Differential Trends of Qinghai Spruce Growth with Elevation in Northwestern China during the Recent Warming Hiatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Plots
2.2. Tree-Ring Sampling and Laboratory Treatment
2.3. Climate Data
2.4. Statistical Analysis
3. Results
3.1. Climate Ttrends in the Middle Qilian Mountains in Recent Decades
3.2. Patterns of Tree Growth along Elevation
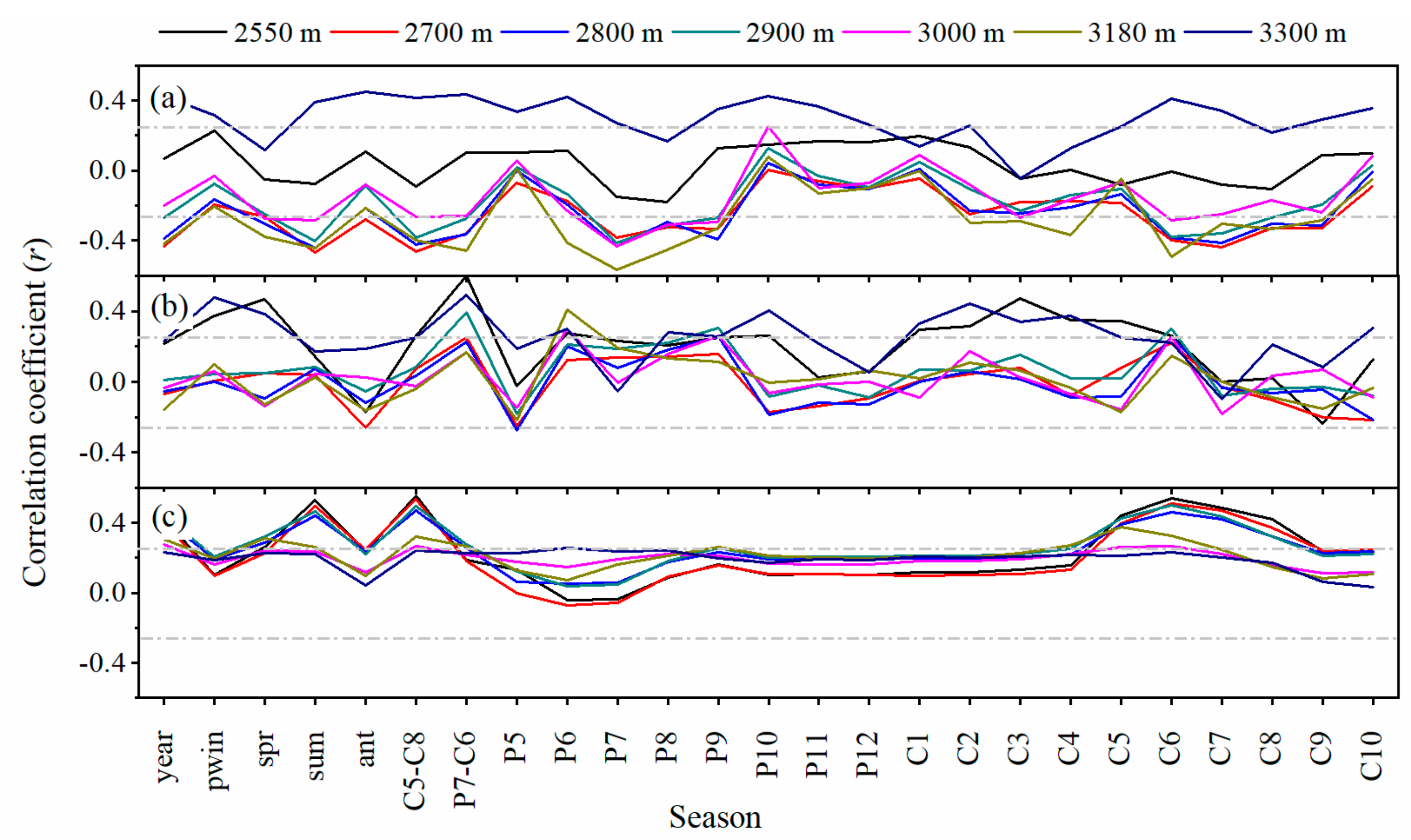
3.3. Growth-Climate Relationships
3.4. Impacts of Main Climate Factor Changes on Elevation-Related Tree Growth
4. Discussion
4.1. The Role of Climate Change on the Three Sub-Periods of Tree Growth
4.2. Variation in the Growth–Climate Relationship along Elevations
4.3. The Implications for Forest Management
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andreu, L.; Gutierrez, E.; Macias, M.; Ribas, M.; Bosch, O.; Camarero, J.J. Climate increases regional tree-growth variability in Iberian pine forests. Glob. Chang. Biol. 2007, 13, 804–815. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Consortium, P.K. Continental-scale temperature variability during the past two millennia. Nat. Geosci. 2015, 8, 981–982. [Google Scholar] [CrossRef]
- Gou, X.H.; Chen, F.H.; Jacoby, G.; Cook, E.; Yang, M.X.; Peng, H.F.; Zhang, Y. Rapid tree growth with respect to the last 400 years in response to climate warming, northeastern Tibetan Plateau. Int. J. Climatol. 2007, 27, 1497–1503. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Penuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef]
- Wilmking, M.; Juday, G.P.; Barber, V.A.; Zald, H.S.J. Recent climate warming forces contrasting growth responses of white spruce at treeline in Alaska through temperature thresholds. Glob. Chang. Biol. 2004, 10, 1724–1736. [Google Scholar] [CrossRef]
- Pasho, E.; Toromani, E.; Alla, A.Q. Climatic impact on tree-ring widths in Abies borisii-regis forests from South-East Albania. Dendrochronologia 2014, 32, 237–244. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Wang, X.C.; Pederson, N.; Chen, Z.J.; Lawton, K.; Zhu, C.; Han, S.J. Recent rising temperatures drive younger and southern Korean pine growth decline. Sci. Total Environ. 2019, 649, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.L.; Gou, X.H.; Deng, Y.; Yang, M.X.; Zhang, F. Assessing the influences of tree species, elevation and climate on tree-ring growth in the Qilian Mountains of northwest China. Trees Struct. Funct. 2017, 31, 393–404. [Google Scholar] [CrossRef]
