Contrasting Response to Drought and Climate of Planted and Natural Pinus pinaster Aiton Forests in Southern Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Sampling Methods
2.3. Climatic Data
2.4. Tree-Ring Methods
2.5. Climatic Response Indices
2.6. Statistical Analysis
3. Results
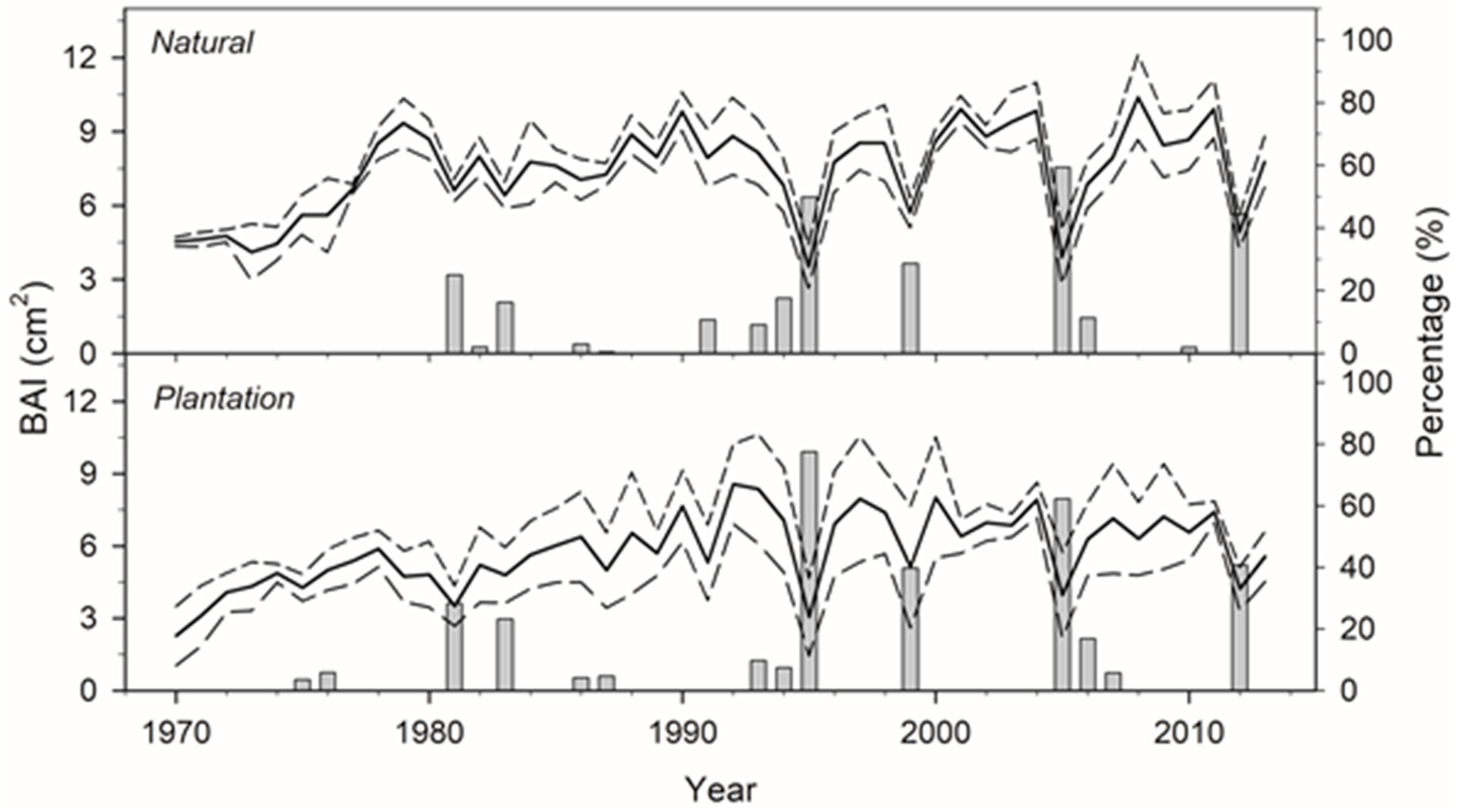
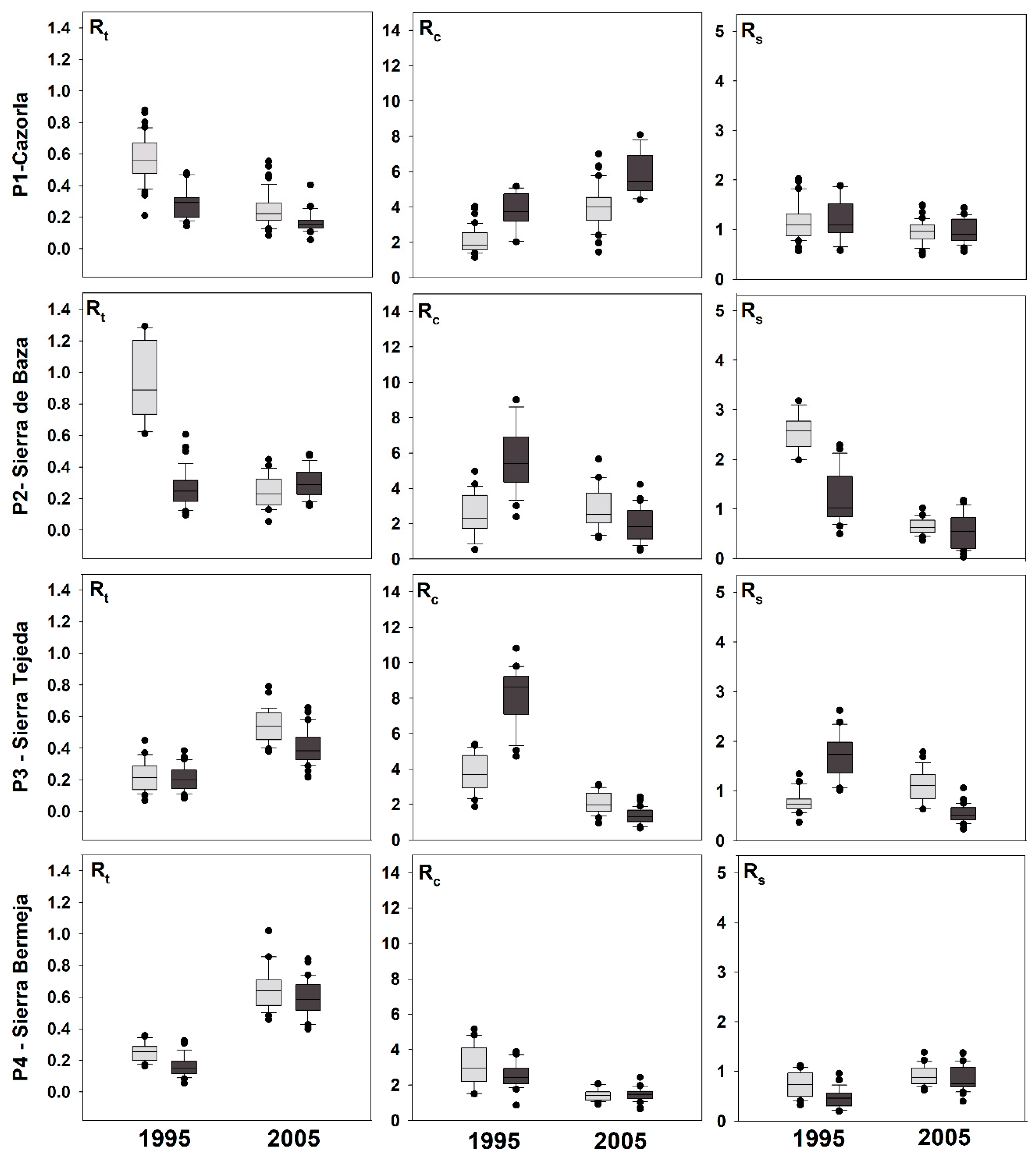
3.1. Structure, Growth, and Sensitivity to Drought
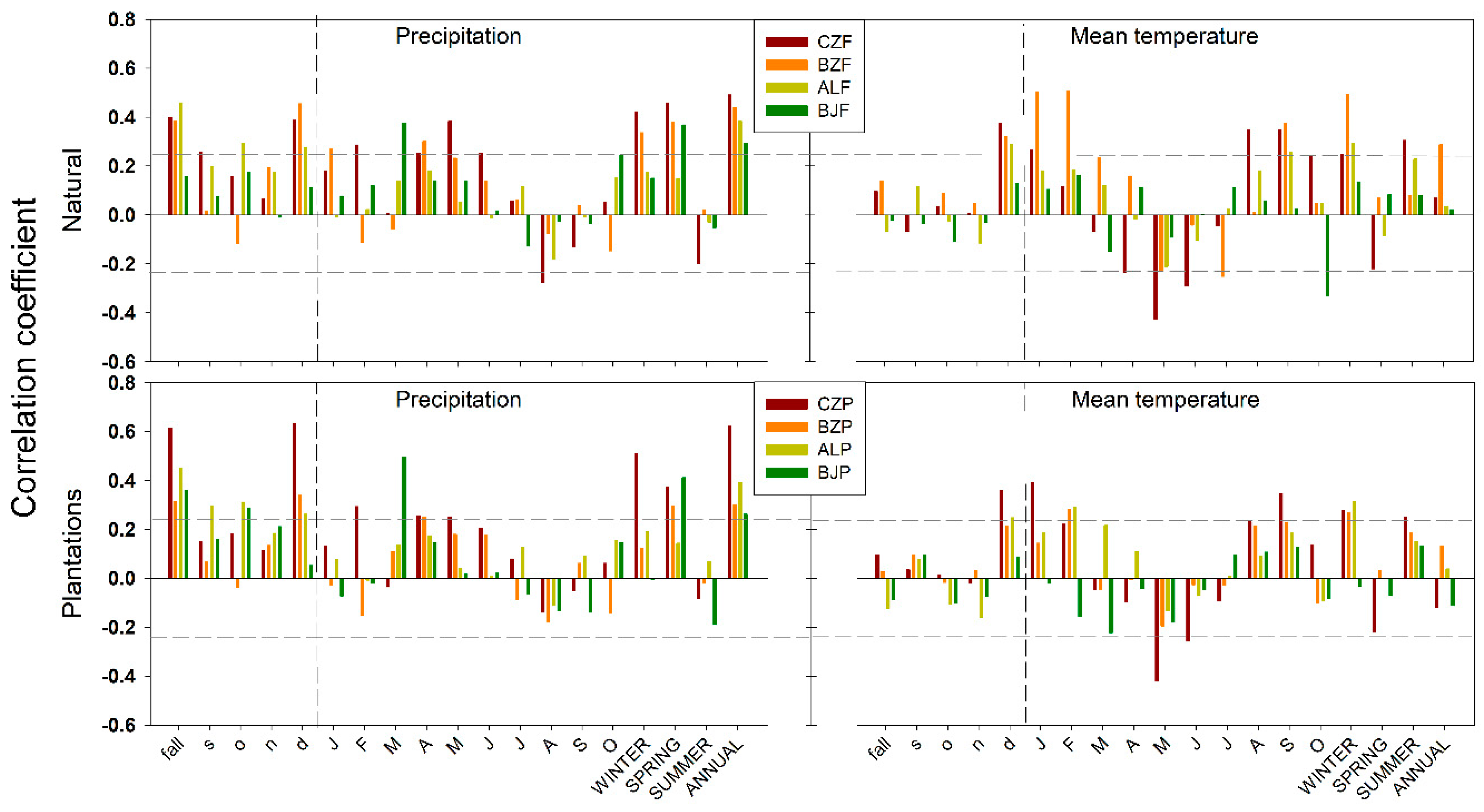
3.2. Climate-Growth Relationships
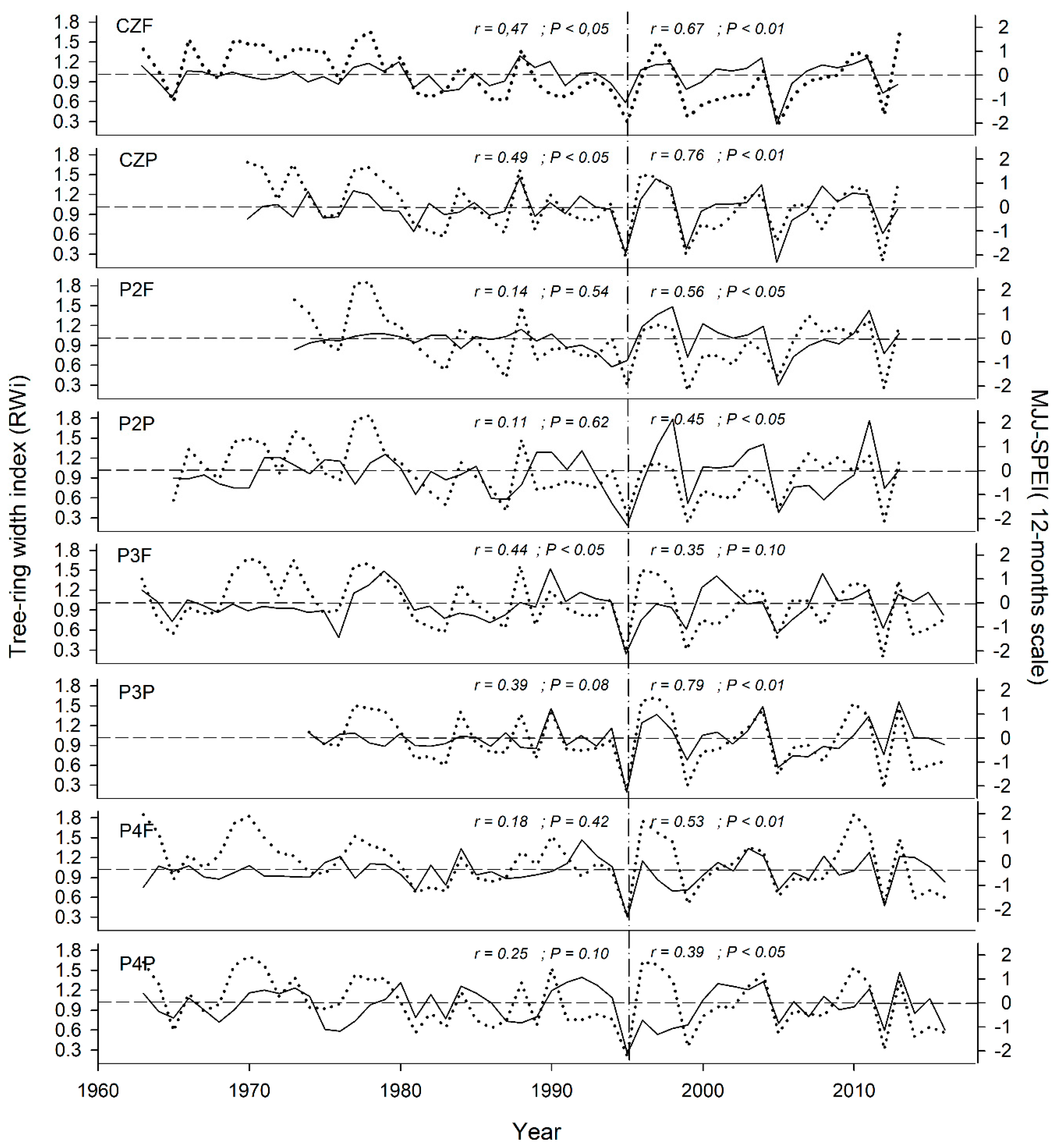
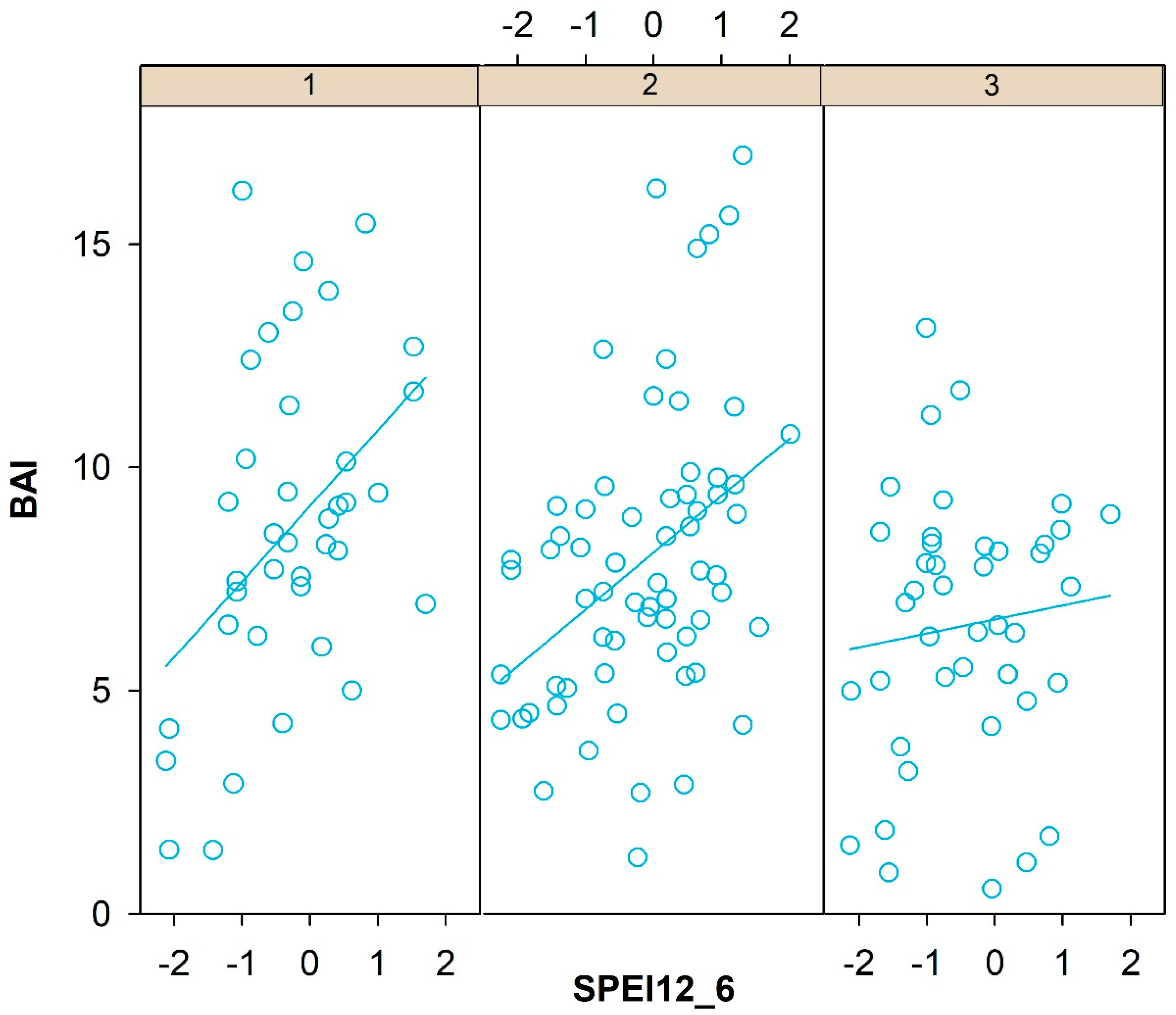
3.3. Climatic Response Indices
3.4. Effects of Defoliation on Growth
4. Discussion
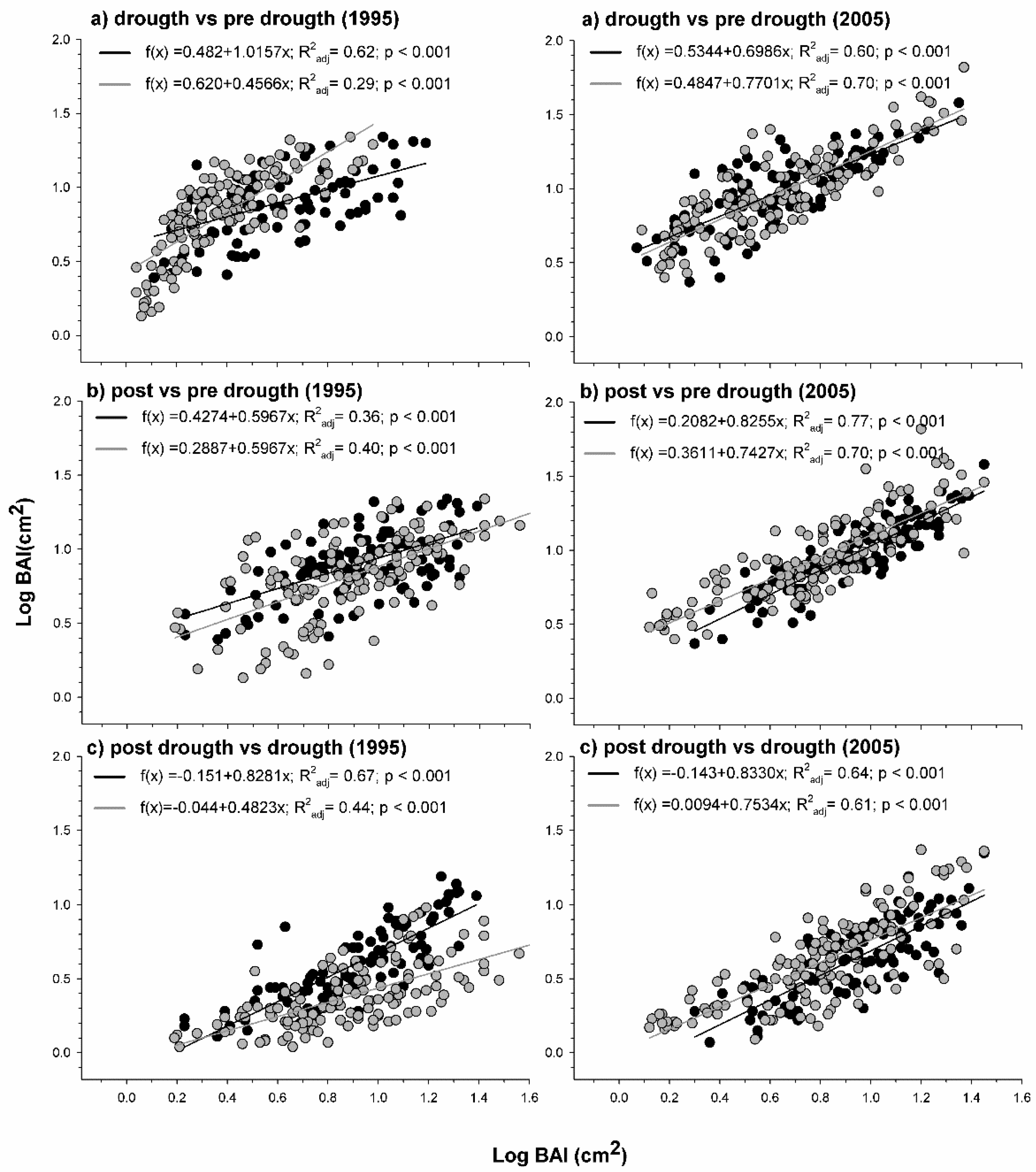
4.1. Contrasting Growth Responses to Drought in Planted and Natural Stands
4.2. Climate Response Indices
4.3. Effect of Defoliation on Growth
4.4. Management Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in Spatiotemporal Patterns of 20th Century Spruce Budworm Outbreaks in Eastern Canadian Boreal Forests. Front. Plant Sci. 2018, 9, 1905. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Bennett, S.; Wernberg, T.; Arackal Joy, B.; de Bettignies, T.; Campbell, A.H. Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 2015, 6, 10280. [Google Scholar] [CrossRef]
