Nitrogen Deposition and Responses of Forest Structure to Nitrogen Deposition in a Cool-Temperate Deciduous Forest
Abstract
1. Introduction
2. Materials and Methods
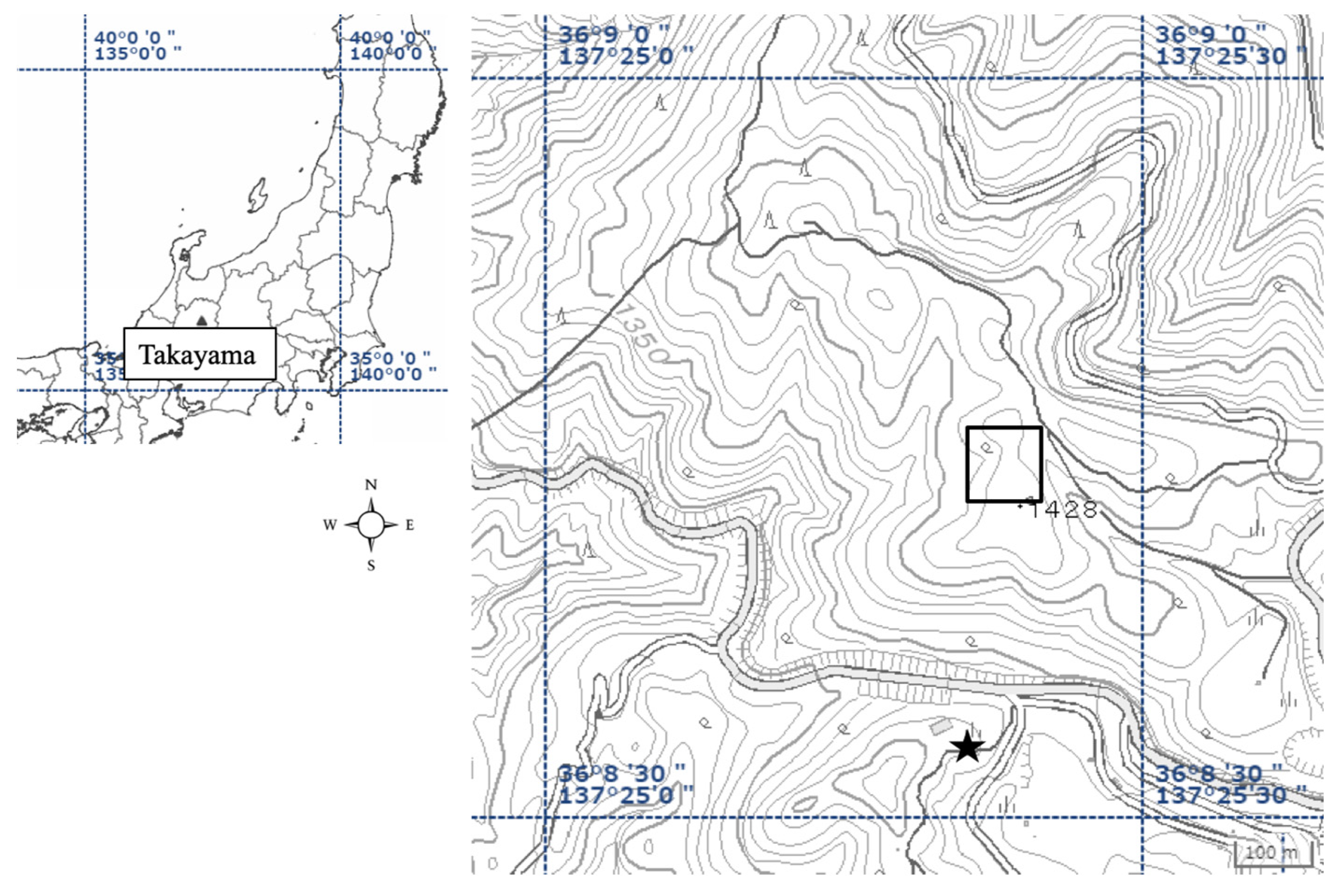
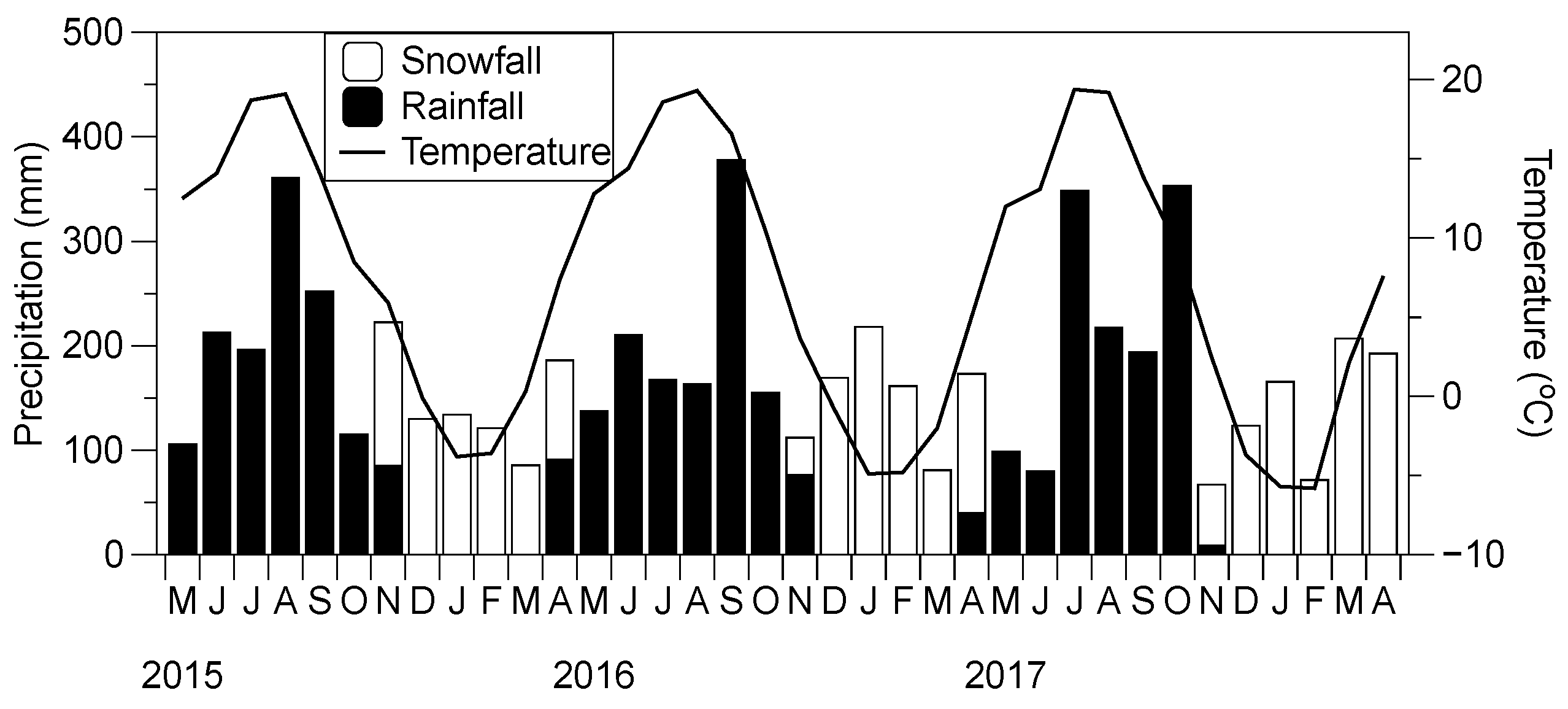
2.1. Study Site
2.2. Sampling of N fluxes in Different Water Fluxes
2.3. Chemical Analysis
2.4. Flux Calculation
2.5. Statistical Analysis
3. Results
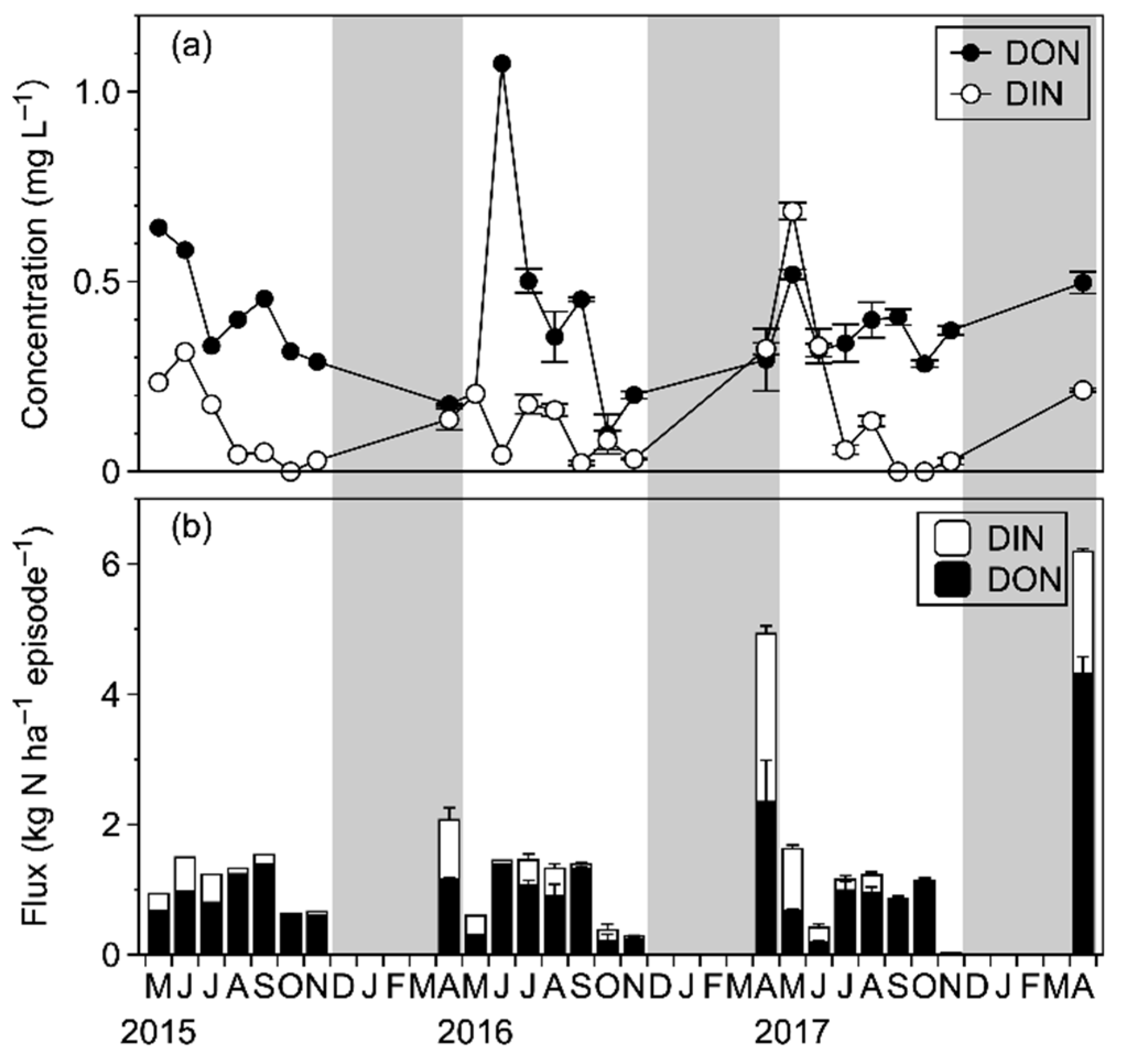
3.1. N Input via Bulk Precipitation
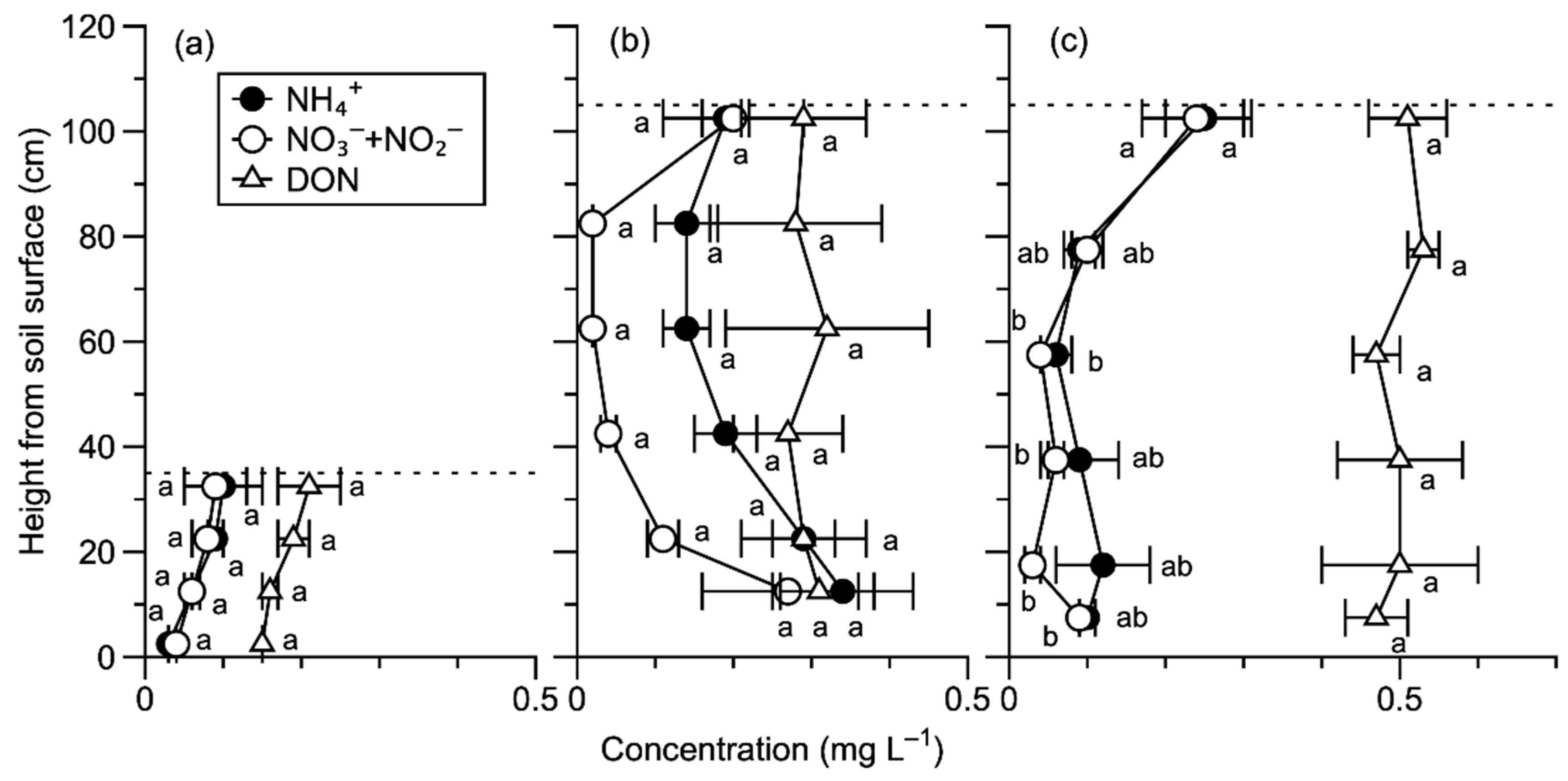
3.2. N Fluxes in the Internal Forest Ecosystem
4. Discussion
4.1. N Deposition into the Takayama Forest Site
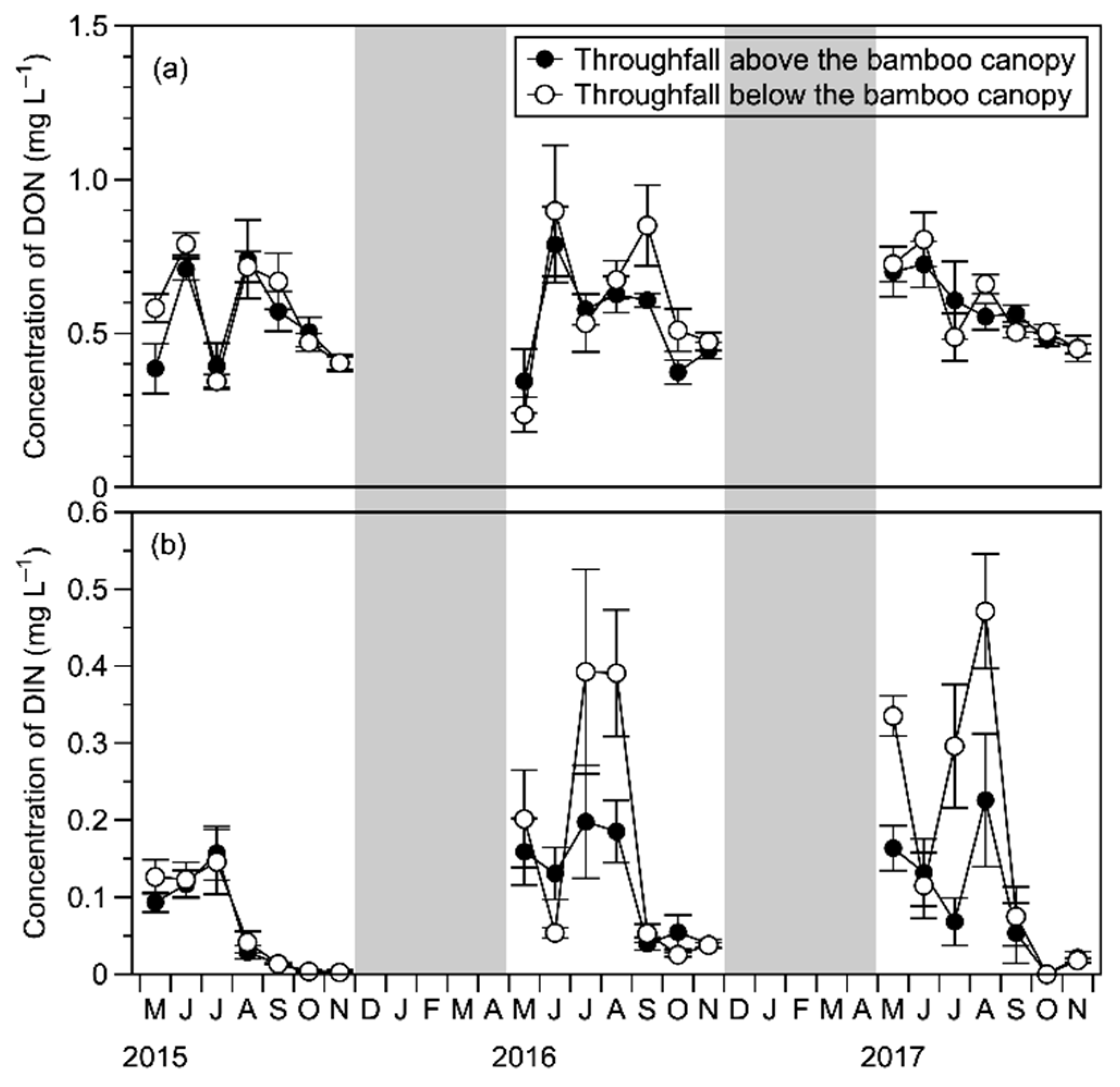
4.1.1. DIN and DON Concentrations during the Growing Season
4.1.2. Snow Contribution to N Deposition
4.1.3. Characteristics of Annual Dissolved N Fluxes
4.2. Responses of Forest Structure to N Deposition in the Internal N Cycle
4.2.1. Responses of Tree Canopy to N Deposition
4.2.2. Responses of Dwarf Bamboo Canopy to N Deposition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, G.K.; Hicks, W.K.; Cinderby, S.; Kuylenstierna, J.C.I.; Stock, W.D.; Dentener, F.J.; Giller, K.E.; Austin, A.T.; Lefroy, R.D.B.; Gimeno, B.S.; et al. Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Glob. Chang. Biol. 2006, 12, 470–476. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems. Bioscience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature 2007, 447, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.A.; Braswell, B.H.; Sulzman, J.; Lamarque, J.F. Nitrogen deposition onto the United States and Western Europe: Synthesis of observations and models. Ecol. Appl. 2005, 15, 38–57. [Google Scholar] [CrossRef]
- Kopaček, J.; Procházková, L.; Hejzlar, J.; Blažka, P. Trends and seasonal patterns of bulk deposition of nutrients in the Czech Republic. Atmos. Environ. 1997, 31, 797–808. [Google Scholar] [CrossRef]
- Rodà, F.; Avila, A.; Rodrigo, A. Nitrogen deposition in Mediterranean forests. Environ. Pollut. 2002, 118, 205–213. [Google Scholar] [CrossRef]
