Space, Habitat and Isolation are the Key Determinants of Tree Colonization by the Carpenter Ant in Plantation Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Studied Variables
- 1.
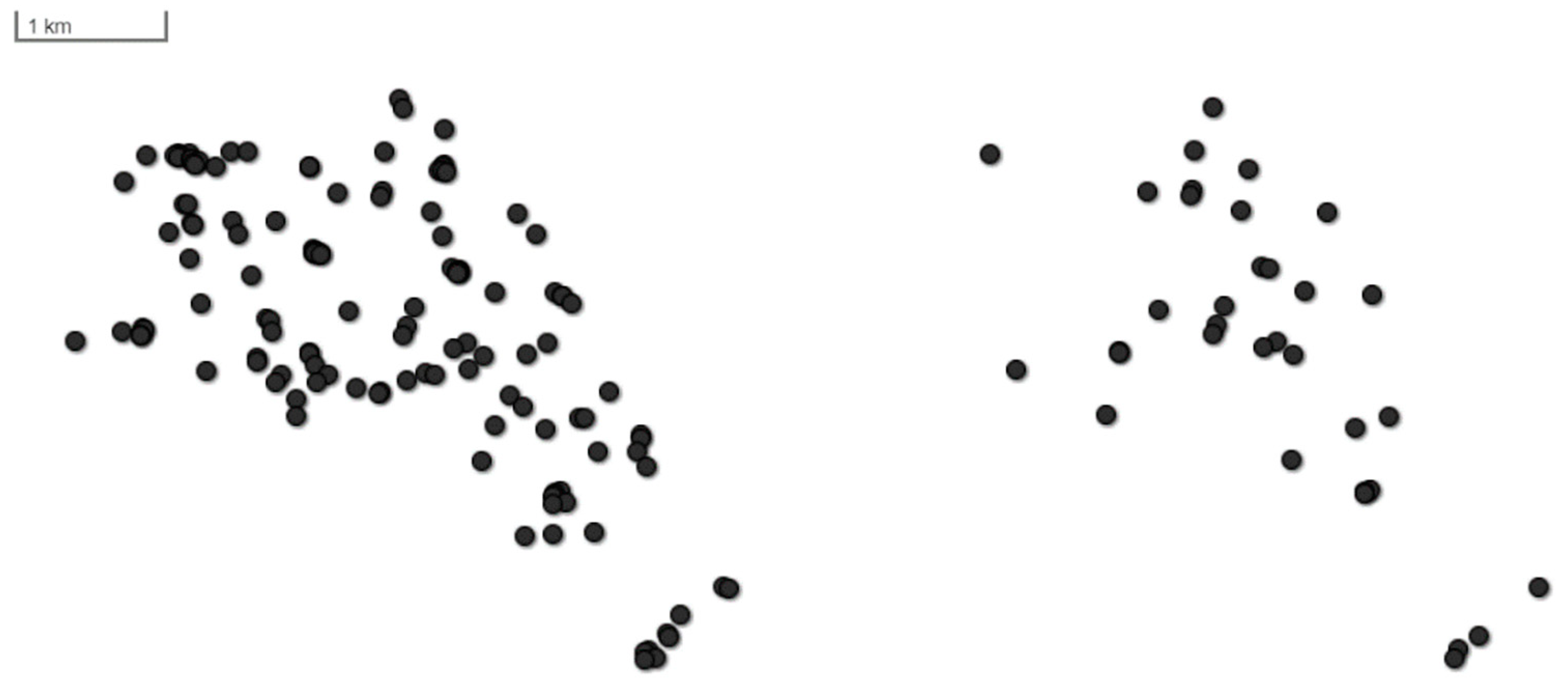
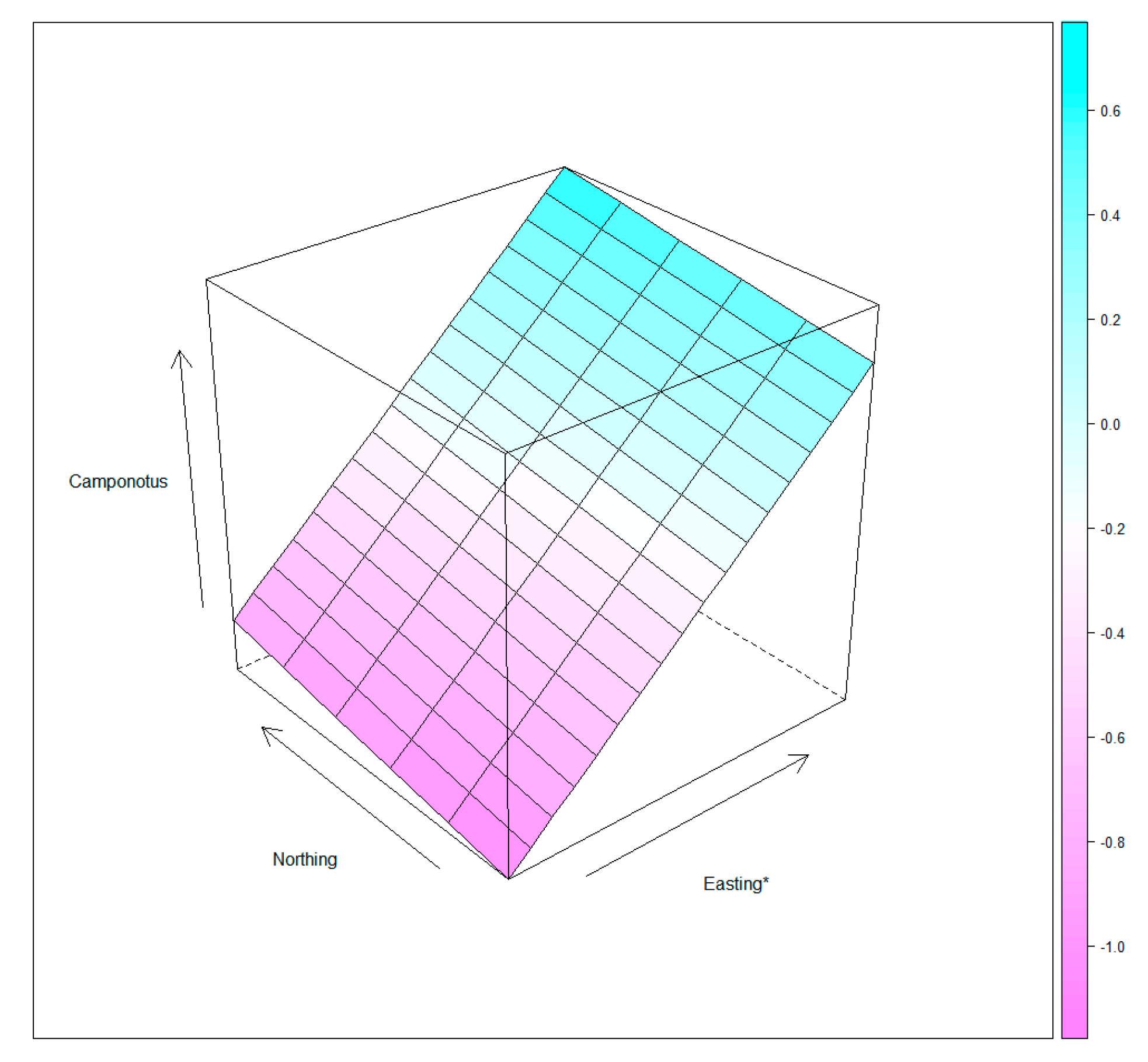
- The first two independent variables collected were regarding the spatial distribution of nests. Each snapped tree was localized using a geographical positioning system (GPS) using geographical coordinates of (a) northing and (b) easting in WGS84 format (center: 50.0058; 16.1780).
- 2.
