Leaf and Soil δ15N Patterns Along Elevational Gradients at Both Treelines and Shrublines in Three Different Climate Zones
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Chemical Analysis
2.3. Statistical Analyses
3. Results
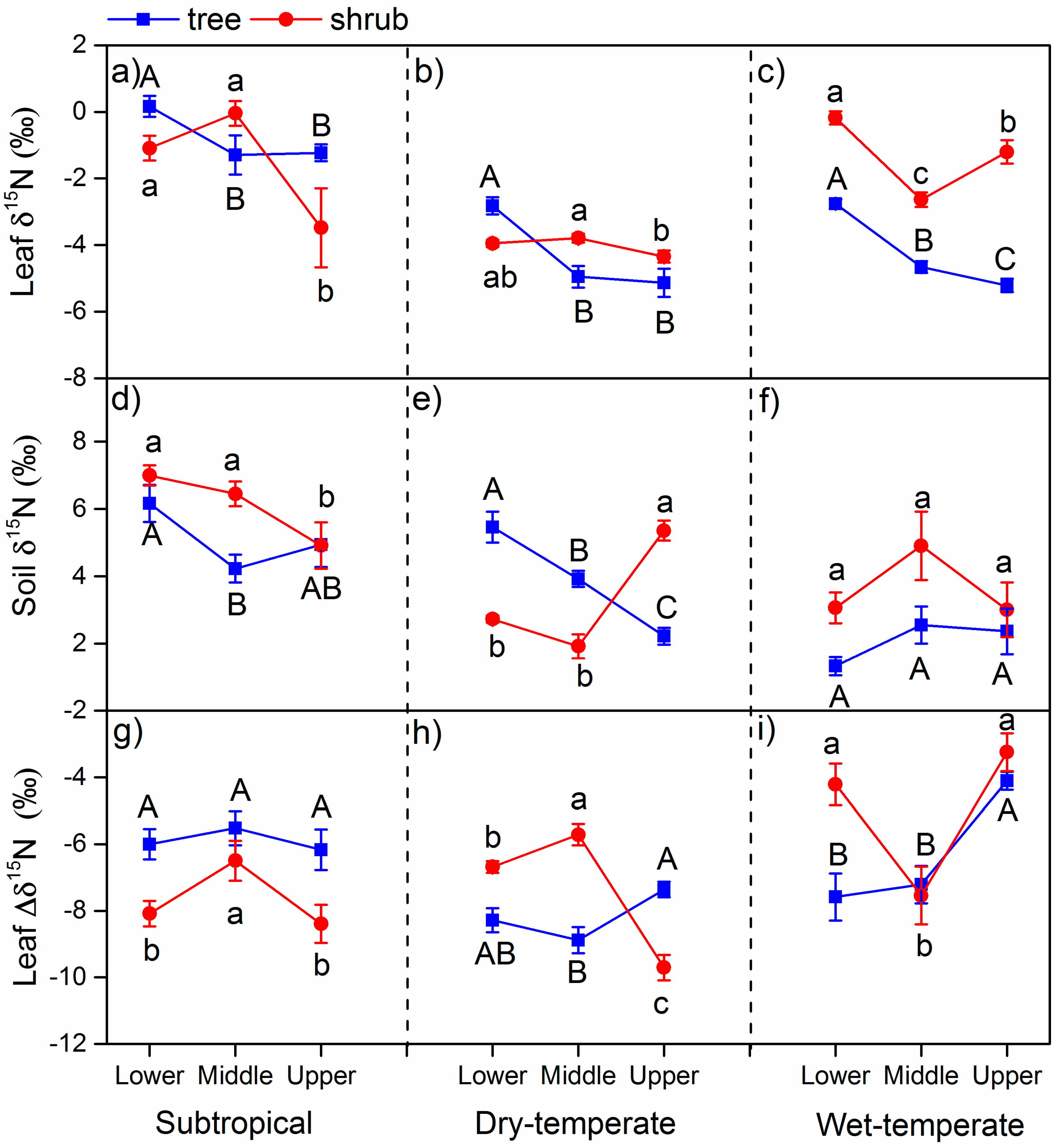
3.1. δ15N in Leaves and Soils
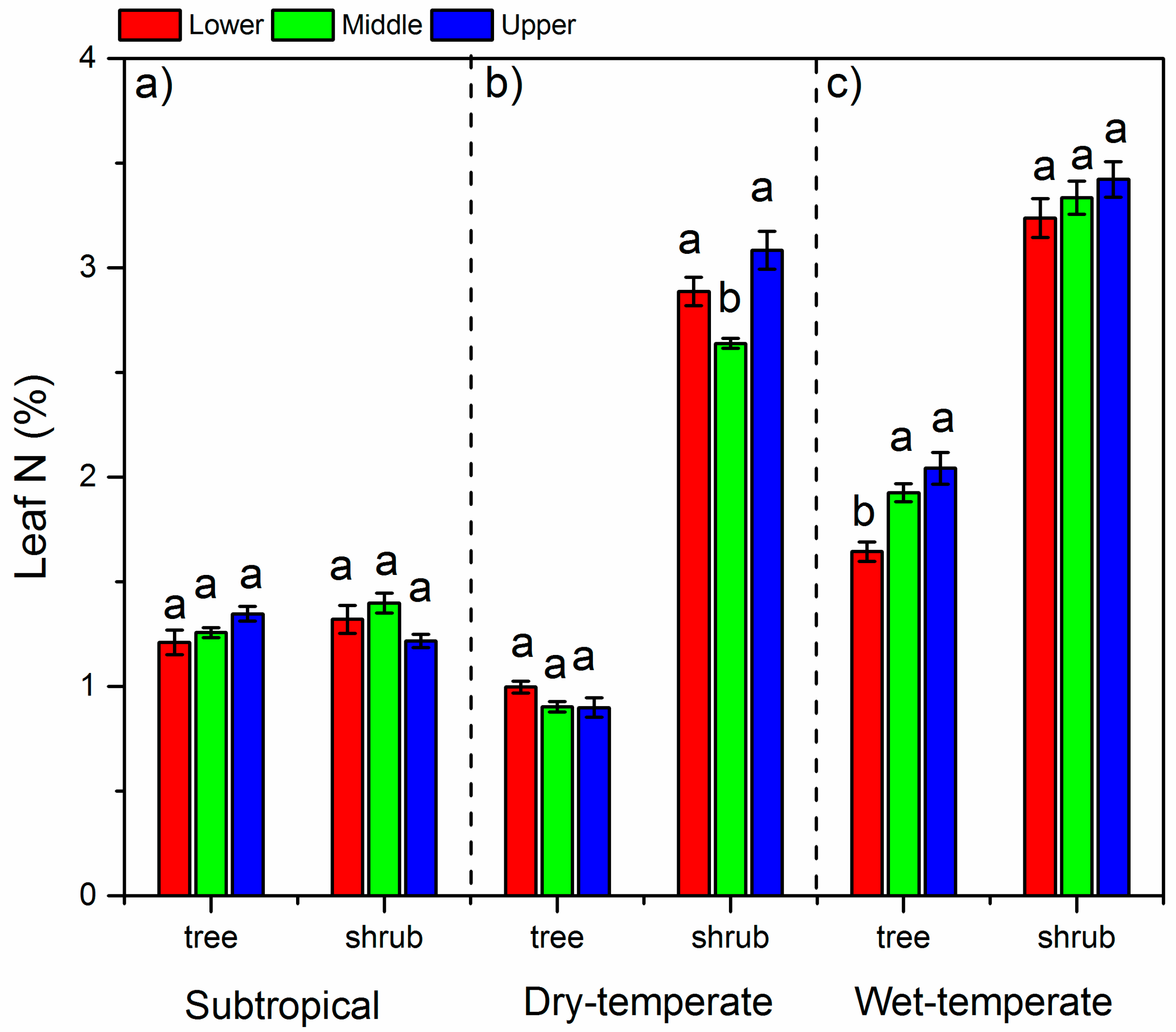
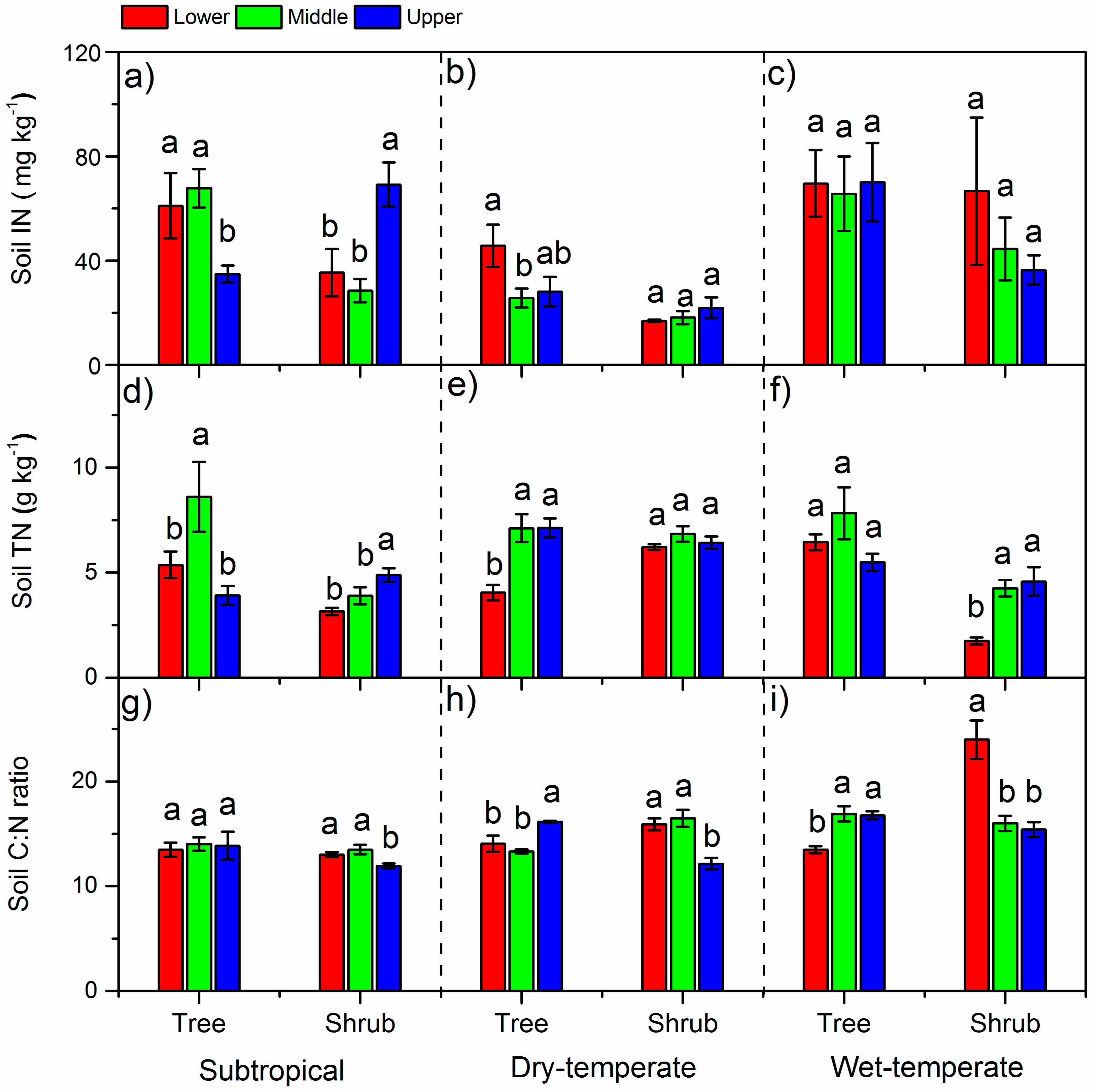
3.2. N Concentrations in Leaves and Soils and Soil C:N Ratios
3.3. Correlations between δ15N and Parameters in Leaves and Soils
4. Discussion
4.1. The δ15N and N in Different Climate Zones
4.2. Variation of δ15N and N Along the Altitude
4.3. Variation of δ15N and N between Life Forms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Högberg, P. 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Austin, A.; Vitousek, P.M. Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 1998, 113, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.M.; Shearer, G.; Kohl, D.H. Foliar 15N natural abundance in Hawaiian rainforest: Patterns and possible mechanisms. Oecologia 1989, 78, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Handley, L.L.; Austin, A.T.; Stewart, G.R.; Robinson, D.; Scrimgeour, C.M.; Raven, J.A.; Schmidt, S. The 15-N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Aust. J. Plant. Phys. 1999, 26, 185–199. [Google Scholar]
- Mizutani, H.; Kabaya, Y.; Wada, E. Linear correlation netween latitude and soil 15N enrichment at seabird rookeries. Naturwissenschaften 1991, 78, 34–36. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Gavazov, K.; Hagedorn, F.; Buttler, A.; Siegwolf, R.; Bragazza, L. Environmental drivers of carbon and nitrogen isotopic signatures in peatland vascular plants along an altitude gradient. Oecologia 2016, 180, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bai, E.; Boutton, T.W.; Liu, F.; Wu, X.B.; Archer, S.R.; Hallmark, C.T. Spatial variation of the stable nitrogen isotope ratio of woody plants along a topoedaphic