- Young, D.J.N.; Stevens, J.T.; Earles, J.M.; Moore, J.; Ellis, A.; Jirka, A.L.; Latimer, A.M. Long-term climate and competition explain forest mortality patterns under extreme drought. Ecol. Lett. 2017, 20, 78–86. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, art129. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Lloret, F. Drought-induced vegetation shifts in terrestrial ecosystems: The key role of regeneration dynamics. Glob. Planet. Chang. 2016, 144, 94–108. [Google Scholar] [CrossRef]
- Sapes, G.; Serra-Diaz, J.M.; Lloret, F. Species climatic niche explains drought-induced die-off in a Mediterranean woody community. Ecosphere 2017, 8, e01833. [Google Scholar] [CrossRef]
- Adams, H.D.; Macalady, A.K.; Breshears, D.D.; Allen, C.D.; Stephenson, N.L.; Saleska, S.R.; Huxman, T.E.; McDowell, N.G. Climate-Induced Tree Mortality: Earth System Consequences. Eos 2010, 91, 153–154. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Greenwood, S.; Ruiz-Benito, P.; Martínez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bönisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Gazol, A.; Ribas, M.; Gutiérrez, E.; Camarero, J.J. Aleppo pine forests from across Spain show drought-induced growth decline and partial recovery. Agric. For. Meteorol. 2017, 232, 186–194. [Google Scholar] [CrossRef]
- Madrigal-González, J.; Andivia, E.; Zavala, M.A.; Stoffel, M.; Calatayud, J.; Sánchez-Salguero, R.; Ballesteros-Cánovas, J. Disentangling the relative role of climate change on tree growth in an extreme Mediterranean environment. Sci. Total. Environ. 2018, 642, 619–628. [Google Scholar] [CrossRef]
