Warming Alters Plant Chemical and Nutrient Compositions by Affecting Metabolites in Cunninghamia lanceolata (Lamb.) Hook
Abstract
1. Introduction
2. Methods
2.1. Study Site and Experimental Setup
2.2. Sampling and Processing of Leaves and Roots
2.3. Carbon, Nitrogen, and Phosphorus Analyses
2.4. Determination of the Concentrations of Reactive Oxygen Species and Osmotic Adjustment Substances
2.5. Determination of the Activities of Antioxidant Enzymes
2.6. Metabolite Extraction for LC-MS
2.7. Statistical Analyses
3. Results
3.1. Tree Height and Plant Nutrients
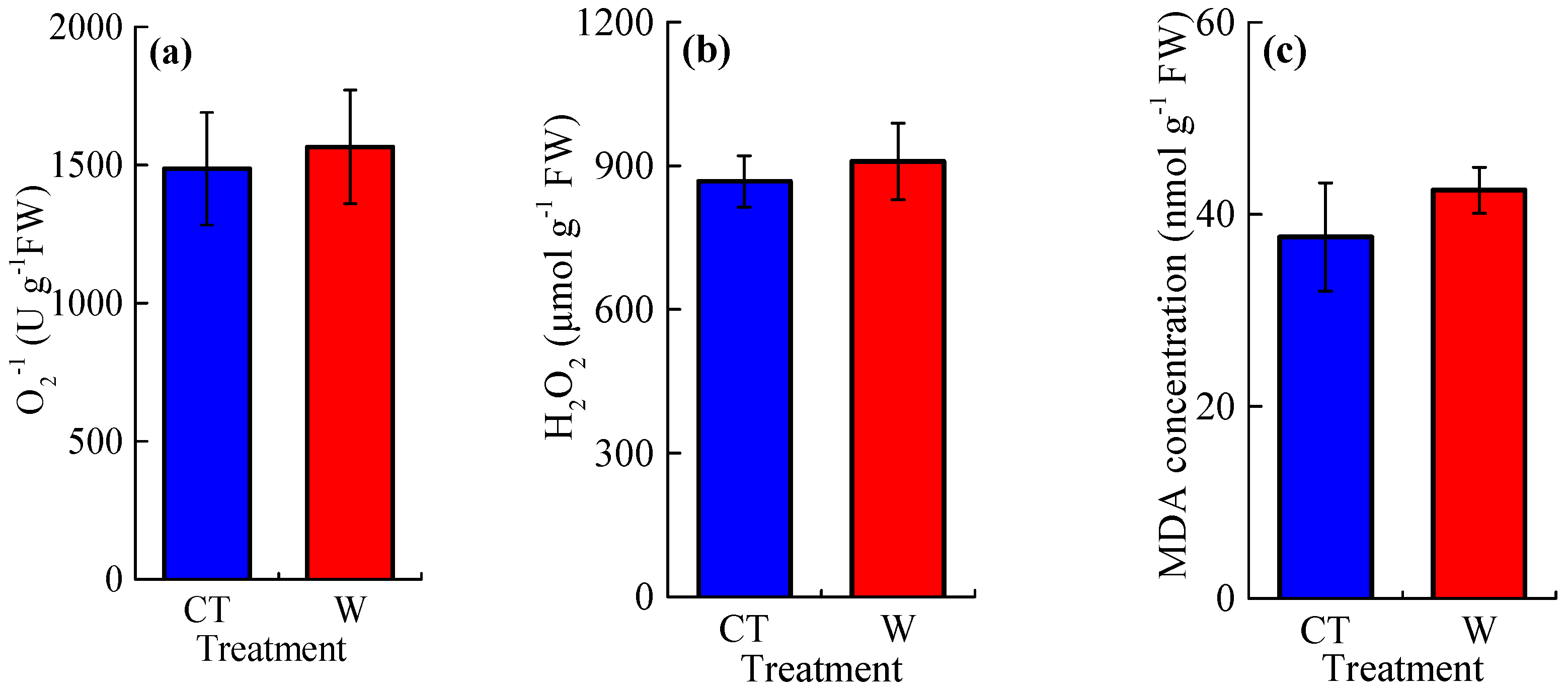
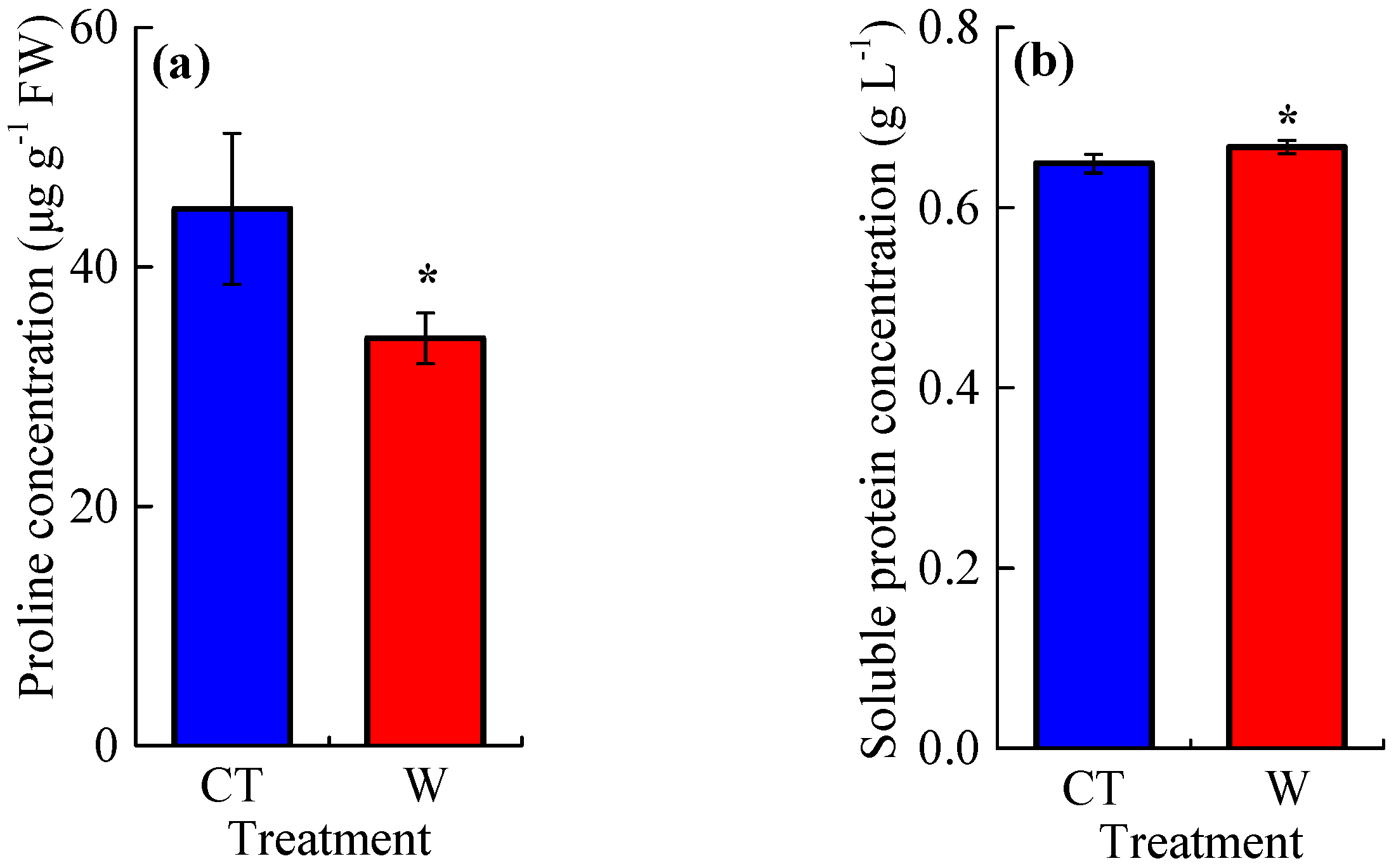
3.2. The Concentrations of Reactive Oxygen Species and Osmotic Adjustment Substances, and the Activities of Antioxidant Enzymes
3.3. Metabolite Profiling
4. Discussion
4.1. Differential Nutrient Status in Response to Warming
4.2. Warming Effects on Reactive Oxygen Species and Antioxidant Enzymes
4.3. Variations of Warming-Induced Metabolite Response
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavaleri, M.A.; Reed, S.C.; Smith, W.K.; Wood, T.E. Urgent need for warming experiments in tropical forests. Glob. Change Biol. 2015, 21, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the world’s forests. Ann. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Anderson, B.T. Intensification of seasonal extremes given a 2 degrees C global warming target. Clim. Change 2012, 112, 325–337. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Working Group I: Contribution to the Intergovernmental Panel on Climate Change Fifth Assessment Report; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The projected timing of climate departure from recent variability. Nature 2013, 502, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Piper, S.C.; Keeling, C.D.; Clark, D.B. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. PNAS 2003, 100, 5852–5857. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.X.; Tian, Z.W.; Gu, S.L.; Guo, H.; Fan, Y.H.; Abid, M.; Chen, K.; Jiang, D.; Cao, W.X.; Dai, T.B. Winter and spring night-warming improve root extension and soil nitrogen supply to increase nitrogen uptake and utilization of winter wheat (Triticum aestivum, L.). Eur. J. Agron. 2018, 96, 96–107. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Ge, L.T.; Yang, H.S.; Ren, W.Z. A meta-analysis of experimental warming effects on woody plant growth and photosynthesis in forests. J. For. Res. 2018, 29, 1–7. [Google Scholar] [CrossRef]