- We searched for four habitat characteristics. We identified (a) the tree species of the snapped tree, and five tree species were snapped. All were coniferous: the Norway spruce (N = 102), Scots pine (N = 17), larch (N = 9), white pine (N = 1), and silver fir (Abies alba; N = 1). We also searched for (b) the presence of rot in the place of the snap. We divided the type of rot into two commonly used categories: white (N = 8) and brown (N = 69) rot. The white rot was most probably caused by Armillaria and brown by Heterobasidion [24]. The rest of the trees were without any indication of the presence of rot. We also checked for (c) the presence of resin on the stem, but this was highly correlated with the presence of rot (Rs = 0.37; p < 0.001). This has been confirmed by current research [25], and we thus did not use resin indication for further analyses. We measured (d) the height of the breakage on the stem (mean = 246.57 ± 1.63 cm SE), and the height of snapped of trees inhabited by carpenter ant nests was 93.71 ± 12.20 SE (5–230) cm.
- 3.
- We also collected two patch-based characteristics. We estimated (a) the canopy closure as a percentage (80.04% ± 1.63%). (b) The composition of the same tree species as the snapped tree was also investigated (57.81% ± 2.66%).
- 4.
- The stand-based characteristic was the age of the stand (73.83 ± 1.93 years).
- 5.
- Two characteristics that reflected isolation were measured. The first was (a) the distance to the forest track (67.36 ± 5.31 m) as a permanently open area. (b) The second variable was the distance to clear-cut or non-forest land (93.65 ± 12.57 m)—the gaps created by actual windstorm were not measured.
2.4. Statistics
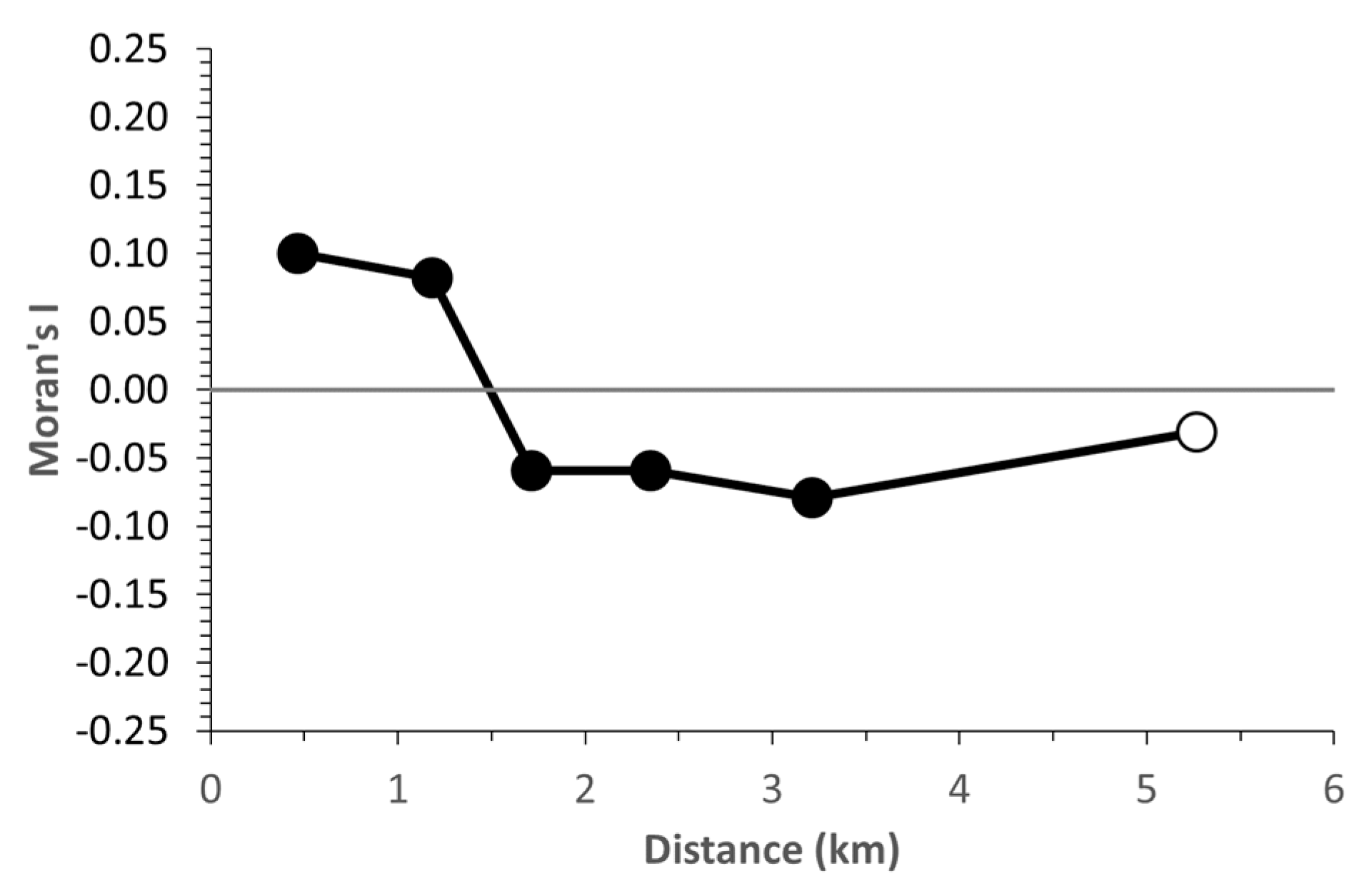
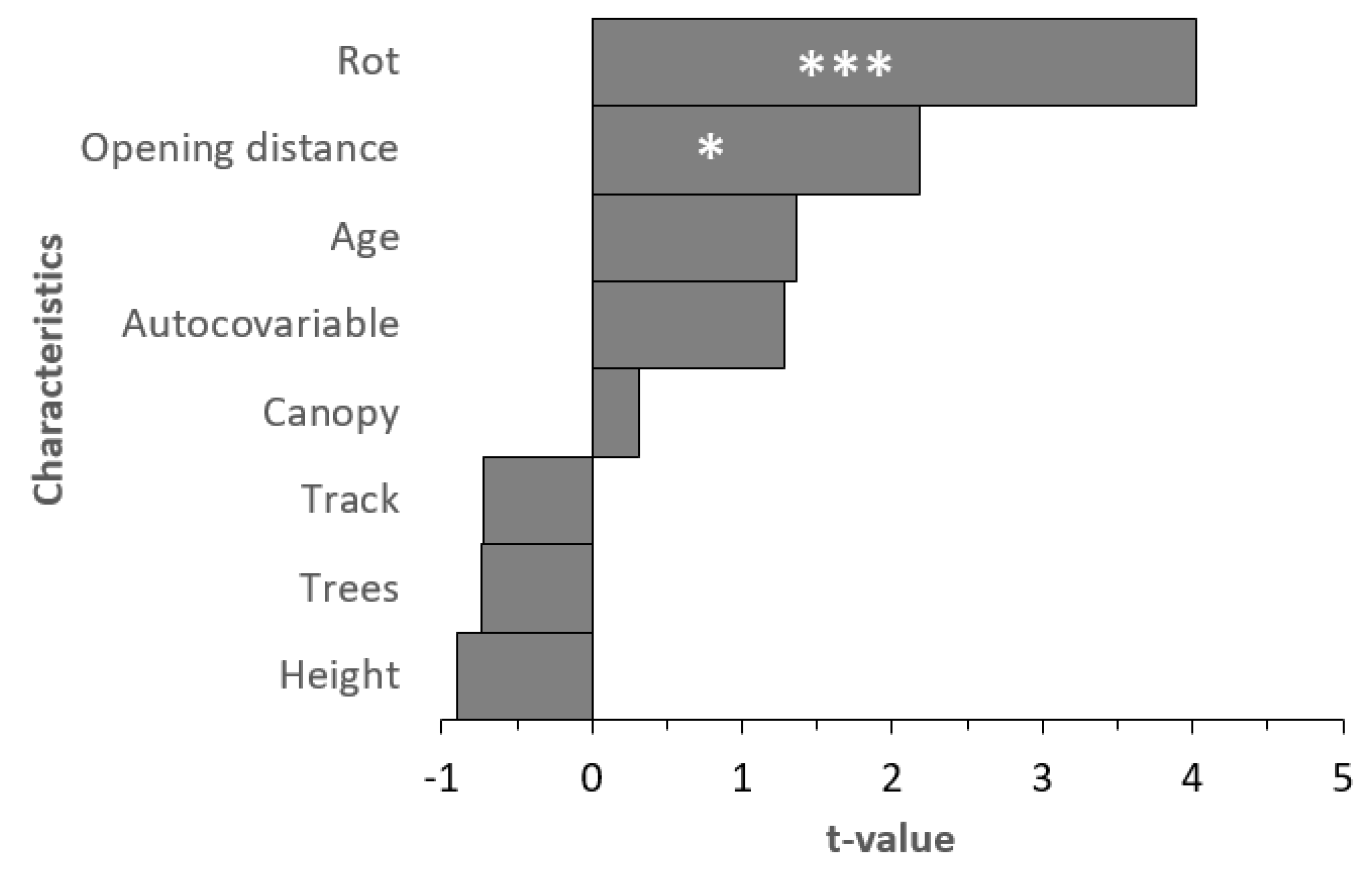
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messenger, A.S. Spruce plantation effects on soil moisture and chemical element distribution. For. Ecol. Manag. 1980, 3, 113–125. [Google Scholar] [CrossRef]
- Prévost, M.; Raymond, P. Effect of gap size, aspect and slope on available light and soil temperature after patch-selection cutting in yellow birch–conifer stands, Quebec, Canada. For. Ecol. Manag. 2012, 274, 210–221. [Google Scholar] [CrossRef]