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bladé, C.; Valdecantos, A.; Seva, J.P.; Fuentes, D.; Alloza, J.A.; Vilagrosa, A.; Bautista, S.; Cortina, J.; Vallejo, R. Pines and oaks in the restoration of Mediterranean landscapes of Spain: New perspectives for an old practice—a review. Plant Ecol. 2004, 171, 209–220. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Dobbertin, M.; Fernández-Cancio, Á.; Vilà-Cabrera, A.; Manzanedo, R.D.; Zavala, M.A.; Navarro-Cerrillo, R.M. Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. natural rear-edge Pinus nigra forests. For. Ecol. Manag. 2013, 310, 956–967. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Navarro, R.M.; Camarero, J.J.; Fernández-Cancio, Á. Drought-induced growth decline of Aleppo and maritime pine forests in south-eastern Spain. For. Syst. 2010, 19, 458. [Google Scholar] [CrossRef]
- de Luis, M.; Čufar, K.; Filippo, A.D.; Novak, K.; Papadopoulos, A.; Piovesan, G.; Rathgeber, C.B.K.; Raventós, J.; Saz, M.A.; Smith, K.T. Plasticity in Dendroclimatic Response across the Distribution Range of Aleppo Pine (Pinus halepensis). PLoS ONE 2013, 8, e83550. [Google Scholar] [CrossRef]