gradient in a subtropical savanna. Oecologia 2009, 159, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.L.; Baisden, W.T. Global patterns of theisotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Aidar, M.P.M.; Bustamante, M.; Dawson, T.E.; Hobbie, E.A.; Kahmen, A.; Mack, M.C.; McLauchlan, K.K.; Michelsen, A.; et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009, 183, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Rustad, L.; Campbell JLMarion, G.; Norby, R.; Mitchell, M.; Hartley, A. A meta-analysis of the response of soil respiration, net nitrogenmineralization, and aboveground plant growth to experimental ecosystemwarming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.F.; Yang, Y.Q. Enrichment of stable carbon and nitrogen isotopes of plant populations and plateau pikas along altitudes. J. Anim. Feed Sci. 2006, 15, 661–667. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Peter, H. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; Xu, M.G.; Qiu, G.Y.; Zhou, J.B. Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J. Plant Ecol. 2009, 2, 107–118. [Google Scholar] [CrossRef]
- Michelsen, A.; Schmidt, I.K.; Jonasson, S.; Quarmby, C.; Sleep, D. Leaf 15 N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non-and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 1996, 105, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Wang, G.; Yang, Y.; Chen, X. Experimental warming increased soil nitrogen sink in the Tibetan permafrost. J. Geophys. Res. Biogeosci. 2017, 122, 1870–1879. [Google Scholar] [CrossRef]
- Dial, R.J.; Smeltz, T.S.; Sullivan, P.F.; Rinas, C.L.; Timm, K.; Geck, J.E.; Tobin, S.C.; Golden, T.S.; Berg, E.C. Shrubline but not treeline advance matches climate velocity in montane ecosystems of south-central Alaska. Glob. Chang. Biol. 2016, 22, 1841–1856. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Hik, D.S. Climate warming as a driver of tundra shrubline advance. J. Ecol. 2018, 106, 547–560. [Google Scholar] [CrossRef]
