Growth of Abies sachalinensis Along an Urban Gradient Affected by Environmental Pollution in Sapporo, Japan
Abstract
:1. Introduction
- What was the growth of A. sachalinensis in the past decades in the urban and rural areas of Sapporo, northern Japan?
- Are the growth trends of A. sachalinensis similar to worldwide trends of urban tree growth?
- Can differences in the growth of A. sachalinensis be found regarding the sampling sites?
2. Materials and Methods
2.1. Climate of Sapporo
2.2. Environmental Pollution Data
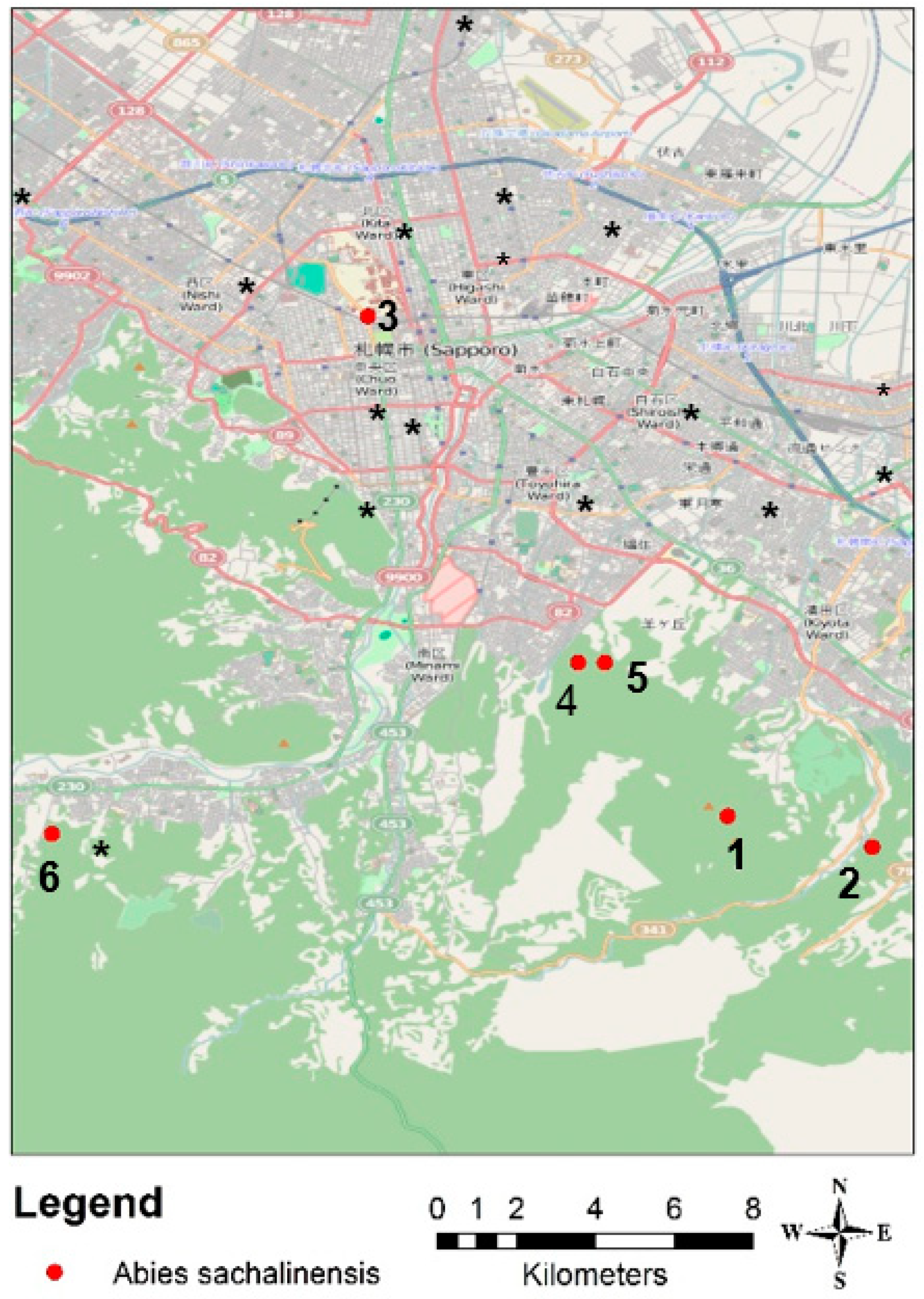
2.3. Sample Tree Species
2.4. Data Collection
2.5. Core and Data Processing
2.6. Statistics
2.7. Trend Analysis (Long-Term Trends)
3. Results
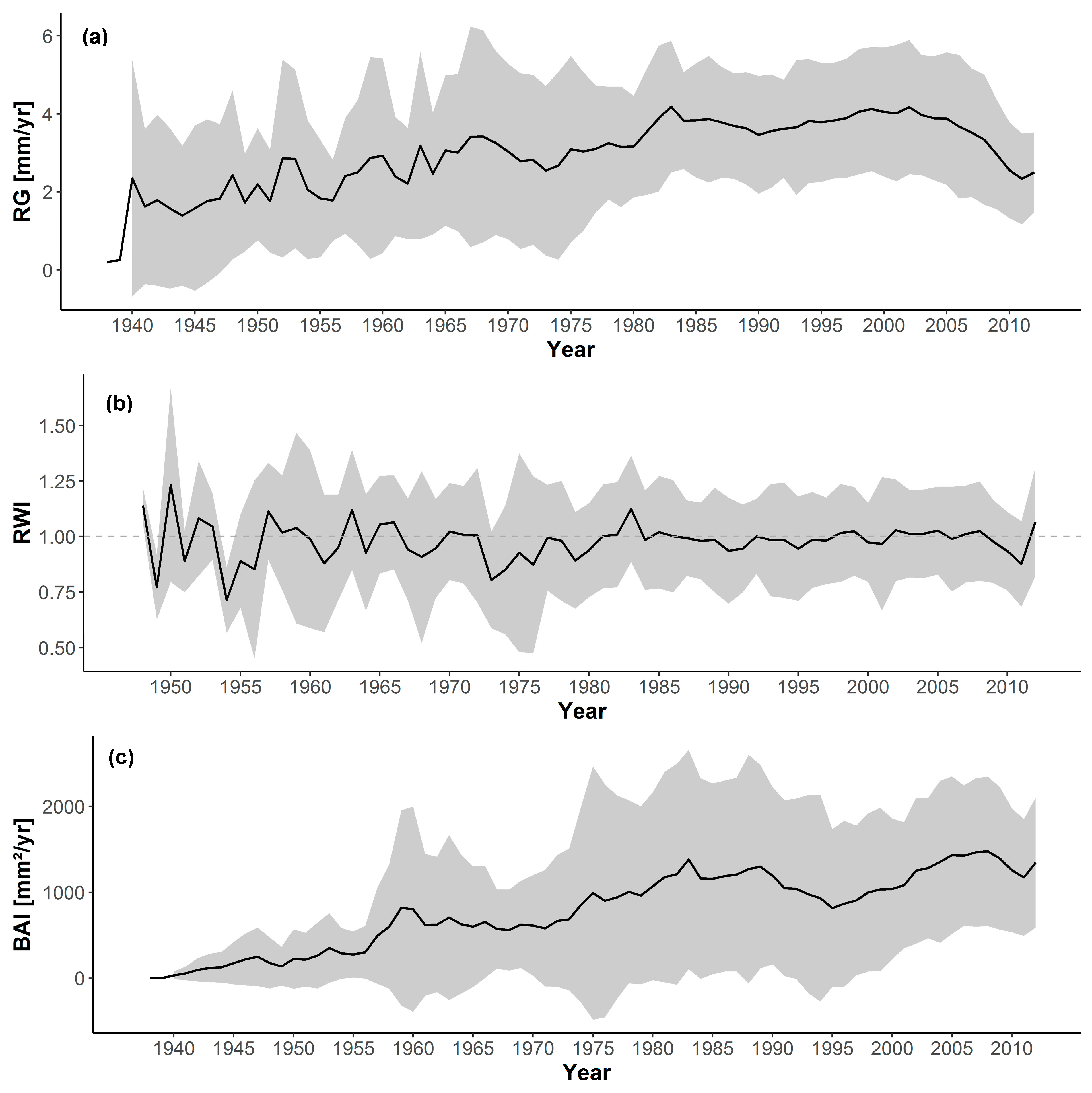
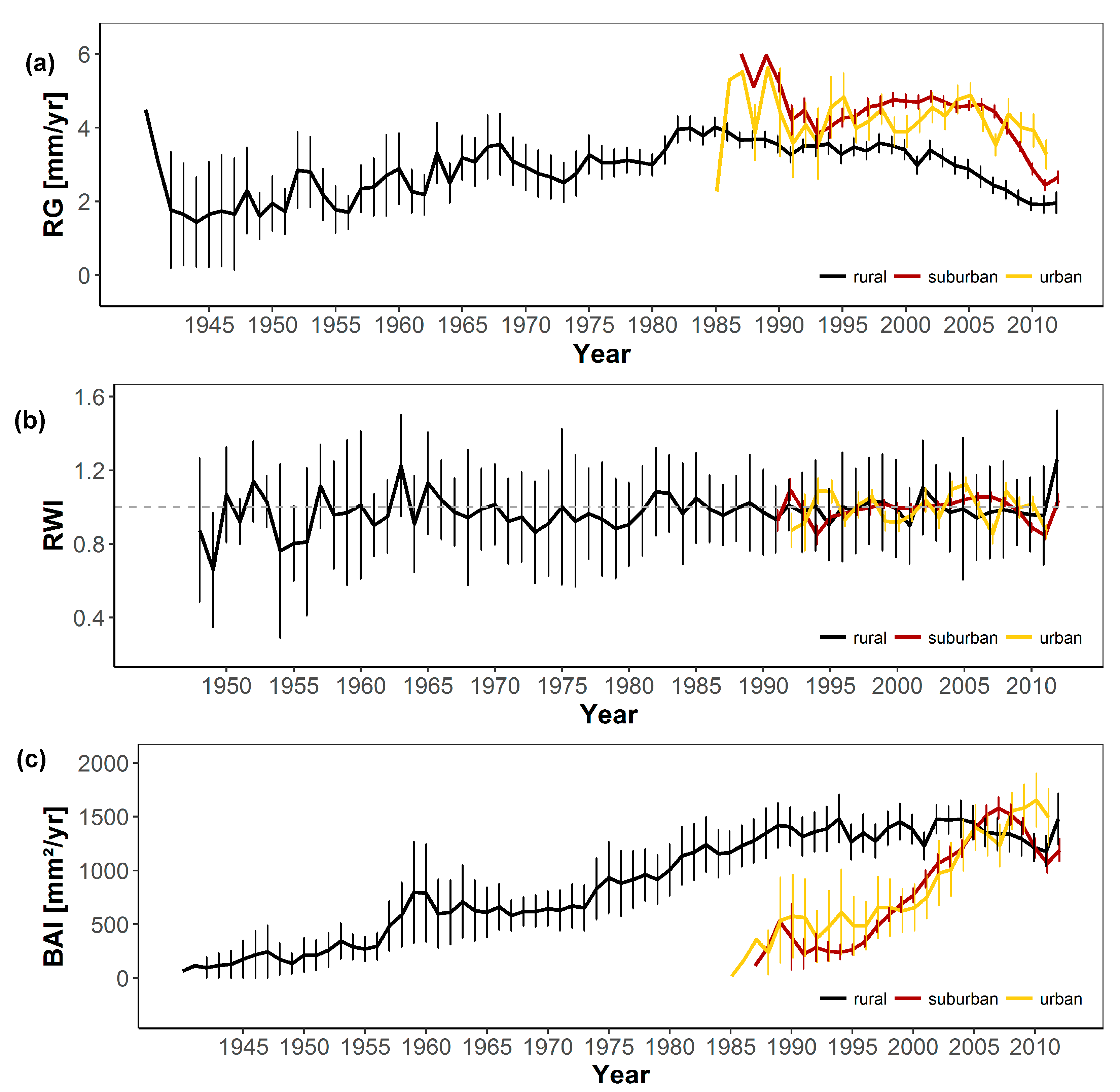
3.1. Short-Term Growth Trends
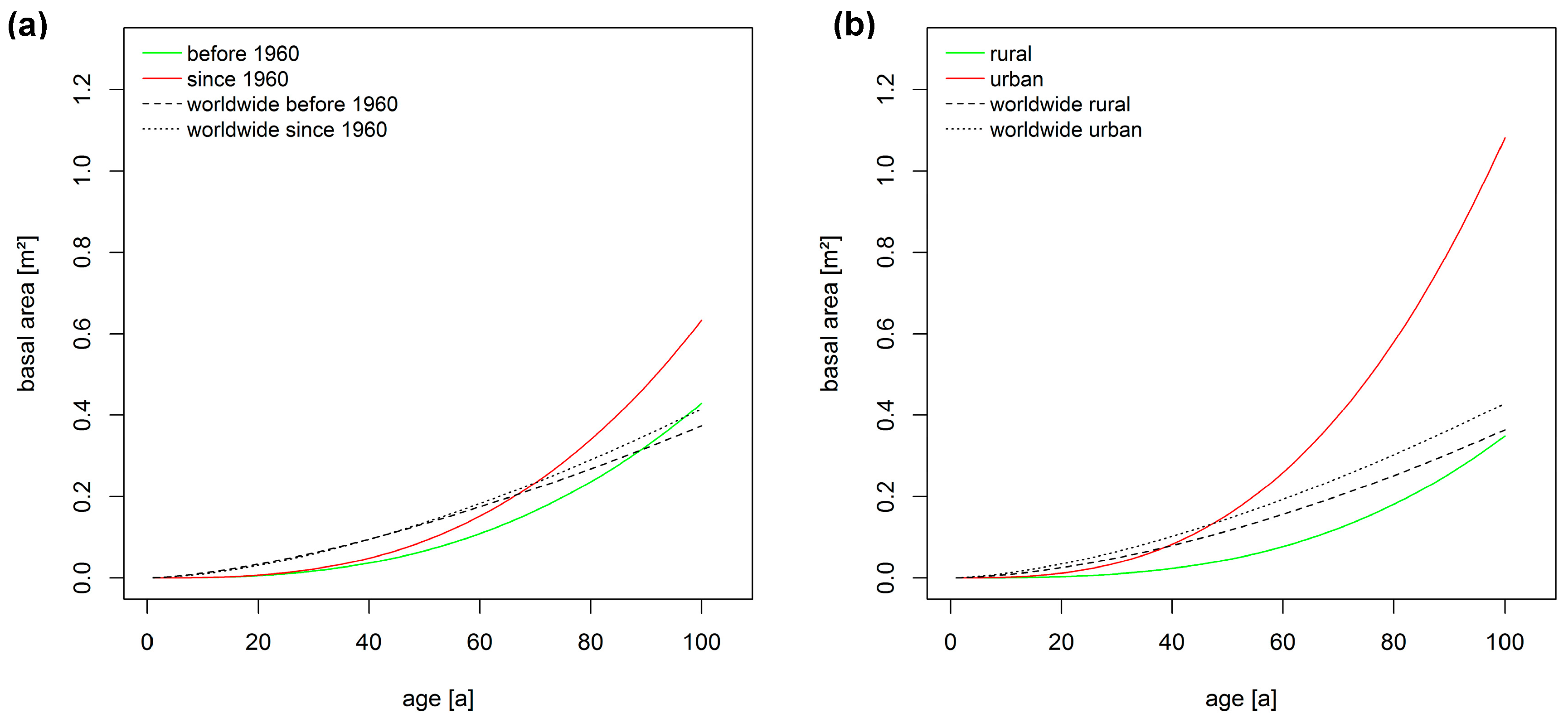
3.2. Long-Term Growth Trends
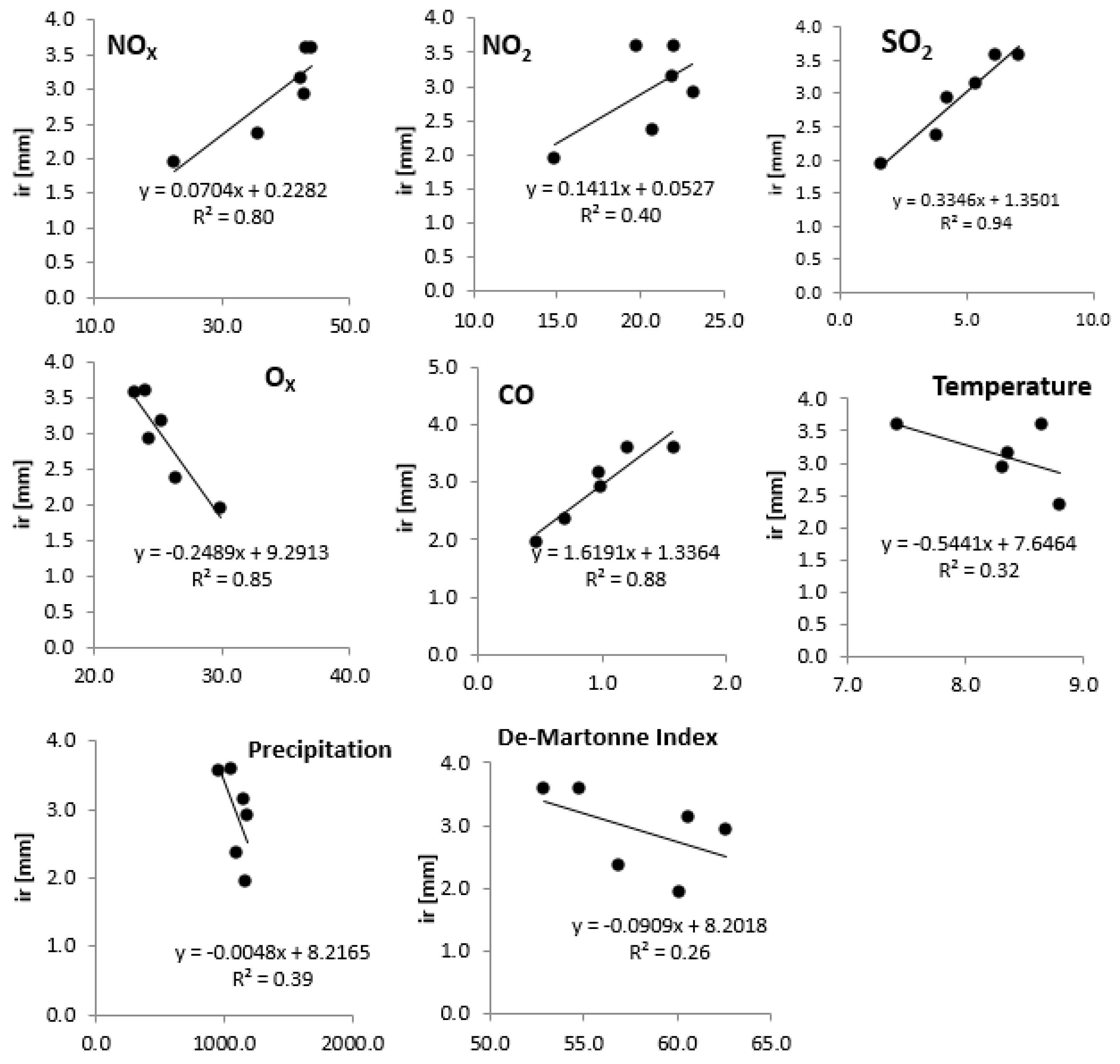
3.3. Relationships of Growth with Environmental Pollution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Bauer, L.I.; Krupa, S.V. The Valley of Mexico: Summary of Observational Studies on its Air Quality and Effects on Vegetation. Environ. Pollut. 1990, 65, 109–118. [Google Scholar] [CrossRef]
- Guardans, R. Estimation of climate change influence on the sensitivity of trees in Europe to air pollution concentrations. Environ. Sci. Policy 2002, 5, 319–333. [Google Scholar] [CrossRef]
- Juknys, R.; Vencloviene, J.; Stravinskiene, V.; Augustaitis, A.; Bartkevicius, E. Scots pine (Pinus sylvestris L.) growth and condition in a polluted environment: From decline to recovery. Environ. Pollut. 2003, 125, 205–212. [Google Scholar] [CrossRef]
- Kint, V.; Aertsen, W.; Campioli, M.; Vansteenkiste, D.; Delcloo, A.; Muys, B. Radial growth change of temperate tree species in response to altered regional climate and air quality in the period 1901–2008. Clim. Chang. 2012, 115, 343–363. [Google Scholar] [CrossRef]
- Legge, A.H.; Jager, H.J.; Krupa, S.V. Sulfur dioxide. In Recognition of Air Pollution Injury to Vegetation: A Pictorial Atlas; Flagler, R., Ed.; Air and Waste Management Association: Pittsburgh, PA, USA, 1999; pp. 3/1–3/42. [Google Scholar]
- Lorenz, M.; Clarke, N.; Paoletti, E.; Bytnerowicz, A.; Grulke, N.; Lukina, N.; Sase, H.; Staelens, J. Air pollution impacts on forests in changing climate. In Forest and Society–Responding to Global Drivers of Change; IUFRO World Series; Mery, G.E.A., Ed.; International Union of Forest Research Organizations: Vienna, Austria, 2010; pp. 55–74. [Google Scholar]