- Natalini, F.; Correia, A.C.; Vázquez-Piqué, J.; Alejano, R. Tree rings reflect growth adjustments and enhanced synchrony among sites in Iberian stone pine (Pinus pinea L.) under climate change. Ann. For. Sci. 2015, 72, 1023–1033. [Google Scholar] [CrossRef]
- Garzón, M.B.; de Dios, R.S.; Ollero, H.S. Effects of climate change on the distribution of Iberian tree species. Appl. Veg. Sci. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Sanguesa-Barreda, G.; Linares, J.C.; Camarero, J.J. Mistletoe effects on Scots pine decline following drought events: Insights from within-tree spatial patterns, growth and carbohydrates. Tree Physiol. 2012, 32, 585–598. [Google Scholar] [CrossRef]
- Viñas, R.; Caudullo, G.; De Rigo, D. Pinus pinaster in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Mazza, G.; Cutini, A.; Manetti, M.C. Influence of tree density on climate-growth relationships in a Pinus pinaster Ait. forest in the northern mountains of Sardinia (Italy). iForest 2014, 8, 456–463. [Google Scholar] [CrossRef]
- Kurz-Besson, C.B.; Lousada, J.L.; Gaspar, M.J.; Correia, I.E.; David, T.S.; Soares, P.M.M.; Cardoso, R.M.; Russo, A.; Varino, F.; Mériaux, C.; et al. Effects of Recent Minimum Temperature and Water Deficit Increases on Pinus pinaster Radial Growth and Wood Density in Southern Portugal. Front. Plant Sci. 2016, 7, 1170. [Google Scholar] [CrossRef]