- Körner, C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Ellison, S.B.Z.; McNown, R.W.; Brownlee, A.H.; Sveinbjoernsson, B. Evidence of soil nutrient availability as the proximate constraint on growth of treeline trees in northwest Alaska. Ecology 2015, 96, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Mohl, P.; Morsdorf, M.A.; Dawes, M.A.; Hagedorn, F.; Bebi, P.; Viglietti, D.; Freppaz, M.; Wipf, S.; Korner, C.; Thomas, F.M.; et al. Twelve years of low nutrient input stimulates growth of trees and dwarf shrubs in the treeline ecotone. J. Ecol. 2019, 107, 768–780. [Google Scholar] [CrossRef]
- Myers-Smith, I. Shrub Line Advance in Alpine Tundra of the Kluane Region: Mechanisms of Expansion and Ecosystem Impacts. Arctic 2007, 60, 447–451. [Google Scholar] [CrossRef]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, F.; Shiyatov, S.G.; Mazepa, V.S.; Devi, N.M.; Grigor’ev, A.A.; Bartysh, A.A.; Fomin, V.V.; Kapralov, D.S.; Terent’ev, M.; Bugman, H.; et al. Treeline advances along the Urals mountain range—Driven by improved winter conditions? Glob. Chang. Biol. 2014, 20, 3530–3543. [Google Scholar] [CrossRef] [PubMed]
- Dawes, M.A.; Schleppi, P.; Hattenschwiler, S.; Rixen, C.; Hagedorn, F. Soil warming opens the nitrogen cycle at the alpine treeline. Glob. Chang. Biol. 2017, 23, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, X.; Tognetti, R.; Lei, J.P.; Pan, H.L.; Liu, X.L.; Jiang, Y.; Wang, X.Y.; He, P.; Yu, F.H.; et al. Elevation alters carbon and nutrient concentrations and stoichiometry in Quercus aquifolioides in southwestern China. Sci. Total Environ. 2018, 622, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.Y.; Wang, X.L.; Chen, T.; Feng, H.Y.; An, L.Z.; He, Y.Q.; Wang, G. Variations of stomatal density and carbon isotope values of Picea crassifolia at different altitudes in the Qilian Mountains. Trees 2003, 17, 258–262. [Google Scholar]