- Du, E.; Jiang, Y.; Fang, J.; de Vries, W. Inorganic nitrogen deposition in China’s forests: Status and characteristics. Atmos. Environ. 2014, 98, 474–482. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, G.; He, N.; Zhan, X.; Fang, H.; Sheng, W.; Zuo, Y.; Zhang, D.; Wang, Q. Spatial and decadal variations in inorganic nitrogen wet deposition in China induced by human activity. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Waldner, P.; Marchetto, A.; Thimonier, A.; Schmitt, M.; Rogora, M.; Granke, O.; Mues, V.; Hansen, K.; Pihl Karlsson, G.; Žlindra, D.; et al. Detection of temporal trends in atmospheric deposition of inorganic nitrogen and sulphate to forests in Europe. Atmos. Environ. 2014, 95, 363–374. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Chen, H.; Mo, J. Impacts of nitrogen deposition on soil nitrogen cycle in forest ecosystems: A review. Acta Ecol. Sin. 2015, 35, 35–43. [Google Scholar] [CrossRef]
- Thomas, R.Q.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar] [CrossRef]
- Bobbink, R.; Ashmore, M.R.; Braun, S.; Flückiger, W.; Van den Wyngaert, I.J. Empirical nitrogen critical loads for natural and semi-natural ecosystems: 2002 update. In Book Empirical Critical Loads for Nitrogen; Swiss Agency for Environment, Forest and Landscape: Berne, Switzerland, 2003; pp. 33–34. [Google Scholar]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.L.; Gundersen, P.; Callesen, I.; Reinds, G.J. Throughfall nitrogen deposition has different impacts on soil solution nitrate concentration in European coniferous and deciduous forests. Ecosystems 2004, 7, 180–192. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 24019. [Google Scholar] [CrossRef]
- Dise, N.B.; Wright, R.F. Nitrogen leaching from European forests in relation to nitrogen deposition. For. Ecol. Manag. 1995, 71, 153–169. [Google Scholar] [CrossRef]
- Wright, R.F.; Alewell, C.; Cullen, J.M.; Evans, C.D.; Marchetto, A.; Moldan, F.; Prechtel, A.; Rogora, M. Trends in nitrogen deposition and leaching in acid-sensitive streams in Europe. Hydrol. Earth Syst. Sci. 2001, 5, 299–310. [Google Scholar] [CrossRef]