- Caminero, L.; Génova, M.; Camarero, J.J.; Sánchez-Salguero, R. Growth responses to climate and drought at the southernmost European limit of Mediterranean Pinus pinaster forests. Dendrochronologia 2018, 48, 20–29. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J. Cumulative Drought Stress Leads to a Loss of Growth Resilience and Explains Higher Mortality in Planted than in Naturally Regenerated Pinus pinaster Stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Bogino, S.M.; Bravo, F. Growth response of Pinus pinaster Ait. to climatic variables in central Spanish forests. Ann. For. Sci. 2008, 65, 506. [Google Scholar] [CrossRef]
- Puri, E.; Hoch, G.; Körner, C. Defoliation reduces growth but not carbon reserves in Mediterranean Pinus pinaster trees. Trees 2015, 29, 1187–1196. [Google Scholar] [CrossRef]
- Haylock, M.R.; Hofstra, N.; Tank, A.M.G.K.; Klok, E.J.; Jones, P.D.; New, M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Perez Latorre, A.V.; Hidalgo, H.; Cabezudo, B. Los tipos funcionales de la flora y vegetación de las peridotitas de Sierra Bermeja (Málaga): Estudio preliminar. In Las Zonas De Montaña: Gestión Y Biodiversidad; Universitat Autònoma de Barcelona: Barcelona, Spain, 2012. [Google Scholar]
- Red de equilibrios biológicos de Andalucía. Available online: http://www.juntadeandalucia.es/medioambiente/site/portalweb/menuitem.7e1cf46ddf59bb227a9ebe205510e1ca/?vgnextoid=095d7d834f2a1510VgnVCM2000000624e50aRCRD&vgnextchannel=6abe5d95c1191510VgnVCM2000000624e50aRCRD (accessed on 27 May 2019).