- Yu, D.; Wang, Q.; Liu, J.; Zhou, W.; Qi, L.; Wang, X.; Zhou, L.; Dai, L. Formation mechanisms of the alpine Erman’s birch (Betula ermanii) treeline on Changbai Mountain in Northeast China. Trees 2014, 28, 935–947. [Google Scholar] [CrossRef]
- WRB, I.W.G. World Reference Base for Soil Resource 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Mariotti, A. Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 1983, 303, 685–687. [Google Scholar] [CrossRef]
- Wang, R.; Dungait, J.A.J.; Creamer, C.A.; Cai, J.; Bo, L.; Xu, Z.; Zhang, Y.; Ma, Y.; Yong, J. Carbon and nitrogen dynamics in soil aggregates under long-term nitrogen and water addition in a temperate steppe. Soil Sci. Soc. Am. J. 2015, 79, 527–535. [Google Scholar] [CrossRef]
- Fang, Y.; Koba, K.; Yoh, M.; Makabe, A.; Liu, X. Patterns of foliar delta δ15N and their control in Eastern Asian forests. Ecol. Res. 2013, 28, 735–748. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Piccolo, M.C.; Townsend, A.R.; Vitousek, P.M.; Cuevas, E.; Mcdowell, W.; Robertson, G.P.; Santos, O.C.; Treseder, K. Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry 1999, 46, 45–65. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Wang, L.; Augusto, L.; Baisden, W.T.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Hobbie, E.A.; Kahmen, A.; et al. Convergence of soil nitrogen isotopes across global climate gradients. Sci. Rep. 2015, 5, 8280. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.H.; Templer, P.H.; Goodale, C.L.; Duke, S.; Groffman, P.M.; Adams, M.B.; Boeckx, P.; Boggs, J.; Campbell, J.; Colman, B. Regional Assessment of N Saturation using Foliar and Root δ15N. Biogeochemistry 2006, 80, 143–171. [Google Scholar] [CrossRef]