- Bruhn, D.; Egerton, J.J.E.; Loveys, B.R.; Ball, M.C. Evergreen leaf respiration acclimates to long-term nocturnal warming under field conditions. Glob. Change Biol. 2007, 13, 1216–1223. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.X.; Yang, L.D.; Guo, J.Y. Effects of drought and warming on biomass, nutrient allocation, and oxidative stress in Abies fabri in eastern Tibetan Plateau. J. Plant. Growth Regul. 2013, 32, 298–306. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E. Elevated carbon dioxide and warming impact silicon and phenolic-based defences differently in native and exotic grasses. Glob. Change Biol. 2018, 24, 3886–3896. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Dörken, V.M.; Arnold, A.L.M.; Adamczyk, B. Environmental conditions and species identity drive metabolite levels in green leaves and leaf litter of 14 temperate woody species. Forests 2018, 9, 775. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Ayala-Roque, M.; Sardans, J.; Bartrons, M.; Granda, V.; Sigurdsson, B.D.; Leblans, N.I.W.; Oravec, M.; Urban, O.; Janssens, I.A.; et al. Impact of soil warming on the plant metabolome of icelandic grasslands. Metabolites 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A.; Alonso-Rodríguez, A.M.; Cavaleri, M.A.; Reed, S.C.; González, G.; Wood, T.E. Infrared heater system for warming tropical forest understory plants and soils. Ecol. Evol. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Sperry, J.; West, A.; Williams, D.; Yepez, E.A. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? Tansley review. New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Zhang, Y.; Kreidler, N.; Sun, S.; Ardy, R.; Cao, K.; Sack, L. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol. Lett. 2014, 17, 1580–1590. [Google Scholar] [CrossRef]
- Tharayil, N.; Suseela, V.; Triebwasser, D.J.; Preston, C.M.; Gerard, P.D.; Dukes, J.S. Changes in the structural composition and reactivity of Acer rubrum leaf litter tannins exposed to warming and altered precipitation: Climatic stress-induced tannins are more reactive. New Phytol. 2011, 191, 132–145. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk, S.; Smolander, A.; Kitunnen, V.; Simon, J. Plant secondary metabolites—Missing pieces in the soil organic matter puzzle of boreal forests. Soils 2018, 2, 2. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Estiarte, M.; Ogaya, R.; Carnicer, J.; Coll, M.; Barbeta, A.; Rivas-Ubach, A.; Llusià, J.; Garbulsky, M.; et al. Evidence of current impact of climate change on life: A walk from genes to the biosphere. Glob. Change Biol. 2013, 19, 2303–2338. [Google Scholar]