- Clyatt, K.A.; Crotteau, J.S.; Schaedel, M.S.; Wiggins, H.L.; Kelley, H.; Churchill, D.J.; Larson, A.J. Historical spatial patterns and contemporary tree mortality in dry mixed-conifer forests. For. Ecol. Manag. 2016, 361, 23–37. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2009, 23, 1696–1718. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; The Blackburn Press: Caldwell, NJ, USA, 2001. [Google Scholar]
- Stokes, M.A. An Introduction to Tree-ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Mazza, G.; Manetti, M.C. Growth rate and climate responses of Pinus pinea L. in Italian coastal stands over the last century. Clim. Chang. 2013, 121, 713–725. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Clim. Appl. Meteor. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2001. [Google Scholar]
- D’Orangeville, L.; Maxwell, J.; Kneeshaw, D.; Pederson, N.; Duchesne, L.; Logan, T.; Houle, D.; Arseneault, D.; Beier, C.M.; Bishop, D.A.; et al. Drought timing and local climate determine the sensitivity of eastern temperate forests to drought. Glob. Chang. Biol. 2018, 24, 2339–2351. [Google Scholar] [CrossRef]
- Spinoni, J.; Naumann, G.; Vogt, J.V.; Barbosa, P. The biggest drought events in Europe from 1950 to 2012. J. Hydrol. Reg. Stud. 2015, 3, 509–524. [Google Scholar] [CrossRef]
- Guada, G.; Camarero, J.J.; Sánchez-Salguero, R.; Cerrillo, R.M.N. Limited Growth Recovery after Drought-Induced Forest Dieback in Very Defoliated Trees of Two Pine Species. Front. Plant Sci. 2016, 7, 418. [Google Scholar] [CrossRef]
- Hartmann, H.; Adams, H.D.; Anderegg, W.R.L.; Jansen, S.; Zeppel, M.J.B. Research frontiers in drought-induced tree mortality: Crossing scales and disciplines. New Phytol. 2015, 205, 965–969. [Google Scholar] [CrossRef]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Beguería, S.; Trigo, R.; López-Moreno, J.I.; Azorín-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Martinez-Vilalta, J.; Cailleret, M.; Camarero, J.J.; Ewers, B.E.; Galbraith, D.; Gessler, A.; Grote, R.; Huang, C.; Levick, S.R.; et al. When a Tree Dies in the Forest: Scaling Climate-Driven Tree Mortality to Ecosystem Water and Carbon Fluxes. Ecosystems 2016, 19, 1133–1147. [Google Scholar] [CrossRef]
- Zhu, J.J.; Fan, Z.P.; Zeng, D.H.; Jiang, F.Q.; Matsuzaki, T. Comparison of stand structure and growth between artificial and natural forests of Pinus sylvestiris var. mongolica on sandy land. J. For. Res. 2003, 14, 103–111. [Google Scholar]
- Song, L.; Li, M.; Zhu, J.; Zhang, J. Comparisons of radial growth and tree-ring cellulose δ13C for Pinus sylvestris var. mongolica in natural and plantation forests on sandy lands. J. For. Res. 2017, 22, 160–168. [Google Scholar] [CrossRef]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Sánchez-Costa, E.; Poyatos, R.; Sabaté, S. Contrasting growth and water use strategies in four co-occurring Mediterranean tree species revealed by concurrent measurements of sap flow and stem diameter variations. Agric. For. Meteorol. 2015, 207, 24–37. [Google Scholar] [CrossRef]