- Cornell, S.E. Atmospheric nitrogen deposition: Revisiting the question of the importance of the organic component. Environ. Pollut. 2011, 159, 2214–2222. [Google Scholar] [CrossRef]
- Pacheco, M.; Donoso, L.; Sanhueza, E. Soluble organic nitrogen in Venezuelan rains. Tellus Ser. B Chem. Phys. Meteorol. 2004, 56, 393–395. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Liu, X.J.; Li, W.Q.; Lü, S.H.; Zheng, L.X.; Bai, Z.C.; Cai, G.Y.; Zhang, F.S. Atmospheric organic nitrogen deposition in China. Atmos. Environ. 2012, 46, 195–204. [Google Scholar] [CrossRef]
- Lovett, G.M.; Lindberg, S.E. Atmospheric deposition and canopy interactions of nitrogen in forests. Can. J. For. Res. 1993, 23, 1603–1616. [Google Scholar] [CrossRef]
- Pelster, D.E.; Kolka, R.K.; Prepas, E.E. Overstory vegetation influence nitrogen and dissolved organic carbon flux from the atmosphere to the forest floor: Boreal Plain, Canada. For. Ecol. Manag. 2009, 259, 210–219. [Google Scholar] [CrossRef]
- Izquieta-Rojano, S.; García-Gomez, H.; Aguillaume, L.; Santamaría, J.M.; Tang, Y.S.; Santamaría, C.; Valiño, F.; Lasheras, E.; Alonso, R.; Àvila, A.; et al. Throughfall and bulk deposition of dissolved organic nitrogen to holm oak forests in the Iberian Peninsula: Flux estimation and identification of potential sources. Environ. Pollut. 2016, 210, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, A.; Verschelde, P.; De Vos, B.; Neirynck, J.; Cools, N.; Roskams, P.; Hens, M.; Louette, G.; Sleutel, S.; De Neve, S. Increasing trends of dissolved organic nitrogen (DON) in temperate forests under recovery from acidification in Flanders, Belgium. Sci. Total Environ. 2016, 553, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Bellot, J.; Escarre, A. Stemflow and throughfall determination in a resprouted Mediterranean holm-oak forest. In Annales des Sciences Forestières; EDP Sciences: Les Ulis, France, 1998; Volume 55, pp. 847–865. [Google Scholar] [CrossRef]
- Aguillaume, L.; Izquieta-Rojano, S.; García-Gómez, H.; Elustondo, D.; Santamaría, J.M.; Alonso, R.; Avila, A. Dry deposition and canopy uptake in Mediterranean holm-oak forests estimated with a canopy budget model: A focus on N estimations. Atmos. Environ. 2017, 152, 191–200. [Google Scholar] [CrossRef]