- Booth, M.; Stark, J.E. Controls on nitrogen cycling in terrestrial ecosystems: A synthetic analysis of literature data. Ecol. Monogr. 2005, 75, 139–157. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Garten, C.T.J. Nitrogen isotope composition of ammonium and nitrate in bulk precipitation and forest throughfall. Int. J. Environ. Anal. Chem. 1992, 47, 33–45. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Edith, B. Imprint of denitrifying bacteria on the global terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2009, 106, 21713–21716. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.; Brume, R. Altitudinal gradients of natural abundance of stable isotopes of nitrogen and carbon in the needles and soil of a pine forest in Nepal. J. For. Sci. 2003, 49, 19–26. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Wang, G. Measurements of nitrogen isotope composition of plants and surface soils along the altitudinal transect of the eastern slope of Mount Gongga in southwest China. Rapid Commun. Mass Spectrom. 2010, 24, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Wang, G.A. Nitrogen isotope composition characteristics of modern plants and their variations along an altitudinal gradient in Dongling Mountain in Beijing. Sci. China 2010, 53, 128–140. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Colpaert, J.V. Nitrogen Availability and Colonization by Mycorrhizal Fungi Correlate with Nitrogen Isotope Patterns in Plants. New Phytol. 2003, 157, 115–126. [Google Scholar] [CrossRef]
- He, X.H.; Critchley, C.; Bledsoe, C. Nitrogen Transfer Within and Between Plants Through Common Mycorrhizal Networks (CMNs). Crit. Rev. Plant Sci. 2003, 22, 531–567. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Jumpponen, A.J. Foliar and fungal 15N:14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: Testing analytical models. Oecologia 2005, 146, 258–268. [Google Scholar] [CrossRef]
- Schulze, E.D.; Chapin, F.S.; Gebauer, G. Nitrogen nutrition and isotope differences among life forms at the northern treeline of Alaska. Oecologia 1994, 100, 406–412. [Google Scholar] [CrossRef]