- Zhang, W.T.; Jiang, Y.; Dong, M.Y.; Kang, M.Y.; Yang, H.C. Relationship between the radial growth of Picea meyeri and climate along elevations of the Luyashan Mountain in North-Central China. For. Ecol. Manag. 2012, 265, 142–149. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, H.; Wu, X.; Hao, Q. Climate-driven speedup of alpine treeline forest growth in the Tianshan Mountains, Northwestern China. Glob. Chang. Biol. 2015, 21, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Cooper, D.J.; Yang, J.W.; Zhang, X.; Wang, X.C. Rapid warming induces the contrasting growth of Yezo spruce (Picea jezoensis var. microsperma) at two elevation gradient sites of northeast China. Dendrochronologia 2018, 50, 52–63. [Google Scholar] [CrossRef]
- Fyfe, J.C.; Meehl, G.A.; England, M.H.; Mann, M.E.; Santer, B.D.; Flato, G.M.; Hawkins, E.; Gillett, N.P.; Xie, S.P.; Kosaka, Y.; et al. Making sense of the early-2000s warming slowdown. Nat. Clim. Chang. 2016, 6, 224–228. [Google Scholar] [CrossRef]
- Kosaka, Y.; Xie, S.P. Recent global-warming hiatus tied to equatorial Pacific surface cooling. Nature 2013, 501, 403. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.P.; Kosaka, Y. What Caused the Global Surface Warming Hiatus of 1998–2013? Curr. Clim. Chang. Rep. 2017, 3, 128–140. [Google Scholar] [CrossRef]
- Gao, L.L.; Gou, X.H.; Deng, Y.; Wang, Z.Q.; Gu, F.; Wang, F. Increased growth of Qinghai spruce in northwestern China during the recent warming hiatus. Agric. For. Meteorol. 2018, 260, 9–16. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Peng, C.H.; Ma, Z.H.; Lei, X.D.; Zhu, Q.; Chen, H.; Wang, W.F.; Liu, S.R.; Li, W.Z.; Fang, X.Q.; Zhou, X.L. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schutze, G.; Uhl, E.; Rotzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Liu, H.Y.; Williams, A.P.; Allen, C.D.; Guo, D.L.; Wu, X.C.; Anenkhonov, O.A.; Liang, E.Y.; Sandanov, D.V.; Yin, Y.; Qi, Z.H.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Adams, H.D.; Barron-Gafford, G.A.; Minor, R.L.; Gardea, A.A.; Bentley, L.P.; Law, D.J.; Breshears, D.D.; McDowell, N.G.; Huxman, T.E. Temperature response surfaces for mortality risk of tree species with future drought. Environ. Res. Lett. 2017, 12, 10. [Google Scholar] [CrossRef]