- Chen, X.Y.; Mulder, J. Atmospheric deposition of nitrogen at five subtropical forested sites in South China. Sci. Total Environ. 2007, 378, 317–330. [Google Scholar] [CrossRef]
- Saigusa, N.; Yamamoto, S.; Murayama, S.; Kondo, H.; Nishimura, N. Gross primary production and net ecosystem exchange of a cool-temperate deciduous forest estimated by the eddy covariance method. Agric. For. Meteorol. 2002, 112, 203–215. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Mo, W.; Satomura, T.; Inatomi, M.; Koizumi, H. Biometric based carbon flux measurements and net ecosystem production (NEP) in a temperate deciduous broad-leaved forest beneath a flux tower. Ecosystems 2007, 10, 324–334. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Saigusa, N.; Koizumi, H. On linking multiyear biometric measurements of tree growth with eddy covariance-based net ecosystem production. Glob. Chang. Biol. 2009, 15, 1015–1024. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Akiyama, T.; Hashimoto, Y.; Inatomi, M.; Sakai, T.; Jia, S.; Mo, W.; Tsuda, S.; Koizumi, H. Biometric based estimates of net primary production (NPP) in a cool-temperate deciduous forest stand beneath a flux tower. Agric. For. Meteorol. 2005, 134, 27–38. [Google Scholar] [CrossRef]
- Nishimura, N.; Matsui, Y.; Ueyama, T.; Mo, W.; Saijo, Y.; Tsuda, S.; Yamamoto, S.; Koizumi, H. Evaluation of carbon budgets of a forest floor Sasa senanensis community in a cool-temperate forest ecosystem, central Japan. Jpn. J. Ecol. 2004, 54, 143–158. [Google Scholar] [CrossRef]
- Chen, S.; Yoshitake, S.; Iimura, Y.; Asai, C.; Ohtsuka, T. Dissolved organic carbon (DOC) input to the soil: DOC fluxes and their partitions during the growing season in a cool-temperate broad-leaved deciduous forest, central Japan. Ecol. Res. 2017, 32, 713–724. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Huang, L.H.; Xiao, Y.L.; Chen, G.; Hu, H.L.; Liu, L.; Zheng, J.K.; Xu, Z.F.; et al. Nitrogen distribution and cycling through water flows in a subtropical bamboo forest under high level of atmospheric deposition. PLoS ONE 2013, 8, 2–12. [Google Scholar] [CrossRef]