- Scharnweber, T.; Manthey, M.; Criegee, C.; Bauwe, A.; Schroder, C.; Wilmking, M. Drought matters—Declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For. Ecol. Manag. 2011, 262, 947–961. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—a dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Penninckx, V.; Meerts, P.; Herbauts, J.; Gruber, W. Ring width and element concentrations in beech (Fagus sylvatica L.) from a periurban forest in central Belgium. For. Ecol. Manag. 1999, 113, 23–33. [Google Scholar] [CrossRef]
- Chappelka, A.H.; Grulke, N. Disruption of the ‘disease triangle’ by chemical and physical environmental change. Plant Biol. 2016, 18, 5–12. [Google Scholar] [CrossRef]
- Innes, J.L. Forest Health: Its Assessment and Status; CAB International Publishers: Oxon, UK, 1993. [Google Scholar]
- Bytnerowicz, A.; Omasa, K.; Paoletti, E. Integrated effects of air pollution and climate change on forests: A northern hemisphere perspective. Environ. Pollut. 2007, 147, 438–445. [Google Scholar] [CrossRef]
- Spiecker, H.; Mielikäinen, K.; Kölh, M.; Skovsgaard, J.P. Growth Trends in European Forests: Studies from 12 Countries; Springer: Berlin, Germany, 1996. [Google Scholar]
- Fang, J.; Kato, T.; Guo, Z.; Yang, Y.; Hu, H.; Shen, H.; Zhao, X.; Kisimoto-Mo, A.W.; Tang, Y.; Houghton, R.A. Evidence for environmentally enhanced forest growth. Proc. Natl. Acad. Sci. USA 2014, 111, 9527–9532. [Google Scholar] [CrossRef] [Green Version]
- Churkina, G.; Zaehle, S.; Hughes, J.; Viovy, N.; Chen, Y.; Jung, M.; Heumann, B.W.; Ramankutty, N.; Heimann, M.; Jones, C. Interactions between nitrogen deposition, land cover conversion, and climate change determine the contemporary carbon balance of Europe. Biogeosciences 2010, 7, 2749–2764. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5 (Suppl. 1), 4967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dahlhausen, J.; Schütze, G.; Perkins, D.; Rötzer, T.; Caldentey, J.; Koike, T.; van Con, T.; et al. Climate change accelerates growth of urban trees in metropolises worldwide. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rötzer, T.; Biber, P.; Moser, A.; Schäfer, C.; Pretzsch, H. Stem and root diameter growth of European beech and Norway spruce under extreme drought. For. Ecol. Manag. 2017, 406, 184–195. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Di Filippo, A.; Alessandrini, A.; Maugeri, M. Drought-driven growth reduction in old beech (Fagus Sylvatica L.) forests of the central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Alaimo, M.G.; Lipani, B.; Lombardo, M.G.; Orecchio, S.; Turano, M.; Melati, M.R. The mapping of stress in the predominant plants in the city of Palermo by lead dosage. Aerobiología 2000, 16, 47–54. [Google Scholar] [CrossRef]