- Pardo, L.H.; McNulty, S.G.; Boggs, J.L.; Duke, S. Regional patterns in foliar 15N across a gradient of nitrogen deposition in the northeastern US. Environ. Pollut. 2007, 149, 293–302. [Google Scholar] [CrossRef]
- Kang, H.; Liu, C.; Yu, W.; Wu, L.; Chen, D.; Sun, X.; Ma, X.; Hu, H.; Zhu, X. Variation in foliar δ 15N among oriental oak (Quercus variabilis) stands over eastern China: Patterns and interactions. J. Geochem. Explor. 2011, 110, 8–14. [Google Scholar] [CrossRef]

| Mountain | Climate Zone | MAT (°C) | MAP (mm) | Longitude (E) | Latitude (N) | Species | Life Form | Elevation (m) a.s.l.) * | Height (m) |
|---|---|---|---|---|---|---|---|---|---|
| Balang | Sup-tropical | 12.8 | 995 | 102°58′ | 30°51′ | Abies faxoniana Rehder & E.H.Wilson | Tree | 2860 | 14 ± 1.7 |
| 3290 | 9.2 ± 0.9 | ||||||||
| 3670 | 11.5 ± 0.4 | ||||||||
| 102°45′ | 30°53′ | Quercus aquifolioides Rehd. et Wils. | Shrub | 2840 | 4.9 ± 0.1 | ||||
| 3160 | 2.8 ± 0.4 | ||||||||
| 3590 | 2.9 ± 0.2 | ||||||||
| Qilian | Dry-temperate | 0.6 | 435 | 100°17′ | 38°34′ | Picea crassifolia Kom. | Tree | 2540 | 13.7 ± 0.9 |
| 2870 | 15.5 ± 1.4 | ||||||||
| 3250 | 11.2 ± 0.5 | ||||||||
| 100°18′ | 38°31′ | Salix gilashanica C. Wang, P.Y. Fu | Shrub | 3000 | 1.4 ± 0.2 | ||||
| 3250 | 1.2 ± 0.0 | ||||||||
| 3550 | 1.5 ± 0.2 | ||||||||
| Changbai | Wet-temperate | −2.3–−3.8 | 1000 | 128°04′ | 42°03′ | Betula ermanii Cham. | Tree | 1700 | 18.9 ± 5.2 |
| 1860 | 13 ± 0.8 | ||||||||
| 2030 | 6.4 ± 4.5 | ||||||||
| 128°04′ | 42°02′ | Vaccinium uliginosum Linn. | Shrub | 1430 | 0.5 ± 0.0 | ||||
| 2000 | 0.2 ± 0.0 | ||||||||
| 2380 | 0.1 ± 0.0 |
| Source | Leaf δ15N | Soil δ15N | Leaf Δδ15N | Leaf N | Soil IN | Soil TN | Soil C:N | |
|---|---|---|---|---|---|---|---|---|
| df | F | F | F | F | F | F | F | |
| Site (S) | 2 | 83.4 *** | 44.2 *** | 26.0 *** | 790.6 *** | 27.7 *** | 4.5 * | 45.3 *** |
| Life form (L) | 1 | 17.8 *** | 7.7 ** | 0.2ns | 1776.8 *** | 19.8 *** | 0.0 ns | 2.2 ns |
| Altitude (A) | 2 | 26.7 *** | 1.4 ns | 8.2 *** | 7.7 *** | 0.8 ns | 1.1 ns | 4.1 * |