- Lovett, G.M.; Lindberg, S.E. Dry deposition and canopy exchange in a mixed oak forest as determined by analysis of throughfall. J. Appl. Ecol. 1984, 21, 1013–1027. [Google Scholar] [CrossRef]
- Cape, J.N.; Tang, Y.S.; González-Beníez, J.M.; Mitošinková, M.; Makkonen, U.; Jocher, M.; Stolk, A. Organic nitrogen in precipitation across Europe. Biogeosciences 2012, 9, 4401–4409. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Iwatsubo, G.; Ohrui, K.; Nakagawa, Y. Nitrogen saturation in Japanese forests: An evaluation. For. Ecol. Manag. 1997, 97, 39–51. [Google Scholar] [CrossRef]
- Asman, W.A.H.; Sutton, M.A.; Schjørring, J.K. Ammonia: Emission, atmospheric transport and deposition. New Phytol. 1998, 139, 27–48. [Google Scholar] [CrossRef]
- Krupa, S.V. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Ferm, M. Atmospheric ammonia and ammonium transport in Europe and critical loads: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 5–17. [Google Scholar] [CrossRef]
- Song, L.; Kuang, F.; Skiba, U.; Zhu, B.; Liu, X.; Levy, P.; Dore, A.; Fowler, D. Bulk deposition of organic and inorganic nitrogen in southwest China from 2008 to 2013. Environ. Pollut. 2017, 227, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hiltbrunner, E.; Schwikowski, M.; Körner, C. Inorganic nitrogen storage in alpine snow pack in the Central Alps (Switzerland). Atmos. Environ. 2005, 39, 2249–2259. [Google Scholar] [CrossRef]
- Bowman, W.D. Inputs and storage of nitrogen in winter snowpack in an alpine ecosystem. Arct. Alp. Res. 1992, 24, 211–215. [Google Scholar] [CrossRef]
- Fibiger, D.L.; Dibb, J.E.; Chen, D.; Thomas, J.L.; Burkhart, J.F.; Huey, L.G.; Hastings, M.G. Analysis of nitrate in the snow and atmosphere at Summit, Greenland: Chemistry and transport. J. Geophys. Res. Atmos. 2016, 5010–5030. [Google Scholar] [CrossRef]