- Baycu, G.; Tolunay, D.; Özden, H.; Günebakan, S. Ecophysiological and seasonal variations in Cd, Pb, Zn, and Ni concentrations in the leaves of urban deciduous trees in Istanbul. Environ. Pollut. 2006, 143, 545–554. [Google Scholar] [CrossRef]
- Bhatti, G.H.; Iqbal, M.Z. Investigations into the effect of automobile exhausts on the phenology, periodicity and productivity of some roadside trees. Acta Soc. Bot. Pol. 1988, 57, 395–399. [Google Scholar] [CrossRef]
- Koike, T.; Watanabe, M.; Hoshika, Y.; Kitao, M.; Matumura, H.; Funada, R.; Izuta, T. Effects of ozone and forest ecosystems in East and Southeast Asia. Dev. Environ. Sci. 2013, 13, 371–390. [Google Scholar]
- Pourkhabbaz, A.; Rastin, N.; Olbrich, A.; Langenfeld-Heyser, E.; Polle, A. Influence of Environmental Pollution on Leaf Properties of Urban Plane Trees, Platanus orientalis L. Bull. Environ. Contam. Toxicol. 2010, 85, 251–255. [Google Scholar] [CrossRef]
- Izuta, T. Air Pollution Impacts on Plants in East Asia; Springer: Tokyo, Japan, 2017. [Google Scholar]
- Joshi, P.C.; Abhishek, S. Physiological responses of some tree species under roadside automobile pollution stress around city of Haridwar, India. Environmentalist 2007, 27, 365–374. [Google Scholar] [CrossRef]
- Pandey, J.; Agrawal, M. Evaluation of air pollution phytotoxicity in a seasonally dry tropical urban environment using three woody perennials. New Phytol. 1994, 126, 53–61. [Google Scholar] [CrossRef]
- Williams, R.J.H.; Lloyd, M.M.; Ricks, G.R. Effects of atmospheric pollution on deciduous woodland I: Some effects on leaves of Quercus petraea (Mattuschka) Leibl. Environ. Pollut. 1971, 2, 57–68. [Google Scholar] [CrossRef]
- Kozlowski, T.T. The impact of environmental pollution on shade trees. J. Arboric. 1986, 12, 29–37. [Google Scholar]
- Moser, A.; Rötzer, T.; Pauleit, S.; Pretzsch, H. Structure and ecosystem services of small-leaved lime (Tilia cordata Mill.) and black locust (Robinia pseudoacacia L.) in urban environments. Urban For. Urban Green. 2015, 14, 1110–1121. [Google Scholar] [CrossRef]
- Morgenroth, J.; Buchan, G.D. Soil moisture and aeration beneath pervious and impervious pavements. Arboric. Urban For. 2009, 35, 135–141. [Google Scholar]
- Bühler, O.; Kristoffersen, P.; Larsen, S.U. Growth of Street Trees in Copenhagen With Emphasis on the Effect of Different Establishment Concepts. Arboric. Urban For. 2007, 5, 330–337. [Google Scholar]