- Schelker, J.; Kuglerová, L.; Eklöf, K.; Bishop, K.; Laudon, H. Hydrological effects of clear-cutting in a boreal forest—Snowpack dynamics, snowmelt and streamflow responses. J. Hydrol. 2013, 484, 105–114. [Google Scholar] [CrossRef]
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Hlásny, T.; Turčáni, M. Persisting bark beetle outbreak indicates the unsustainability of secondary Norway spruce forests: Case study from Central Europe. Ann. For. Sci. 2013, 70, 481–491. [Google Scholar] [CrossRef]
- Drever, C.R.; Peterson, G.; Messier, C.; Bergeron, Y.; Flannigan, M. Can forest management based on natural disturbances maintain ecological resilience? Can. J. For. Res. 2006, 36, 2285–2299. [Google Scholar] [CrossRef] [Green Version]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity. Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Heiskanen, J.; Mäkitalo, K. Soil water-retention characteristics of Scots pine and Norway spruce forest sites in Finnish Lapland. For. Ecol. Manag. 2002, 162, 137–152. [Google Scholar] [CrossRef]
- Dai, W.; Fu, W.; Jiang, P.; Zhao, K.; Li, Y.; Tao, J. Spatial pattern of carbon stocks in forest ecosystems of a typical subtropical region of southeastern China. For. Ecol. Manag. 2018, 409, 288–297. [Google Scholar] [CrossRef]
- Horák, J.; Brestovanská, T.; Mladenović, S.; Kout, J.; Bogusch, P.; Halda, J.P.; Zasadil, P. Green desert?: Biodiversity patterns in forest plantations. For. Ecol. Manag. 2019, 433, 343–348. [Google Scholar] [CrossRef]
- Hartley, M.J. Rationale and methods for conserving biodiversity in plantation forests. For. Ecol. Manag. 2002, 155, 81–95. [Google Scholar] [CrossRef]
- Gjerde, I.; Sætersdal, M.; Nilsen, T. Abundance of two threatened woodpecker species in relation to the proportion of spruce plantations in native pine forests of western Norway. Biodivers. Conserv. 2005, 14, 377–393. [Google Scholar] [CrossRef]
- Gittings, T.; O’Halloran, J.; Kelly, T.; Giller, P.S. The contribution of open spaces to the maintenance of hoverfly (Diptera, Syrphidae) biodiversity in Irish plantation forests. For. Ecol. Manag. 2006, 237, 290–300. [Google Scholar] [CrossRef]
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Kenis, M.; Hurley, B.P.; Hajek, A.E.; Cock, M.J.W. Classical biological control of insect pests of trees. Biol. Invasions 2017, 19, 3401–3417. [Google Scholar] [CrossRef]