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J.S. Warming and drought differentially influence the production and resorption of elemental and metabolic nitrogen pools in Quercus rubra. Glob. Change Biol. 2015, 21, 4177–4195. [Google Scholar] [CrossRef] [PubMed]
- Suseela, V.; Tharayil, N. Decoupling the direct and indirect effects of climate on plant litter decomposition: Accounting for stress-induced modifications in plant chemistry. Glob. Change Biol. 2018, 24, 1428–1451. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J.; Rivas-Ubach, A. Ecological metabolomics: Overview of current developments and future challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Oravec, M.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.; Parella, T.; Peñuelas, J. Warming differently influences the effects of drought on stoichiometry and metabolomics in shoots and roots. New Phytol. 2015, 207, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Guidi, L.; Lorenzini, G.; Massai, R.; Nali, C.; Landi, M. Cross-talk between physiological and metabolic adjustments adopted by Quercus cerris to mitigate the effects of severe drought and realistic future ozone concentrations. Forests 2017, 8, 148. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Barbeta, A.; Sardans, J.; Guenther, A.; Ogaya, R.; Oravec, M.; Urban, O.; Peñuelas, J. Topsoil depth substantially influences the responses to drought of the foliar metabolomes of Mediterranean forests. Perspect. Plant. Ecol. 2016, 21, 41–54. [Google Scholar] [CrossRef]
- Li, R.R.; Lu, Y.; Wan, F.X.; Wang, Y.M.; Pan, X.C. Impacts of a high nitrogen load on foliar nutrient status, N metabolism, and photosynthetic capacity in a Cupressus lusitanica Mill. Plantation. Forests 2018, 9, 483. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Liu, Q. Effects of soil warming and nitrogen fertilization on leaf physiology of Pinus tabulaeformis seedlings. Acta Physiol. Plant. 2012, 34, 1837–1846. [Google Scholar] [CrossRef]
- Long, J.R.D.; Sundqvist, M.K.; Gundale, M.J.; Giesler, R.; Wardle, D.A. Effects of elevation and nitrogen and phosphorus fertilization on plant defence compounds in subarctic tundra heath vegetation. Funct. Ecol. 2016, 30, 314–325. [Google Scholar] [CrossRef]
- Yao, L.H.; Kang, W.X.; Zhao, Z.H.; He, J.N. Carbon fixed characteristics of plant of Chinese fir (Cunninghamia lanceolata) plantation at different growth stages in Huitong. Acta Ecol. Sinica 2015, 35, 1187–1197. [Google Scholar]