- Rahman, M.A.; Stringer, P.; Ennos, A.R. Effect of Pit Design and Soil Composition on Performance of Pyrus calleryana Street Trees in the Establishment Period. Arboric. Urban For. 2013, 39, 256–266. [Google Scholar]
- Akbari, H.; Pomerantz, M.; Taha, H. Cool surfaces and shade trees to reduce energy use and improve air quality in urban areas. Sol. Energy 2001, 70, 295–310. [Google Scholar] [CrossRef]
- Beatty, R.A.; Heckman, C.T. Survey of urban tree programs in the United States. Urban Ecol. 1981, 5, 81–102. [Google Scholar] [CrossRef]
- Rodríguez Martín, J.A.; De Arana, C.; Ramos-Miras, J.J.; Gil, C.; Boluda, R. Impact of 70 years urban growth associated with heavy metalpollution. Environ. Pollut. 2015, 196, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, F.J.; Nowak, D.J. Spatial heterogeneity and air pollution removal by an urban forest. Landsc. Urban Plan. 2009, 90, 102–110. [Google Scholar] [CrossRef]
- Ding, H.; Pretzsch, H.; Schütze, G.; Rötzer, T. Size dependency of tree growth response to drought among Norway spruce and European beech individuals in monospecific and mixed-species stands. Plant Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Köppen, W.; Geiger, G. Handbuch der Klimatologie; Gebrüder Borntraeger: Berlin, Germany, 1930–1939. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Chmielewski, F.M.; Rötzer, T. Response of tree phenology to climate change across Europe. Agric. For. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Moser, A.; Uhl, E.; Rötzer, T.; Biber, P.; Dahlhausen, J.; Lefer, B.; Pretzsch, H. Effects of Climate and the Urban Heat Island Effect on Urban Tree Growth in Houston. Open J. For. 2017, 7, 428–445. [Google Scholar] [CrossRef] [Green Version]
- De Martonne, E. Une novelle fonction climatologique: L’indice d’aridité. La Météorol. 1926, 21, 449–458. [Google Scholar]
- Chittendon, F. RHS Dictionary of Plants Plus Supplement; Oxford University Press: Oxford, UK, 1951–1956. [Google Scholar]
- Matsuda, K.; Shibuya, M.; Koike, T. Maintenance and rehabilitation of the mixed conifer-broadleaf forests in Hokkaido, northern Japan. Eur. J. For. Res. 2002, 5, 119–130. [Google Scholar]
- Sugai, T.; Kitao, M.; Watanabe, T.; Koike, T. Can needle nitrogen content explain the interspecific difference in ozone sensitivities of photosynthesis between Japanese larch (Larix kaempferi) and Sakhalin fir (Abies sachalinensis)? Photosynthetica 2019, 57, 540–547. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Watanabe, M.; Matsumura, H.; Kohno, Y.; Izuta, T. Experimental studies on the effects of ozone on growth and photosynthetic activity of Japanese forest tree species. Asian J. Atmos. Environ. 2011, 5, 65–67. [Google Scholar] [CrossRef]
