for P. menziesii a 2 ha forestry stand | 0 | n.a. | n.a. | n.a. | n.a. |
| Finland, 2007–2017 | 1782–2434.2 ha (2007–2010); 0 ha (2011–2018) | 0 | 10 places, 500 seed/place (2008); 0 (other years) | n.a. | all negative | n.a. |
| Country and Years | Total Hectares/Number in Your Member State | Visually Surveyed Locations Hectares/Number | Number of Laboratory Samples Taken | Results for Visually Surveyed Locations +/− | Results for Laboratory Analyses+/− | In case of Positive Findiings Size (ha) and Location of Demarcated Area (Infected Zone + Buffer Zone) |
|---|---|---|---|---|---|---|
| Spain, Castilla y León, 2009–2017 | 1,291,800 ha per year (11,626,200 in total) | 341–5738 sites per year (21,840 in total) | 42–212 per year (1222 in total) | 5–45 sites with symptoms per year (166 in total) | 5 positive (2009–2013) | data not available |
| Spain, Cantabria, 2009–2017 | 12,213–33,129 ha | 1160–2694 ha per year (12,446 ha in total) | 34 in total | 53 sites with symptoms | 5 positive in total | 10,666 ha in 16 demarcated areas since the detection of the pathogen in 2005 |
| Portugal, 2009–2017 | 794,500–1,015,000 ha per year (8487,616 in total) | 677–11,125 sites per year (46,696 in total) | 4–77 per year (315 in total) | 2–66 sites with symptoms per year (281 in total) | 1 positive (2016); other years negative | 550 ha located in 1 region (2016); other years n.a. |
| Italy, 2010–2017 | 614,000–793,894 ha per year | 1799–42,294 ha + 719–12 sites per year (164,184 ha + 1596 sites in total) | 30–160 (714 in total) | all negative | all negative | n.a. |
| France, 2013–2017 | 3,079,000 ha | 48-111 forest sites inspected yearly; 104–191 inspections in parks and gardens yearly (939 sites in total) | 3–17 per year | 0 | 0 | n.a. |
| Bulgaria, 2015–2016 | 240,638 ha productive forests covered by Pinus spp. 7372 ha of P. menziesii plantations. it is also present in mixed forests, parks, gardens etc. | in 263.01–386.30 ha forests covered by Pinus spp. 8–394.55 ha plantations covered by P. menziesii | 0–6 | all negative | all negative | not applicable |
| Northern Macedonia, 2007–2017 | 80,000 ha | 20,000 ha (surveyed area) Pinus spp.: 26 locations and P. menziesii: 3 locations | 0 | n.a. | n.a. | n.a. |
| Slovenia, 2007–2017 | cca. 80,000 ha of Pinus spp. and Pseudotsuga stands (around 6% of total wood stock) | 98–900 ha (surveyed area); 28–7781 locations | 0–55 | all negative | all negative | n.a. |
| Great Britain, 2007–2017 | 104 ha (P. radiata only); 416,424–448,284 ha | 19–77 sites each year; 40.4–104.257 ha each year | 0–88 (per year) | all negative | all negative or n.a. | n.a. |
| Sweden, 2014–2017 | >9.2 million ha productive forests where at least 70% of the area is covered by Pinus spp. | 0 | 0 | n.a. | n.a. | n.a. |
| Finland, 2007–2017 | 17,000,000 ha | 0 | 0 | n.a. | n.a. | n.a. |
| Country and Years | Number of Inspections in Garden Centers | Number of Laboratory Samples Taken | Results for Laboratory Analyses+/− |
|---|---|---|---|
| Spain, Castilla y León, 2009–2017 | data not available | data not available | data not available |
| Spain, Cantabria, 2009–2017 | data not available | data not available | data not available |
| Portugal, 2009–2017 | data not available | data not available | data not available |
| Italy, 2010–2017 | included in production sites for planting material—nurseries | included in production sites for planting material—nurseries | included in production sites for planting material - nurseries |
| France, 2013–2017 | 616 | 37 | 0 |
| Bulgaria, 2015–2016 | data not available | data not available | data not available |
| Northern Macedonia, 2007–2017 | included in forest survey | included in forest survey | included in forest survey |
| Slovenia, 2007–2017 | from 6 to 32 | 0–23 (per year; some samples taken from plants for planting, originating from other Member States) | n.a. or negative |
| Great Britain, 2007–2017 | 71–308 | included in production sites for planting material—nursery surveys | all negative or n.a. |