- Fettig, C.J.; Mortenson, L.A.; Bulaon, B.M.; Foulk, P.B. Tree mortality following drought in the central and southern Sierra Nevada, California, U.S. For. Ecol. Manag. 2019, 432, 164–178. [Google Scholar] [CrossRef]
- Peña-Gallardo, M.; Vicente-Serrano, S.M.; Camarero, J.J.; Gazol, A.; Sánchez-Salguero, R.; Domínguez-Castro, F.; El Kenawy, A.; Beguería-Portugés, S.; Gutiérrez, E.; De Luis, M.; et al. Drought Sensitiveness on Forest Growth in Peninsular Spain and the Balearic Islands. Forests 2018, 9, 524. [Google Scholar] [CrossRef]
- Montoro Girona, M.; Morin, H.; Lussier, J.M.; Walsh, D. Radial Growth Response of Black Spruce Stands Ten Years after Experimental Shelterwoods and Seed-Tree Cuttings in Boreal Forest. Forests 2016, 7, 240. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- McDowell, N.; Allen, C.D.; Anderson-Teixeira, K.; Brando, P.; Brienen, R.; Chambers, J.; Christoffersen, B.; Davies, S.; Doughty, C.; Duque, A.; et al. Drivers and mechanisms of tree mortality in moist tropical forests. New Phytol. 2018, 219, 851–869. [Google Scholar] [CrossRef]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Chang. Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Chang. Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Sánchez-Salguero, R.; Rodriguez, C.; Duque Lazo, J.; Moreno-Rojas, J.M.; Palacios-Rodriguez, G.; Camarero, J.J. Is thinning an alternative when trees could die in response to drought? The case of planted Pinus nigra and P. Sylvestris stands in southern Spain. For. Ecol. Manag. 2019, 433, 313–324. [Google Scholar] [CrossRef]
- Newman, B.D.; Wilcox, B.P.; Archer, S.R.; Breshears, D.D.; Dahm, C.N.; Duffy, C.J.; McDowell, N.G.; Phillips, F.M.; Scanlon, B.R.; Vivoni, E.R. Ecohydrology of water-limited environments: A scientific vision. Wat. Res. Res. 2006, 42. [Google Scholar] [CrossRef]
- Camarero, J.J.; Linares, J.C.; Sangüesa-Barreda, G.; Sánchez-Salguero, R.; Gazol, A.; Navarro-Cerrillo, R.M.; Carreira, J.A. The Multiple Causes of Forest Decline in Spain: Drought, Historical Logging, Competition and Biotic Stressors. In Dendroecology: Tree-Ring Analyses Applied to Ecological Studies; Amoroso, M.M., Daniels, L.D., Baker, P.J., Camarero, J.J., Eds.; Ecological Studies; Springer International Publishing: Cham, Switzerland, 2017; pp. 307–323. [Google Scholar]
- Girona, M.M.; Rossi, S.; Lussier, J.M.; Walsh, D.; Morin, H. Understanding tree growth responses after partial cuttings: A new approach. PLoS ONE 2017, 12, e0172653. [Google Scholar]
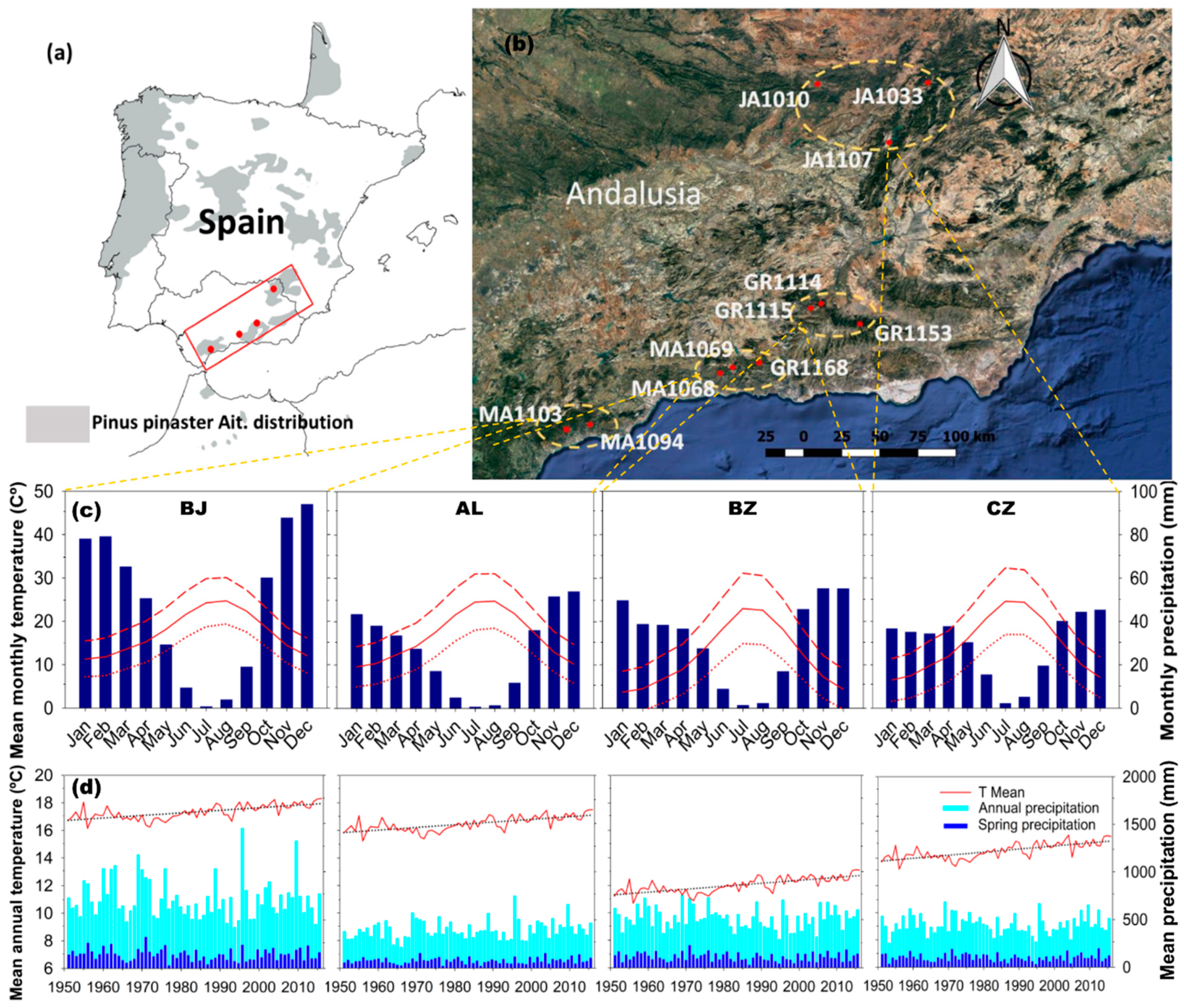
| Provenance Zone | Stand Type | Site (Code) | Lat (N) | Long (W) | Elev | Exp | Slope (%) | Soil Type | DBH (cm) | Height (m) | G (m2/ha) | S | Density (Tree/ha) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sierra de Cazorla, Segura y las Villas (CZ) | P | JA1010 | 38.41 | 3.42 | 1090 | SE | 10–35 | L | 34.0 ± 1.1 | 14.4 ± 0.6 | 16.5 | 290 | 160 |
| F | JA1033 | 38.44 | 2.59 | 980 | SE | 0–10 | L/D | 31.0 ± 1.8 | 14.0 ± 0.8 | 39.7 | 730 | 500 | |