- Zhang, D.; Katsuki, T.; Rushforth, K. Abies sachalinensis. IUCN 2013. [Google Scholar] [CrossRef]
- Cook, E.R.; Briffa, K.R.; Meko, D.M.; Graybill, D.A.; Funkhouser, G. The “segment length curse” in long tree-ring chronology development for palaeoclimatic studies. Holocene 1995, 5, 229–237. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1976. [Google Scholar]
- Schweingruber, F.H. Tree Rings. Basics and Applications of Dendrochronology; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Bunn, A. Dendrochronology Program Library in R. Dendrochronologia 2015, 26, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Nowak, D.J.; Kuroda, M.; Crane, D.E. Tree mortality rates and tree population projections in Baltimore, Maryland, USA. Urban For. Urban Green. 2004, 2, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Skiera, B.; Moll, G. The sad state of city trees. Am. For. (March/April) 1992, 98, 61–64. [Google Scholar]
- Hiura, T.; Sano, J.; Konno, Y. Age structure and response to fine-scale disturbances of Abies sachalinensis, Picea jezoensis, Picea glehnii, and Betula ermanii growing under the influence of a dwar bamboo understory in northern Japan. Can. J. For. Res. 1996, 26, 289–297. [Google Scholar] [CrossRef]
- Pretzsch, H. Modellierung des Waldwachstums; Parey Buchverlag, Blackwell Wissenschafts-Verlag GmbH: Berlin, Germany, 2001. [Google Scholar]
- Hasenauer, H. Dimensional relationship of open-grown trees in Austria. For. Ecol. Manag. 1997, 96, 197–206. [Google Scholar] [CrossRef]
- Umeki, K. Growth characteristics of six tree species on Hokkaido Island, northern Japan. Ecol. Res. 2001, 16, 435–450. [Google Scholar] [CrossRef]
- Peper, P.J.; McPherson, E.G.; Mori, S.M. Predictive equations for dimensions and leaf area of coastal Southern California street trees. J. Arboric. 2001, 27, 169–180. [Google Scholar]
- Schweingruber, F.H. Tree Rings and Environment; Swiss Federal Institute for Forest, Snow and Landscape Research: Birmensdorf, Switzerland, 1996. [Google Scholar]
- Funakoshi, S. Shoot growth and winter bud formation in Abies sachalinensis MAST. Res. Bull. Coll. Exp. For. Hokkaido Univ. 1985, 42, 785–808. [Google Scholar]