| Sweden, 2014–2017 | included in production sites for planting material—nurseries | 0 | n.a. |
| Finland, 2007–2017 | 0–186 | 0 | n.a. |
| Medium Name | Abbreviated Name | Purpose | Ingredients for 1 L of Medium |
|---|---|---|---|
| Potato dextrose agar | PDA | Isolation, identification and routine culturing | 20 g dextrose, 20 g agar, broth from 250 g white potatoes, distilled water to 1 L (a commercial preparation of PDA can also be used) |
| Potato dextrose agar supplemented with streptomycin | PDAS | Isolation | PDA ingredients, 0.5 g streptomycin, distilled water to 1 L |
| Potato dextrose agar supplemented dichloran, streptomycin and pentachloronitrobenzene | PDAspd | Isolation | PDA ingredients, 2 mg dichloran, 0.5 g streptomycin, 0.2 g pentachloronitrobenzene (PCNB), distilled water to 1 L |
| Dichloran chloramphenicol peptone agar | DCPA | Isolation | 15 g peptone, 1 g KH2PO4, 0.5 g MgSO4·7H2O, 20 g agar, 2 mg dichloran (2,6-dichloro-4-nitroanilin), 0.2 g chloramphenicol, 0.05 g violet crystal, distilled water to 1 L |
| Komada’s medium | - | Isolation | 1 g K2HPO4, 0.5 g KCl, 0.5 g MgSO4·7H2O, 10 mg Fe-Na-EDTA, 2 g L-Asparagine, 20 g D-Galactose, 15 g agar, 0.5 g PCNB, 0.25 g oxgall, 0.2 g chloramphenicol (or 0.15 g streptomycin), distilled water to 1 L (pH 3.8) |
| Fusarium selective medium | FSM | Isolation | 1.0 g KH2PO4, 0.5 g MgSO4·7H2O, 0.2 g PCNB, 10 mL of a streptomycin sulfate stock solution (30 mg/mL), 15.0 g peptone, 20.0 g agar, distilled water to 1 L |
| Modified Fusarium selective medium | Modified FSM | Isolation | The same as FSM, but containing 3 g streptomycin, 50 mg Rose Bengal, 1 g PCNB, and 1 g neomycin |
| Spezieller-Nährstoffarmer agar | SNA | Morphological identification | 1 g KH2PO4, 1 g KNO3, 0.5 g MgSO4·7H2O, 0.5 g KCl, 0.3 D-glucose, 0.2 sucrose, 20 g agar, distilled water to 1 L |
| Water agar | WA | Identification | 20 g agar, distilled water to 1 L |
| Carnation leaf agar | CLA | Morphological identification | WA overlaid with sterile pieces of carnation leaves |
| Complete medium | CM | Sporulation medium | 30 g sucrose, 2 g NaNO3, 2.5 g N-Z Amine, 1 g yeast extract 1 g, Vitamin stock solution 10 mL, 1 L of distilled water (Vitamin stock: 4 g inositol, 200 mg Ca pantothenate, 200 mg Choline·Cl, 100 mg thiamine, 75 mg pyridoxine, 75 mg nicotinamide, 50 mg ascorbic acid, 30 mg riboflavin, 5 mg p-aminobenzoic acid, 5 mg folic acid, 5 mg biotin, 50:50 ethanol:H2O to 1 L) |
| Carrot agar | CA | Sexual compatibility tests | 400 g peeled and diced carrots, 20 g agar, distilled water to 1 L |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vainio, E.J.; Bezos, D.; Bragança, H.; Cleary, M.; Fourie, G.; Georgieva, M.; Ghelardini, L.; Hannunen, S.; Ioos, R.; Martín-García, J.; et al. Sampling and Detection Strategies for the Pine Pitch Canker (PPC) Disease Pathogen Fusarium circinatum in Europe. Forests 2019, 10, 723. https://doi.org/10.3390/f10090723
Vainio EJ, Bezos D, Bragança H, Cleary M, Fourie G, Georgieva M, Ghelardini L, Hannunen S, Ioos R, Martín-García J, et al. Sampling and Detection Strategies for the Pine Pitch Canker (PPC) Disease Pathogen Fusarium circinatum in Europe. Forests. 2019; 10(9):723. https://doi.org/10.3390/f10090723
Chicago/Turabian StyleVainio, Eeva J., Diana Bezos, Helena Bragança, Michelle Cleary, Gerda Fourie, Margarita Georgieva, Luisa Ghelardini, Salla Hannunen, Renaud Ioos, Jorge Martín-García, and et al. 2019. "Sampling and Detection Strategies for the Pine Pitch Canker (PPC) Disease Pathogen Fusarium circinatum in Europe" Forests 10, no. 9: 723. https://doi.org/10.3390/f10090723
APA StyleVainio, E. J., Bezos, D., Bragança, H., Cleary, M., Fourie, G., Georgieva, M., Ghelardini, L., Hannunen, S., Ioos, R., Martín-García, J., Martínez-Álvarez, P., Mullett, M., Oszako, T., Papazova-Anakieva, I., Piškur, B., Romeralo, C., Sanz-Ros, A. V., Steenkamp, E. T., Tubby, K., ... Diez, J. J. (2019). Sampling and Detection Strategies for the Pine Pitch Canker (PPC) Disease Pathogen Fusarium circinatum in Europe. Forests, 10(9), 723. https://doi.org/10.3390/f10090723