- Fahey, T.J.; Yavitt, J.B.; Pearson, J.A.; Knight, D.H. The nitrogen cycle in lodgepole pine forests, southeastern Wyoming. Biogeochemistry 1985, 1, 257–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, X.; Jickells, T.; Neil Cape, J.; Goulding, K.; Fangmeier, A.; Zhang, F. Evidence for organic N deposition and its anthropogenic sources in China. Atmos. Environ. 2008, 42, 1035–1041. [Google Scholar] [CrossRef][Green Version]
- Ban, S.; Matsuda, K.; Sato, K.; Ohizumi, T. Long-term assessment of nitrogen deposition at remote EANET sites in Japan. Atmos. Environ. 2016, 146, 70–78. [Google Scholar] [CrossRef]
- Li, J.; Fang, Y.; Yoh, M.; Wang, X.; Wu, Z.; Kuang, Y.; Wen, D. Organic nitrogen deposition in precipitation in metropolitan Guangzhou city of southern China. Atmos. Res. 2012, 113, 57–67. [Google Scholar] [CrossRef]
- Ham, Y.S.; Tamiya, S.; Choi, I.S. Contribution of dissolved organic nitrogen deposition to nitrogen saturation in a forested mountainous watershed in Tsukui, Central Japan. Water. Air. Soil Pollut. 2007, 178, 113–120. [Google Scholar] [CrossRef]
- Balestrini, R.; Tagliaferri, A. Atmospheric deposition and canopy exchange processes in alpine forest ecosystems (northern Italy). Atmos. Environ. 2001, 35, 6421–6433. [Google Scholar] [CrossRef]
- Fenn, M.E.; Ross, C.S.; Schilling, S.L.; Baccus, W.D.; Larrabee, M.A.; Lofgren, R.A. Atmospheric deposition of nitrogen and sulfur and preferential canopy consumption of nitrate in forests of the Pacific Northwest, USA. For. Ecol. Manag. 2013, 302, 240–253. [Google Scholar] [CrossRef]
- Guerrieri, R.; Vanguelova, E.I.; Michalski, G.; Heaton, T.H.E.; Mencuccini, M. Isotopic evidence for the occurrence of biological nitrification and nitrogen deposition processing in forest canopies. Glob. Chang. Biol. 2015, 21, 4613–4626. [Google Scholar] [CrossRef] [PubMed]
- Gaige, E.; Dail, D.B.; Hollinger, D.Y.; Davidson, E.A.; Fernandez, I.J.; Sievering, H.; White, A.; Halteman, W. Changes in canopy processes following whole-forest canopy nitrogen fertilization of a mature spruce-hemlock forest. Ecosystems 2007, 10, 1133–1147. [Google Scholar] [CrossRef]