- Moser, A.; Uhl, E.; Rötzer, T.; Biber, P.; Caldentey, J.M.; Pretzsch, H. Effects of climate trends and drought events on urban tree growth in Santiago de Chile. Cienc. Investig. Agrar. 2018, 45, 35–50. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Synthesis Report; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Rötzer, T.; Rahman, F.; Moser, A.; Pauleit, S.; Pretzsch, H. Process based simulation of tree growth and ecosystem services of urban trees under present and future climate conditions. Sci. Total Environ. 2019, 676, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Temperate Plants Database. Ken Fern. Temperate.Theferns.info. Available online: http://temperate.theferns.info/plant/Taraxacum+officinale (accessed on 8 April 2019).
- Aihara, K.; Aso, T.; Takeda, M.; Koshiji, T. Actual condition of forest decline and approach (II) The phenomena of forest decline at Tanzawa Mountain in Kanagawa (in Japanese) prefecture. J. Jpn. Soc. Atmos. Environ. Plan. A 2004, 39, A29–A39. [Google Scholar]
- Kohno, Y.; Matsumura, H.; Ishii, T.; Izuta, T. Establishing critical levels of air pollutants for protecting East Asian vegetation—A challenge. In Plant Responses to Air Pollution and Global Change; Omasa, K., Nouchi, I., De Kok, L.J., Eds.; Springer: Tokyo, Japan, 2005; pp. 243–250. [Google Scholar]
- Kume, A.; Numata, S.; Watanabe, K.; Honoki, H.; Nakajima, H.; Ishida, M. Influence of air pollution on the mountain forests along the Tateyama-Kurobe Alpine route. Ecol. Res. 2009, 24, 821–830. [Google Scholar] [CrossRef]
- Takeda, M.; Aihara, K. Effects of ambient ozone concentrations on beech (Fagus crenanta) seedlings in the Tanzawa Mountains, Kanagawa Prefecture, Japan (in Japanese with English summary). J. Jpn. Soc. Atmos. Environ. 2007, 42, 1007–1117. [Google Scholar]
- Furukawa, A. Inhibition of photosynthesis of Populus euramericana and Helianthus annuus by SO2, NO2 and O3. Ecol. Res. 1991, 6, 79–86. [Google Scholar] [CrossRef]
- Yonekura, T.; Yoshidome, M.; Watanabe, M.; Honda, Y.; Ogiwara, I.; Izuta, T. Carry-over effects of ozone and water stress on leaf phenological characteristics and bud frost hardiness of Fagus crenata seedlings. Trees 2004, 18, 581–588. [Google Scholar] [CrossRef]
- Gregg, J.W.; Jones, C.G.; Dawson, T.E. Urbanization effects on tree growth in the vicinity of New York City. Nature 2003, 424, 183–187. [Google Scholar] [CrossRef] [PubMed]

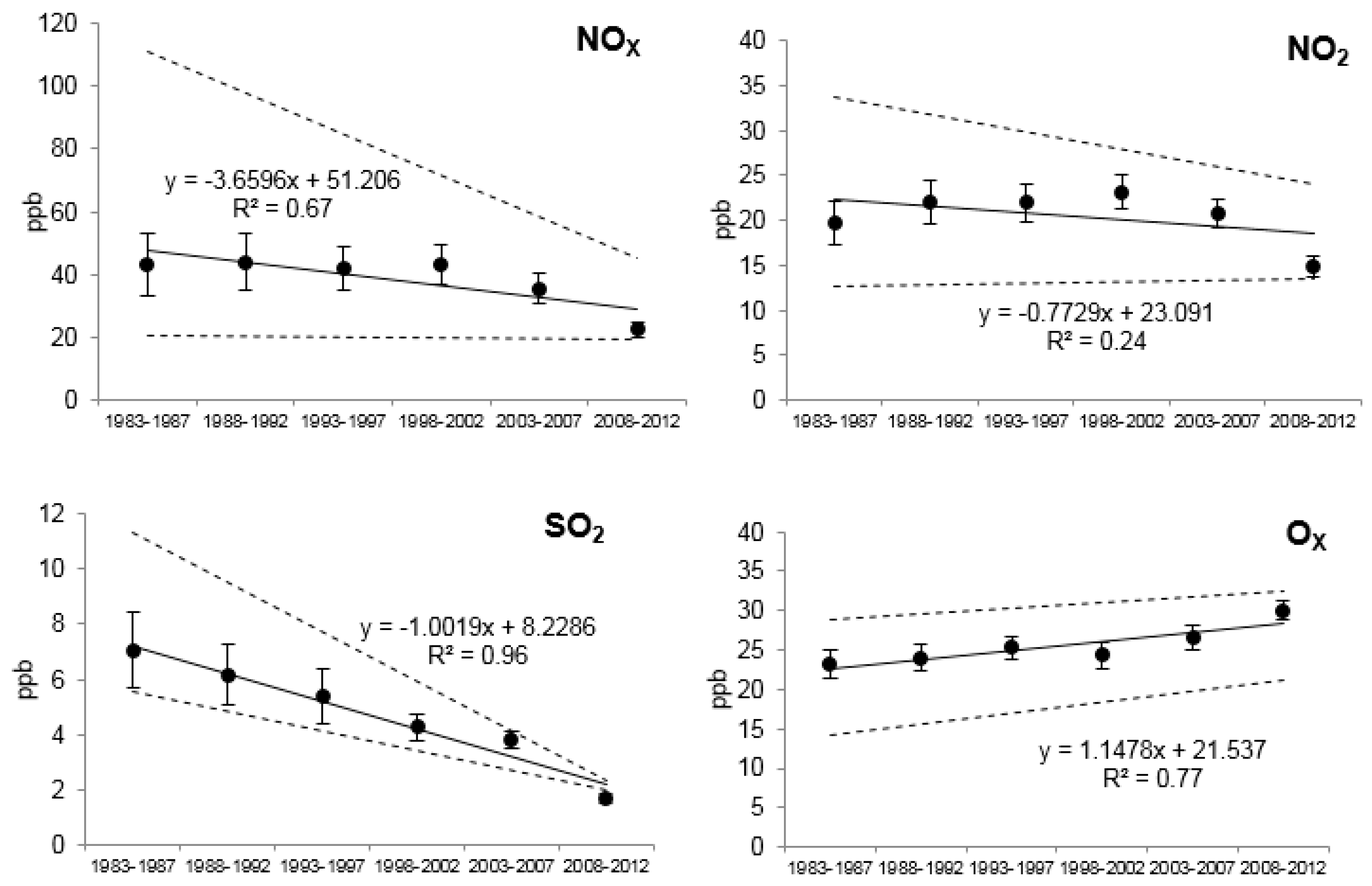
| Station | NO2 | Ox | SO2 | NOx | Temperature | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atsubetsu-SE* | 21.9 | + | 29.4 | + | 3.2 | − | 38.7 | − | 9.1 | + |
| EastDistrict | 21.1 | + | 25.3 | − | 5.9 | + | 36.8 | − | 8.6 | + |
| Fushimi-SW | 18.5 | − | 26.0 | − | - | 31.0 | − | 8.6 | + | |