- Adlung, K.G. A Critical Evaluation of the European Research on Use of Red Wood Ants (Formica rufa Group) for the Protection of Forests against Harmful Insects. Z. Für Angew. Entomol. 1966, 57, 167–189. [Google Scholar] [CrossRef]
- Vélová, L.; Véle, A. The importance of woodpeckers in forest protection: Review. Rep. For. Res. 2019, 64. in press. [Google Scholar]
- Bezděčka, P.; Bezděčková, K. Ants in the Collections of Czech, Moravian and Silesian Museums, 1st ed.; Muzeum Vysočiny Jihlava: Jihlava, Czech Republic, 2011; ISBN 978-80-86382-38-8. [Google Scholar]
- Křístek, J.; Urban, J. Lesnická Entomologie; Vyd. 2., upr.; Academia: Praha, Czech Republic, 2013; ISBN 978-80-200-2237-0. [Google Scholar]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsäläinen, K. The Ants of Poland, 1st ed.; with reference to the myrmecofauna of Europe; Natura Optima dux Foundation: Warszawa, Poland, 2012; ISBN 83-930773-4-6. [Google Scholar]
- Hansen, L.; Klotz, J. Carpenter Ants of the United States and Canada, 1st ed.; Comstock Publishing Associates: Ithaca, NY, USA, 2005; ISBN 978-0-8014-4262-9. [Google Scholar]
- Seifert, B. The ecology of Central European non-arboreal ants—37 years of a broad-spectrum analysis under permanent taxonomic control. Soil Org. 2017, 89, 1–67. [Google Scholar]
- Ayre, G.L. Feeding Behaviour and Digestion in Camponotus Herculeanus (L.) (hymenoptera: Formicidae). Entomol. Exp. Et Appl. 1963, 6, 165–170. [Google Scholar] [CrossRef]
- Černý, A. Parazitické Dřevokazné houby, 1st ed.; SZN: Praha, Czech Republic, 1989; ISBN 80-209-0090-X. [Google Scholar]
- Holuša, J.; Lubojacký, J.; Čurn, V.; Tonka, T.; Lukášová, K.; Horák, J. Combined effects of drought stress and Armillaria infection on tree mortality in Norway spruce plantations. For. Ecol. Manag. 2018, 427, 434–445. [Google Scholar] [CrossRef]
- Rangel, T.F.; Diniz-Filho, J.A.F.; Bini, L.M. SAM. Ecography 2010, 33, 46–50. [Google Scholar] [CrossRef]
- De Frutos, Á.; Olea, P.P.; Vera, R. Analyzing and modelling spatial distribution of summering lesser kestrel: The role of spatial autocorrelation. Ecol. Model. 2007, 200, 33–44. [Google Scholar] [CrossRef]