| F | JA1107 | 38.11 | 2.90 | 1200 | S | 10–35 | LD | 41.1 ± 3.9 | 14.3 ± 0.7 | 23.3 | 390 | 140 | |
| Sierra Nevada, de Huetor y Baza (BZ) | P | GR1153 | 37.14 | 3.07 | 1500 | W | 35–65 | D | 19.1 ± 1.2 | 6.3 ± 0.2 | 32.4 | 750 | 1150 |
| F | GR1114 | 37.23 | 3.45 | 1320 | S | 35–65 | L | 30.0 ± 1.5 | 10.2 ± 0.5 | 14.1 | 285 | 240 | |
| P | GR1115 | 37.25 | 3.36 | 1350 | E | 10–35 | L | 24.1 ± 1.1 | 10.6 ± 0.3 | 47.8 | 450 | 930 | |
| Sierra Tejeda y Almijara (AL) | F | GR1168 | 36.91 | 3.75 | 1250 | NE | 10–35 | L | 38.7 ± 2.1 | 9.6 ± 0.4 | 12.5 | 230 | 125 |
| P | MA1068 | 36.87 | 4.02 | 970 | SE | 35–65 | D | 30.9 ± 0.9 | 9.5 ± 0.4 | 16.5 | 300 | 210 | |
| P | MA1069 | 36.88 | 3.93 | 1580 | S | 10–35 | D | 37.1 ± 3.6 | 13.0 ± 0.9 | 32.5 | 576 | 270 | |
| Sierra Bermeja (BJ) | F | MA1103 | 36.59 | 4.96 | 400 | SW | 35–65 | P | 33.4 ± 1.7 | 8.2 ± 0.3 | 11.4 | 250 | 180 |
| P | MA1094 | 36.29 | 5.12 | 260 | S | >65 | P | 32.2 ± 3.2 | 8.8 ± 0.4 | 10.5 | 195 | 130 |
| N Trees (Cores) | Timespan (N Years) | TRW ± SD (mm) | BAI20 | AC1 | MS | Rbar | EPS | ||
|---|---|---|---|---|---|---|---|---|---|
| Sierra de Cazorla, Segura y las Villas (CZ) | CZF | 22 (44) | 1848–2013 (166) | 1.92 ± 0.12 | 8.74 ± 0.52 | 0.598 * | 0.283 *** | 0.371 | 0.913 |
| CZP | 12 (23) | 1970–2013 (44) | 2.40 ± 0.30 | 6.94 ± 0.61 | 0.621 * | 0.406 *** | 0.795 | 0.988 | |
| Sierra Nevada, de Huetor y Baza (BZ) | BZF | 12 (12) | 1973–2013 (41) | 2.36 ± 0.0.35 * | 6.43 ± 0.57 | 0.758 ** | 0.314 | 0.787 | 0.986 |
| BZP | 22 (42) | 1966–2013 (48) | 1.83 ± 0.19 * | 3.54 ± 0.36 | 0.676 ** | 0.445 | 0.560 | 0.976 | |
| Sierra Tejeda y Almijara (AL) | ALF | 14 (22) | 1910–2017 (108) | 1.45 ± 0.13 *** | 7.48 ± 0.62 * | 0.676 ** | 0.295 *** | 0.360 | 0.925 |
| ALP | 36 (64) | 1974–2017 (44) | 3.10 ± 0.22 *** | 10.26 ± 0.72 * | 0.469 ** | 0.383 *** | 0.802 | 0.994 | |
| Sierra Bermeja (BJ) | BJF | 12 (23) | 1960–2017 (58) | 2.54 ± 0.29 * | 8.62 ± 0.58 * | 0.590 ** | 0.339 ** | 0.729 | 0.976 |
| BJP | 18 (30) | 1936–2017 (82) | 1.71 ± 0.17 * | 5.45 ± 0.46 * | 0.606 ** | 0.376 ** | 0.589 | 0.968 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Vallejo, C.; Navarro-Cerrillo, R.M. Contrasting Response to Drought and Climate of Planted and Natural Pinus pinaster Aiton Forests in Southern Spain. Forests 2019, 10, 603. https://doi.org/10.3390/f10070603
Rodriguez-Vallejo C, Navarro-Cerrillo RM. Contrasting Response to Drought and Climate of Planted and Natural Pinus pinaster Aiton Forests in Southern Spain. Forests. 2019; 10(7):603. https://doi.org/10.3390/f10070603
Chicago/Turabian StyleRodriguez-Vallejo, Carlos, and Rafael M. Navarro-Cerrillo. 2019. "Contrasting Response to Drought and Climate of Planted and Natural Pinus pinaster Aiton Forests in Southern Spain" Forests 10, no. 7: 603. https://doi.org/10.3390/f10070603
APA StyleRodriguez-Vallejo, C., & Navarro-Cerrillo, R. M. (2019). Contrasting Response to Drought and Climate of Planted and Natural Pinus pinaster Aiton Forests in Southern Spain. Forests, 10(7), 603. https://doi.org/10.3390/f10070603