- Carroll, C.J.W.; Martin, P.H.; Knapp, A.K.; Ocheltree, T.W. Temperature induced shifts in leaf water relations and growth efficiency indicate climate change may limit aspen growth in the Colorado Rockies. Environ. Exp. Bot. 2019, 159, 132–137. [Google Scholar] [CrossRef]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.K.; Germino, M.J.; Johnson, D.M.; Reinhardt, K. The Altitude of Alpine Treeline: A Bellwether of Climate Change Effects. Bot. Rev. 2009, 75, 163–190. [Google Scholar] [CrossRef]
- Yang, B.; He, M.H.; Melvin, T.M.; Zhao, Y.; Briffa, K.R. Climate Control on Tree Growth at the Upper and Lower Treelines: A Case Study in the Qilian Mountains, Tibetan Plateau. PLoS ONE 2013, 8, 12. [Google Scholar] [CrossRef]
- Barber, V.A.; Juday, G.P.; Finney, B.P. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 2000, 405, 668–673. [Google Scholar] [CrossRef]
- Sidor, C.G.; Popa, I.; Vlad, R.; Cherubini, P. Different tree-ring responses of Norway spruce to air temperature across an altitudinal gradient in the Eastern Carpathians (Romania). Trees Struct. Funct. 2015, 29, 985–997. [Google Scholar] [CrossRef]
- Ballantyne, A.S.W.; Anderegg, W.; Kauppi, P.; Sarmiento, J.; Tans, P.; Shevliakova, E.; Pan, Y.; Poulter, B.; Anav, A.; Friedlingstein, P.; et al. Accelerating net terrestrial carbon uptake during the warming hiatus due to reduced respiration. Nat. Clim. Chang. 2017, 7, 148–152. [Google Scholar] [CrossRef]
- He, H.; Wang, S.; Zhang, L.; Wang, J.; Ren, X.; Zhou, L.; Piao, S.; Yan, H.; Ju, W.; Gu, F.; et al. Altered trends in carbon uptake in China’s terrestrial ecosystems under the enhanced summer monsoon and warming hiatus. Natl. Sci. Rev. 2019, 6, 505–514. [Google Scholar] [CrossRef]
- Li, D.L.; Liu, X.H. Climate in Gansu; China Meteorological Press: Beijing, China, 2000. [Google Scholar]
- Wang, J.Y.; Chang, X.X.; Ge, S.L.; Miao, Y.X.; Chang, Z.Q.; Hu, Z. Vertical Distribution of the Vegetation and Water and Heat Conditions of Qilian Mountain (Northern Slope). J. Northwest For. Coll. 2001, 16, 1–3. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Cook, R.E.; Briffa, K.R.; Shiyatov, S.G.; Mazepa, V.S. Tree-ring standardization and growth-trend estimation. In Methods of Dendrochronology: Applications in the Environmental Science; Springer: Amsterdam, The Netherlands, 1990; Volume 1990, pp. 104–123. [Google Scholar]