| Hassamu-NW* | 16.9 | − | 28.0 | + | 4.2 | − | 26.2 | − | 8.6 | + |
| Higashi 18-chome | 25.4 | + | - | - | 54.6 | + | - | |||
| HigashiEast* | 15.7 | − | 29.0 | + | 3.9 | − | 23.8 | − | 7.7 | − |
| Kita-19-jyo | 19.4 | − | - | - | 35.2 | − | - | |||
| Kita-1-jyo | 35.0 | + | - | - | 85.0 | + | - | |||
| Kita-Shiroishi-E | 18.2 | − | 28.1 | + | 1.0 | − | 28.6 | − | 8.8 | + |
| MiddlePart | 26.9 | + | 17.7 | − | 6.9 | + | 50.5 | + | - | |
| Minami-14-jyo | 22.4 | + | - | - | 47.7 | + | - | |||
| MinamiS | 9.1 | − | - | - | 11.8 | − | - | |||
| ShinoroN* | 13.1 | − | 28.5 | + | 3.9 | − | 19.9 | − | 8.0 | − |
| Teine* | 19.3 | − | 25.8 | − | 4.3 | − | 34.4 | − | 8.5 | − |
| TsukisamuChuoS* | 28.4 | + | - | - | 77.1 | + | - | |||
| WestDistrict | 21.8 | + | 24.7 | − | 6.3 | + | 37.5 | − | 8.8 | + |
| Yamahana | - | 33.0 | + | - | - | 9.1 | + | |||
| mean | 20.8 | 26.9 | 4.4 | 39.9 | 8.6 |
| Plot | Name | Classification | N [mg/g] | P [mg/g] | K [μg/g] | Ca [mg/g] | Mg [mg/g] | Compaction [kg/m3] |
|---|---|---|---|---|---|---|---|---|
| 1 | Shirahata-yama 1 | rural | 2.02 | 4.23 | 148.00 | - | - | 0.50 |
| 2 | Shirahata- yama 2 | rural | 2.11 | 6.81 | 182.00 | - | - | 0.50 |
| 3 | Hokkaoido University Nursery | urban | 3.07 | 1.89 | 152.00 | 2.44 | 0.23 | 0.55 |
| 4 | Hitsujigaoka-7 1 | suburban | 3.02 | 1.69 | 345.00 | 1.88 | 0.22 | 0.52 |
| 5 | Hitsujigaoka-7 2 | suburban | 3.78 | 2.33 | 360.00 | - | - | 0.60 |
| 6 | Misumai | rural | 2.98 | 1.98 | 288.00 | 3.31 | 0.45 | 0.59 |
| Plot | Classification | n | Age [a] | Dbh [cm] | Tree Height [m] | Crown Start [m] | Crown Diameter [m] | CPA [m2] | Crown Length [m] | Crown Volume [m3] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rural | 20 | 47.3 ± 13.42 | 32.8 ± 6.8 | 17.9 ± 1.6 | 6.3 ± 2.4 | 5.5 ± 8.1 | 100.0 ± 207.7 | 11.6 ± 16.1 | 1161.5 ± 2382.3 |
| 2 | rural | 15 | 98.6 ± 23.2 | 49.1 ± 14.5 | 24.3 ± 3.6 | 7.3 ± 3.8 | 8.7 ± 2.4 | 253.6 ± 137.1 | 16.9 ± 4.7 | 4616.6 ± 3480.3 |
| 3 | urban | 18 | 40.4 ± 6.3 | 28.5 ± 4.6 | 15.2 ± 2.0 | 3.5 ± 1.5 | 4.9 ± 1.1 | 78.4 ± 35.9 | 11.7 ± 2.5 | 958.6 ± 529.1 |
| 4 | suburban | 21 | 32.2 ± 3.3 | 26.2 ± 2.9 | 15.3 ± 1.0 | 6.0 ± 2.0 | 5.2 ± 0.9 | 86.6 ± 33.4 | 9.3 ± 2.3 | 823.3 ± 415.9 |
| 5 | suburban | 20 | 35.3 ± 3.2 | 27.7 ± 2.8 | 16.0 ± 1.1 | 7.9 ± 1.4 | 4.5 ± 0.8 | 65.6 ± 19.9 | 8.1 ± 1.6 | 543.1 ± 219.5 |
| 6 | rural | 15 | 100.0 ± 64.1 | 41.9 ± 12.1 | 17.9 ± 2.4 | 6.4 ± 2.9 | 6.5 ± 1.5 | 138.1 ± 66.9 | 11.6 ± 2.9 | 1628.3 ± 1006.0 |
| Site | n | Age [a] | Dbh [cm] | Tree Height [m] | Crown Start [M] | Crown Diameter [M] | CPA [m2] | Crown Length [m] | Crown Volume [m3] |