- Hoelldobler, B. Zur Frage der Oligogynie bei Camponotus ligniperda Latr. und Camponotus herculeanus L. (Hym. Formicidae). J. Appl. Entomol. 1961, 49, 337–352. [Google Scholar]
- Gadau, J.; Gertsch, P.J.; Heinze, J.; Pamilo, P.; Hölldobler, B. Oligogyny by Unrelated Queens in the Carpenter Ant, Camponotus ligniperdus. Behav. Ecol. Sociobiol. 1998, 44, 23–33. [Google Scholar] [CrossRef]
- Mabelis, A.A. Flying as a survival strategy for wood ants in a fragmented landscape (Hymenoptera, Formicidae). Memorab. Zool. 1994, 48, 147–170. [Google Scholar]
- Mabelis, A.A.; Chardon, J.P. Survival of the trunk ant (Formica truncorum Fabricius, 1804; Hymenoptera: Formicidae) in a fragmented habitat. Myrmecol. Nachr. 2006, 2006, 1–11. [Google Scholar]
- Helms, J.A. The flight ecology of ants (Hymenoptera: Formicidae). Myrmecol. News 2018, 26, 19–30. [Google Scholar]
- Kloft, W.; Haisch, A.; Haisch, B. Traceruntersuchungen mr Abgrenzung von Nestarealen holzzerstorender Rossameisen Camponotus herculeanus L. und C. ligniperda Latr.). Entomol. Exp. Et Appl. 1965, 8, 20–26. [Google Scholar] [CrossRef]
- Markó, B.; Kiss, K.; Gallé, L. Mosaic structure of ant communities (Hymenoptera: Formicidae) in Eastern Carpathian marshes: Regional versus local scales. Acta Zool. Acad. Sci. Hung. 2004, 50, 77–95. [Google Scholar]
- Ribas, C.R.; Schoereder, J.H. Are all ant mosaics caused by competition? Oecologia 2002, 131, 606–611. [Google Scholar] [CrossRef]
- Boulay, R.; Cerdá, X.; Simon, T.; Roldan, M.; Hefetz, A. Intraspecific competition in the ant Camponotus cruentatus. Anim. Behav. 2007, 74, 985–993. [Google Scholar] [CrossRef]
- Seppä, P.; Johansson, H.; Gyllenstrand, N.; Pálsson, S.; Pamilo, P. Mosaic structure of native ant supercolonies. Mol. Ecol. 2012, 21, 5880–5891. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Rosengren, R.; Sundström, L. Seasonal polydomy and unicoloniality in a polygynous population of the red wood ant Formica truncorum. Behav. Ecol. Sociobiol. 2005, 57, 339–349. [Google Scholar] [CrossRef]
- Mabelis, A.A. Wood Ant Wars the Relationship Between Aggression and Predation in the Red Wood Ant (Formica Polyctena Forst.) by. Neth. J. Zool. 1978, 29, 451–620. [Google Scholar] [CrossRef] [Green Version]