- Monserud, R.A.; Sterba, H. A basal area increment model for individual trees growing in even- and uneven-aged forest stands in Austria. For. Ecol. Manag. 1996, 80, 57–80. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Begueria, S.; Lopez-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Liu, X. Qinghai Spruce; Lanzhou University Press: Lanzhou, China, 1992; Volume 1992, pp. 48–54. [Google Scholar]
- Leblanc, D.C. Red spruce decline on whiteface mountain, New-York 1. relationships with elevation, tree age, and competition. Can. J. For. Res. Rev. Can. Res. For. 1990, 20, 1408–1414. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe-A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Choi, J.; Lorimer, C.G.; Vanderwerker, J.; Cole, W.G.; Martin, G.L. A crown model for simulating long-term stand and gap dynamics in northern hardwood forests. For. Ecol. Manag. 2001, 152, 235–258. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.G.; Alam, S.A.; Zhai, L.H.; Dawson, A.; Stadt, K.J.; Comeau, P.G. Drought causes reduced growth of trembling aspen in western Canada. Glob. Chang. Biol. 2017, 23, 2887–2902. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Shao, X.M.; Eckstein, D.; Liu, X.H. Spatial variability of tree growth along a latitudinal transect in the Qilian Mountains, northeastern Tibetan Plateau. Can. J. For. Res. Rev. Can. Res. For. 2010, 40, 200–211. [Google Scholar] [CrossRef]
- Liang, E.; Leuschner, C.; Dulamsuren, C.; Wagner, B.; Hauck, M. Global warming-related tree growth decline and mortality on the north-eastern Tibetan plateau. Clim. Chang. 2016, 134, 163–176. [Google Scholar] [CrossRef]
- Zhao, S.D.; Jiang, Y.; Dong, M.Y.; Xu, H.; Manzanedo, R.D.; Pederson, N. Early monsoon failure and mid-summer dryness induces growth cessation of lower range margin Picea crassifolia. Trees Struct. Funct. 2018, 32, 1401–1413. [Google Scholar] [CrossRef]
- Wu, X.C.; Liu, H.Y.; Wang, Y.F.; Deng, M.H. Prolonged limitation of tree growth due to warmer spring in semi-arid mountain forests of Tianshan, northwest China. Environ. Res. Lett. 2013, 8, 9. [Google Scholar] [CrossRef]