|---|---|---|---|---|---|---|---|---|---|
| urban | 18 | 40.4 a ± 6.3 | 28.5 a ± 4.6 | 15.2 a ± 2.0 | 3.5 a ± 1.5 | 4.9 a ± 1.1 | 78.4 a ± 35.9 | 11.7 a ± 2.5 | 958.6 a ± 529.1 |
| suburban | 43 | 34.4 a ± 2.2 | 26.5 a ± 2.9 | 15.6 a ± 1.1 | 7.0 ab ± 2.0 | 4.8 a ± 0.9 | 76.1 a ± 29.2 | 8.6 b ± 2.1 | 678.5 b ± 359.7 |
| rural | 50 | 82.0 b ± 30.03 | 40.4 b ± 12.9 | 19.8 b ± 3.9 | 6.6 b ± 3.8 | 6.7 b ± 2.2 | 157.5 b ± 86.6 | 13.2 a ± 4.1 | 2338.1 b ± 1874.2 |
| Chronology | Growth Rate | Mean Sens. | AR |
|---|---|---|---|
| Urban | 4.22 | 0.23 | 0.33 |
| Suburban | 4.21 | 0.19 | 0.51 |
| Rural | 3.46 | 0.21 | 0.68 |
| overall | 3.88 | 0.20 | 0.56 |
| Parameter | Fixed Effect | Value ± SE | p |
|---|---|---|---|
| a | Intercept | −13.14 ± 0.66 | <0.001 |
| Log (ageij) | Log (age) | 2.67 ± 0.04 | <0.001 |
| timeij | Time of growth | −0.15 ± 0.14 | 0.26 |
| b × timeij × log(ageij) | Log (age) × Time of growth | 0.12 ± 0.04 | <0.001 |
| Random effect dij | 1.07 | - | |
| ε | 0.32 | - | |
| Parameter | Fixed Effect | Value ± SE | p |
|---|---|---|---|
| a | Intercept | −14.58 ± 0.15 | <0.001 |
| Log (ageij) | Log (age) | 2.94 ± 0.02 | <0.001 |
| urbij | Urbanity | 1.79 ± 0.20 | <0.001 |
| b × urbij × log (ageij) | Log (age) × Urbanity | −0.14 ± 0.02 | <0.001 |
| Random effect dij | 0.82 | - | |
| ε | 0.32 | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moser-Reischl, A.; Rötzer, T.; Biber, P.; Ulbricht, M.; Uhl, E.; Qu, L.; Koike, T.; Pretzsch, H. Growth of Abies sachalinensis Along an Urban Gradient Affected by Environmental Pollution in Sapporo, Japan. Forests 2019, 10, 707. https://doi.org/10.3390/f10080707
Moser-Reischl A, Rötzer T, Biber P, Ulbricht M, Uhl E, Qu L, Koike T, Pretzsch H. Growth of Abies sachalinensis Along an Urban Gradient Affected by Environmental Pollution in Sapporo, Japan. Forests. 2019; 10(8):707. https://doi.org/10.3390/f10080707
Chicago/Turabian StyleMoser-Reischl, Astrid, Thomas Rötzer, Peter Biber, Matthias Ulbricht, Enno Uhl, Laiye Qu, Takayoshi Koike, and Hans Pretzsch. 2019. "Growth of Abies sachalinensis Along an Urban Gradient Affected by Environmental Pollution in Sapporo, Japan" Forests 10, no. 8: 707. https://doi.org/10.3390/f10080707
APA StyleMoser-Reischl, A., Rötzer, T., Biber, P., Ulbricht, M., Uhl, E., Qu, L., Koike, T., & Pretzsch, H. (2019). Growth of Abies sachalinensis Along an Urban Gradient Affected by Environmental Pollution in Sapporo, Japan. Forests, 10(8), 707. https://doi.org/10.3390/f10080707