- Mellec, A.; Meesenburg, H.; Michalzik, B. The importance of canopy-derived dissolved and particulate organic matter (DOM and POM)–comparing throughfall solution from broadleaved and coniferous forests. Ann. For. Sci. 2010, 67, 411. [Google Scholar] [CrossRef]
- Adriaenssens, S.; Staelens, J.; Wuyts, K.; De Schrijver, A.; Van Wittenberghe, S.; Wuytack, T.; Kardel, F.; Verheyen, K.; Samson, R.; Boeckx, P. Foliar nitrogen uptake from wet deposition and the relation with leaf wettability and water storage capacity. Water. Air. Soil Pollut. 2011, 219, 43–57. [Google Scholar] [CrossRef]
- Wuyts, K.; Adriaenssens, S.; Staelens, J.; Wuytack, T.; Van Wittenberghe, S.; Boeckx, P.; Samson, R.; Verheyen, K. Contributing factors in foliar uptake of dissolved inorganic nitrogen at leaf level. Sci. Total Environ. 2015, 505, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.A.; Middelburg, J.J.; Stapel, J.; Bouma, T.J. Dissolved organic nitrogen uptake by seagrasses. Limnol. Oceanogr. 2008, 53, 542–548. [Google Scholar] [CrossRef]
- Hinko-Najera Umana, N.; Wanek, W. Large canopy exchange fluxes of inorganic and organic nitrogen and preferential retention of nitrogen by epiphytes in a tropical lowland rainforest. Ecosystems 2010, 13, 367–381. [Google Scholar] [CrossRef]
- Neff, J.C.; Chapin, F.S., III; Vitousek, P.M. Breaks in the cycle: Dissolved organic nitrogen in terrestrial ecosystems. Front. Ecol. Environ. 2007, 1, 205. [Google Scholar] [CrossRef]
- Rodrigo, A.; Àvila, A.; Rodà, F. The chemistry of precipitation, throughfall and stemflow in two holm oak (Quercus ilex L.) forests under a contrasted pollution environment in NE Spain. Sci. Total Environ. 2003, 305, 195–205. [Google Scholar] [CrossRef]


| Rainfall | Snowfall | Total | Contribution of Snowfall | |
|---|---|---|---|---|
| Precipitation (mm) | 1344 (25.6) | 776 (107) | 2120 (117) | 0.37 (0.03) |
| Concentration (mg L−1) | ||||
| DON | 0.41 (0.03) | 0.32 (0.16) | – | – |
| DIN | 0.13 (0.04) | 0.23 (0.09) | – | – |
| TDN | 0.55 (0.04) | 0.53 (0.19) | – | – |
| Flux (kg N ha−1 period−1) | ||||
| DON | 5.29 (0.39) | 2.61 (1.60) | 7.90 (1.22) | 0.32 (0.15) |