- Sanchez-Salguero, R.; Camarero, J.J.; Hevia, A.; Madrigal-Gonzalez, J.; Linares, J.C.; Ballesteros-Canovas, J.A.; Sanchez-Miranda, A.; Alfaro-Sanchez, R.; Sanguesa-Barreda, G.; Galvan, J.D.; et al. What drives growth of Scots pine in continental Mediterranean climates: Drought, low temperatures or both? Agric. For. Meteorol. 2015, 206, 151–162. [Google Scholar] [CrossRef]
- Yu, L.; Huang, L.; Shao, X.M.; Xiao, F.J.; Wilmking, M.; Zhang, Y.X. Warming-Induced Decline of Picea crassifolia Growth in the Qilian Mountains in Recent Decades. PLoS ONE 2015, 10, 13. [Google Scholar] [CrossRef]
- Littell, J.S.; Peterson, D.L.; Tjoelker, M. Douglas-fir growth in mountain ecosystems: Water limits tree growth from stand to region. Ecol. Monogr. 2008, 78, 349–368. [Google Scholar] [CrossRef]
- Kunstler, G.; Albert, C.H.; Courbaud, B.; Lavergne, S.; Thuiller, W.; Vieilledent, G.; Zimmermann, N.E.; Coomes, D.A. Effects of competition on tree radial-growth vary in importance but not in intensity along climatic gradients. J. Ecol. 2011, 99, 300–312. [Google Scholar] [CrossRef]
- Pedersen, B.S. The role of stress in the mortality of midwestern oaks s indicated by growth prior to death. Ecology 1998, 79, 79–83. [Google Scholar] [CrossRef]
- Lutz, D.A.; Shugart, H.H.; Ershov, D.V.; Shuman, J.K.; Isaev, A.S. Boreal forest sensitivity to increased temperatures at multiple successional stages. Ann. For. Sci. 2013, 70, 299–308. [Google Scholar] [CrossRef][Green Version]
- Chang, X.X.; Zhao, W.Z.; He, Z.B. Radial pattern of sap flow and response to microclimate and soil moisture in Qinghai spruce (Picea crassifolia) in the upper Heihe River Basin of arid northwestern China. Agric. For. Meteorol. 2014, 187, 14–21. [Google Scholar] [CrossRef]
- Tian, F.X.; Zhao, C.Y.; Feng, Z.D. Simulating evapotranspiration of Qinghai spruce (Picea crassifolia) forest in the Qilian Mountains, northwestern China. J. Arid Environ. 2011, 75, 648–655. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Im, S.T.; Petrov, I.A. Warming hiatus and evergreen conifers in Altay-Sayan Region, Siberia. J. Mt. Sci. 2018, 15, 2579–2589. [Google Scholar] [CrossRef]