| S × L | 2 | 4.7 * | 2.5 ns | 1.5 ns | 1.0 ns | 4.1 * | 4.4 * | 30.5 *** |
| S × A | 4 | 4.6 ** | 5.7 *** | 12.8 *** | 7.3 *** | 0.6 ns | 5.8 *** | 1.1 ns |
| L × A | 2 | 32.3 *** | 6.5 ** | 15.3 *** | 450.9 *** | 0.5 ns | 0.8 ns | 8.6 *** |
| S × L × A | 4 | 6.7 *** | 10.0 *** | 11.0 *** | 6.6 *** | 3.8 ** | 3.6 ** | 12.7 *** |
| Leaf N | Soil IN | Soil TN | Soil pH | SOC | Soil C:N | |
|---|---|---|---|---|---|---|
| Subtropical | ||||||
| Leaf δ15N | 0.249 | –0.292 | 0.058 | –0.439 ** | 0.036 | –0.015 |
| Soil δ15N | 0.072 | –0.432 ** | –0.508 ** | –0.089 | –0.555 ** | –0.393 * |
| Dry-temperate | ||||||
| Leaf δ15N | 0.172 | 0.496 ** | –0.484 ** | 0.115 | –0.340 * | 0.006 |
| Soil δ15N | –0.05 | 0.476 ** | –0.464 ** | 0.404 * | –0.578 ** | –0.373 * |
| Wet-temperate | ||||||
| Leaf δ15N | 0.682 ** | –0.144 | –0.629 ** | –0.123 | –0.612 ** | 0.402 * |
| Soil δ15N | 0.459 ** | –0.031 | –0.433 ** | –0.087 | –0.388 * | 0.099 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Jiang, Y.; Ren, H.; Yu, F.-H.; Li, M.-H. Leaf and Soil δ15N Patterns Along Elevational Gradients at Both Treelines and Shrublines in Three Different Climate Zones. Forests 2019, 10, 557. https://doi.org/10.3390/f10070557
Wang X, Jiang Y, Ren H, Yu F-H, Li M-H. Leaf and Soil δ15N Patterns Along Elevational Gradients at Both Treelines and Shrublines in Three Different Climate Zones. Forests. 2019; 10(7):557. https://doi.org/10.3390/f10070557
Chicago/Turabian StyleWang, Xue, Yong Jiang, Haiyan Ren, Fei-Hai Yu, and Mai-He Li. 2019. "Leaf and Soil δ15N Patterns Along Elevational Gradients at Both Treelines and Shrublines in Three Different Climate Zones" Forests 10, no. 7: 557. https://doi.org/10.3390/f10070557
APA StyleWang, X., Jiang, Y., Ren, H., Yu, F.-H., & Li, M.-H. (2019). Leaf and Soil δ15N Patterns Along Elevational Gradients at Both Treelines and Shrublines in Three Different Climate Zones. Forests, 10(7), 557. https://doi.org/10.3390/f10070557