| DIN | 1.52 (0.09) | 1.67 (0.85) | 3.19 (0.80) | 0.50 (0.13) |
| TDN | 6.81 (0.32) | 4.28 (1.97) | 11.1 (1.71) | 0.37 (0.13) |
| Contribution of DON | 0.78 (0.02) | 0.59 (0.13) | 0.78 (0.02) | – |
| NH4–N | NO3 + NO2–N | DIN | DON | |
|---|---|---|---|---|
| Concentrations | −0.15 | −0.32 | −0.24 | −0.04 |
| Fluxes | 0.08 | −0.06 | 0.02 | 0.67 * |
| Water Flux (mm) | NH4–N (kg N ha−1 Year−1) | NO3 + NO2–N (kg N ha−1 Year−1) | DIN (kg N ha−1 Year−1) | DON (kg N ha−1 Year−1) | |
|---|---|---|---|---|---|
| BP | 2120 (117) | 1.66 (0.68) | 1.53 (0.12) | 3.19 (0.80) | 7.90 (1.22) |
| SF | 21.4 (3.53) | 0.02 (0.01) | 0.00 (0.00) | 0.02 (0.01) | 0.14 (0.03) |
| TFa | 2048 (114) | 2.05 (0.91) | 0.80 (0.30) | 2.82 (1.21) | 9.87 (1.67) |
| TFb | 1764 (104) | 2.22 (1.12) | 0.98 (0.39) | 3.17 (1.52) | 8.85 (1.86) |
| Net TFa | −50.0 (11.7) | 0.41 (0.24) | −0.73 (0.19) | −0.35 (0.41) | 2.11 (0.42) |
| Net TFb | −283 (57.0) | 0.17 (0.28) | 0.18 (0.18) | 0.35 (0.44) | −1.02 (0.55) |
| NH4–N | NO3 + NO2–N | DIN | DON | |
|---|---|---|---|---|
| Net TFa | 0.11 | −0.01 | 0.36 | 0.46 * |
| Net TFb | 0.04 | 0.08 | 0.20 | −0.63 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, R.; Chen, S.; Yoshitake, S.; Ohtsuka, T. Nitrogen Deposition and Responses of Forest Structure to Nitrogen Deposition in a Cool-Temperate Deciduous Forest. Forests 2019, 10, 631. https://doi.org/10.3390/f10080631
Cao R, Chen S, Yoshitake S, Ohtsuka T. Nitrogen Deposition and Responses of Forest Structure to Nitrogen Deposition in a Cool-Temperate Deciduous Forest. Forests. 2019; 10(8):631. https://doi.org/10.3390/f10080631
Chicago/Turabian StyleCao, Ruoming, Siyu Chen, Shinpei Yoshitake, and Toshiyuki Ohtsuka. 2019. "Nitrogen Deposition and Responses of Forest Structure to Nitrogen Deposition in a Cool-Temperate Deciduous Forest" Forests 10, no. 8: 631. https://doi.org/10.3390/f10080631
APA StyleCao, R., Chen, S., Yoshitake, S., & Ohtsuka, T. (2019). Nitrogen Deposition and Responses of Forest Structure to Nitrogen Deposition in a Cool-Temperate Deciduous Forest. Forests, 10(8), 631. https://doi.org/10.3390/f10080631