- Mabelis, A.A.; Korczyńska, J. Dispersal for survival: Some obesrvations on the trunk ant (Formica truncorum, Fabricius). Neth. J. Zool. 2001, 51, 299–321. [Google Scholar] [CrossRef]
- Véle, A.; Holuša, J.; Horák, J. Ant abundance increases with clearing size. J. For. Res. 2016, 21, 110–114. [Google Scholar] [CrossRef]
- Savolainen, R.; Vepsäläinen, K. A Competition Hierarchy among Boreal Ants. Oikos 1988, 51, 135–155. [Google Scholar] [CrossRef]
- Alinvi, O.; Bohlin, J.; Ball, J.P. Interspecific competition among ants in the boreal forest. Insectes Sociaux 2008, 55, 1–11. [Google Scholar] [CrossRef]
- Blochmann, F. Über das Vorkommen von bakterienähnlichen Gebilden in den Geweben und Eiern verschiedener Insekten. Zentbl. Bakteriol. 1892, 11, 234–240. [Google Scholar]
- De Souza, D.J.; Bézier, A.; Depoix, D.; Drezen, J.-M.; Lenoir, A. Blochmannia endosymbionts improve colony growth and immune defence in the ant Camponotus fellah. BMC Microbiol. 2009, 9, 29. [Google Scholar] [CrossRef]
- Sanders, C.J. Seasonal and daily activity patterns of Carpenter ants (Camponotus spp.) in northwestern Ontario (Hymenoptera: Formicidae). Can. Entomol. 1972, 104, 1681–1687. [Google Scholar] [CrossRef]
- Westerfelt, P.; Widenfalk, O.; Lindelöw, Å.; Gustafsson, L.; Weslien, J.; Leather, S.R.; Jonsell, M. Nesting of solitary wasps and bees in natural and artificial holes in dead wood in young boreal forest stands. Insect Conserv. Divers. 2015, 8, 493–504. [Google Scholar] [CrossRef]
- Horák, J.; Rébl, K. The species richness of click beetles in ancient pasture woodland benefits from a high level of sun exposure. J. Insect Conserv. 2013, 17, 307–318. [Google Scholar] [CrossRef]
- Aussenac, G. Interactions between forest stands and microclimate. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Olchev, A.; Radler, K.; Sogachev, A.; Panferov, O.; Gravenhorst, G. Application of a three-dimensional model for assessing effects of small clear-cuttings on radiation and soil temperature. Ecol. Model. 2009, 220, 3046–3056. [Google Scholar] [CrossRef]
- Gooding, G.V.; Hodges, C.S.; Ross, E.W. Effect of Temperature on Growth and Survival of Fomes annosus. For. Sci. 1966, 12, 325–333. [Google Scholar]