- Brubaker, L.B. Responses of tree poputations to climatic-change. Vegetatio 1986, 67, 119–130. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant Developmental Responses to Climate Change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.Y.; Wang, Y.F.; Piao, S.L.; Lu, X.M.; Camarero, J.J.; Zhu, H.F.; Zhu, L.P.; Ellison, A.M.; Ciais, P.; Penuelas, J. Species interactions slow warming-induced upward shifts of treelines on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2016, 113, 4380–4385. [Google Scholar] [CrossRef]
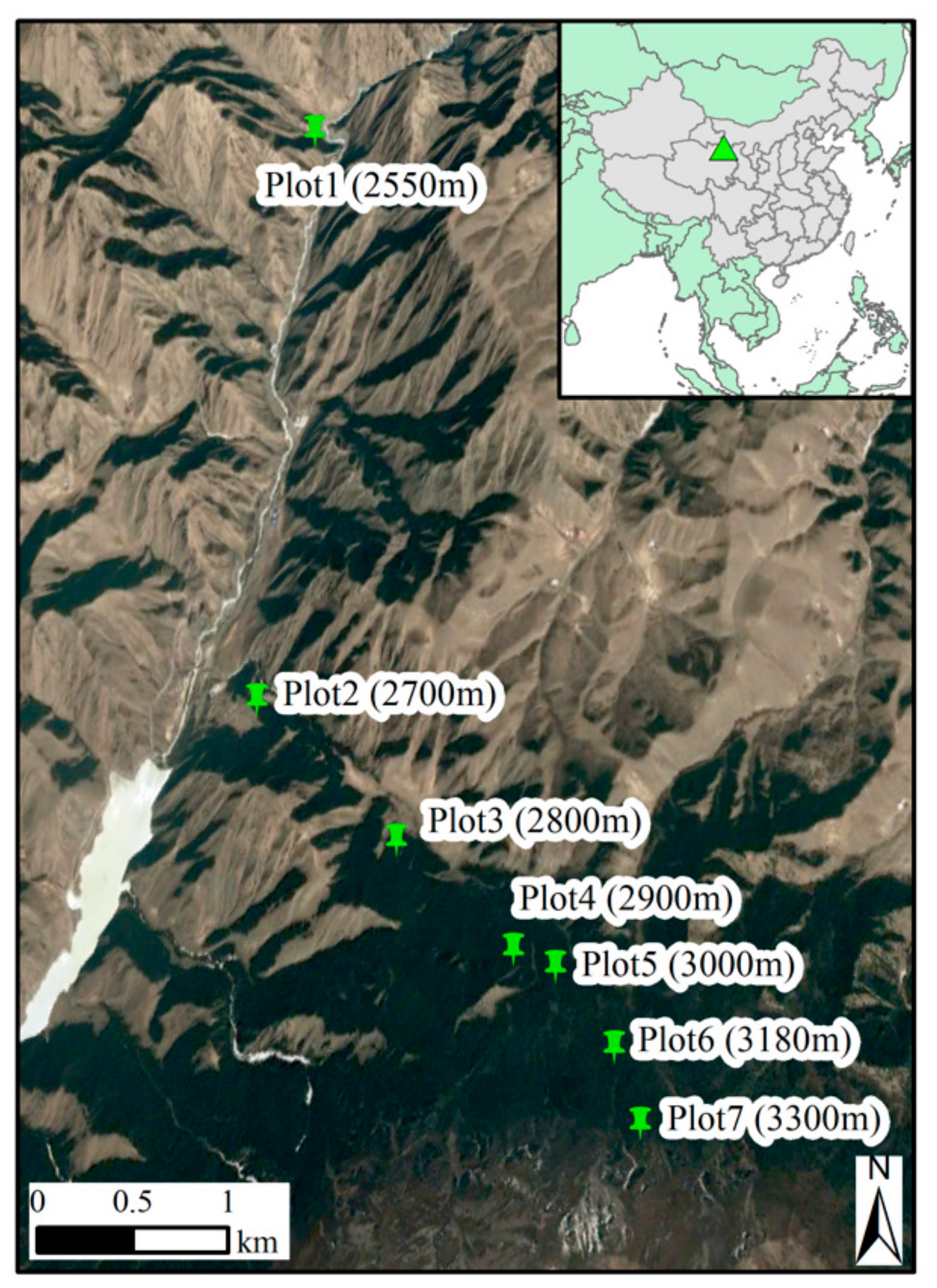



| Plots | Elevation (m a.s.l.) | Aspect (°) | Slope (°) | Canopy Density | Stand Density (Trees/ha) | Age (Years) | DBH (cm) | Number of Cores Analyzed |
|---|---|---|---|---|---|---|---|---|
| P1 | 2550 | NE12 | 41 | 0.55 | 1375 | 64 (31–129) | 11.6 (5.9–27.2) | 26 |
| P2 | 2700 | NE45 | 33 | 0.75 | 1625 | 68 (45–115) | 12.3 (5.6–24.8) | 59 |
| P3 | 2800 | NE7 | 23 | 0.85 | 3025 | 66 (39–116) | 14.1 (6.6–29.5) | 122 |
| P4 | 2900 | NE24 | 32 | 0.90 | 2725 | 65 (33–109) | 12.7 (5.5–31.9) | 107 |
| P5 | 3000 | NW7 | 23 | 0.75 | 2125 | 58 (30–102) | 12.6 (5.5–31.9) | 48 |
| P6 | 3180 | NE25 | 32 | 0.55 | 825 | 113 (30–197) | 28.9 (6.3–39.1) | 22 |
| P7 | 3300 | NE32 | 35 | 0.20 | 300 | 80 (41–169) | 15.1 (5.3–35.2) | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yu, P.; Zhang, L.; Wang, Y.; Yu, Y.; Wang, S. Differential Trends of Qinghai Spruce Growth with Elevation in Northwestern China during the Recent Warming Hiatus. Forests 2019, 10, 712. https://doi.org/10.3390/f10090712
Wang B, Yu P, Zhang L, Wang Y, Yu Y, Wang S. Differential Trends of Qinghai Spruce Growth with Elevation in Northwestern China during the Recent Warming Hiatus. Forests. 2019; 10(9):712. https://doi.org/10.3390/f10090712
Chicago/Turabian StyleWang, Bin, Pengtao Yu, Lei Zhang, Yanhui Wang, Yipeng Yu, and Shunli Wang. 2019. "Differential Trends of Qinghai Spruce Growth with Elevation in Northwestern China during the Recent Warming Hiatus" Forests 10, no. 9: 712. https://doi.org/10.3390/f10090712
APA StyleWang, B., Yu, P., Zhang, L., Wang, Y., Yu, Y., & Wang, S. (2019). Differential Trends of Qinghai Spruce Growth with Elevation in Northwestern China during the Recent Warming Hiatus. Forests, 10(9), 712. https://doi.org/10.3390/f10090712