- Ross, E.W. Thermal inactivation of conidia and basidiospores of Fomes annosus. Phytopathology 1969, 59, 1798–1801. [Google Scholar]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood. Its Biology and Ecology, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 1988; ISBN 0-471-10310-1. [Google Scholar]
- Edman, M.; Jönsson, M.; Jonsson, B.G. Fungi and wind strongly influence the temporal availability of logs in an old-growth spruce forest. Ecol. Appl. 2007, 17, 482–490. [Google Scholar] [CrossRef]
- Abreau, H.S.; Nascimento, A.M.; Maria, M.A. Lignin structure and wood properties. Wood Fiber Sci. J. Soc. Wood Sci. Technol. 1999, 31, 426–433. [Google Scholar]
- Spiecker, H. Silvicultural management in maintaining biodiversity and resistance of forests in Europe—Temperate zone. J. Environ. Manag. 2003, 67, 55–65. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Holuša, J. Health condition of Norway spruce Picea abies (L.) Karst. stands in the Beskid Mts. Dendrobiology 2004, 51, 11–15. [Google Scholar]
- Jactel, H.; Nicoll, B.C.; Branco, M.; Gonzalez-Olabarria, J.R.; Grodzki, W.; Långström, B.; Moreira, F.; Netherer, S.; Orazio, C.; Piou, D.; et al. The influences of forest stand management on biotic and abiotic risks of damage. Ann. For. Sci. 2009, 66, 701–719. [Google Scholar] [CrossRef]
- Green, G.W.; Sullivan, C.R. Ants Attacking Larvae of the Forest Tent Caterpillar, Malacosoma disstria Hbn. (Lepidoptera: Lasiocampidae). Can. Entomol. 1950, 82, 194–195. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Véle, A.; Horák, J. Space, Habitat and Isolation are the Key Determinants of Tree Colonization by the Carpenter Ant in Plantation Forests. Forests 2019, 10, 630. https://doi.org/10.3390/f10080630
Véle A, Horák J. Space, Habitat and Isolation are the Key Determinants of Tree Colonization by the Carpenter Ant in Plantation Forests. Forests. 2019; 10(8):630. https://doi.org/10.3390/f10080630
Chicago/Turabian StyleVéle, Adam, and Jakub Horák. 2019. "Space, Habitat and Isolation are the Key Determinants of Tree Colonization by the Carpenter Ant in Plantation Forests" Forests 10, no. 8: 630. https://doi.org/10.3390/f10080630
APA StyleVéle, A., & Horák, J. (2019). Space, Habitat and Isolation are the Key Determinants of Tree Colonization by the Carpenter Ant in Plantation Forests. Forests, 10(8), 630. https://doi.org/10.3390/f10080630