- Liao, Y.C.; Fan, H.B.; Wei, X.H.; Wu, J.P.; Duan, H.L.; Fu, X.L.; Liu, W.F.; Wang, H.M.; Zhan, X.W.; Tang, P.; et al. Competition increased fine root biomass in Chinese fir (Cunninghamia lanceolata) plantations in Subtropical China. For. Ecol. Manag. 2019, 435, 151–157. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, S.D.; Yang, Z.J.; Lin, C.F.; Xiong, D.C.; Lin, W.S.; Xu, C.; Chen, G.S.; Xie, J.S.; Li, Y.Q.; et al. Will heterotrophic soil respiration be more sensitive to warming than autotrophic respiration in subtropical forests? Eur. J. Soil Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Kong, L.G.; Xie, Y.; Hu, L.; Si, J.S.; Wang, Z.S. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed. Sci. Rep.-UK. 2017, 7, 43363. [Google Scholar] [CrossRef] [PubMed]
- Brawn, K.; Fridovich, I. Superoxide radical and superoxide dismutases: Threat and defense. In Oxygen and Living Processes; Springer: New York, NY, USA, 1981; pp. 97–112. [Google Scholar]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant. Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Walter, T.; John, L. A photometric methods for the determination of proline. J. Biol. Chem. 1955, 215, 655–660. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. I: Occurrence in higher plants. Plant. Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Kochhar, S.; Kochhar, V.K.; Khanduja, S.D. Changes in the pattern of isoperoxidases during maturation of grape berries cv gulabi as affected by ethephon (2-chloroethy1) phosphoric acid. Am. J. Enol. Viticult. 1979, 30, 275–277. [Google Scholar]
- Trevor, E.; Kraus, R.; Austin, F. Paclobutrazol protects wheat seedlings from heat and paraquat injury is detoxification of active oxygen involved. Plant. Cell Physiol. 1994, 35, 45–52. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Dinis, T.C. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Cheminform 2010, 28, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Lin, W.S.; Yang, Z.R.; Lin, T.W.; Liu, X.F.; Chen, Y.M.; Yang, Y.S. Effects of soil warming and nitrogen deposition on available nitrogen in a young Cunninghamia lanceolata stand in mid-subtropical China. Acta Ecol. Sinica 2017, 37, 44–53. [Google Scholar]
- Bei, Z.X.; Zhang, Q.F.; Zheng, W.; Yang, L.M.; Chen, Y.M.; Yang, Y.S. Effects of simulated warming on soil phosphorus availability in subtropical Chinese fir plantation. Acta Ecol. Sinica 2018, 38, 1106–1113. [Google Scholar]
- Bai, K.D.; Lv, S.L.; Ning, S.J.; Zeng, D.J.; Guo, Y.L.; Wang, B. Leaf nutrient concentrations associated with phylogeny, leaf habit and soil chemistry in tropical karst seasonal rainforest tree species. Plant. Soil 2019, 434, 305–326. [Google Scholar] [CrossRef]
- Costa, M.G.; Gama-Rodrigues, A.C.; de Moraes Gonçalves, J.L.; Gama-Rodrigues, E.F.; da Silva Sales, M.V.; Aleixo, S. Labile and non-labile fractions of phosphorus and its transformations in soil under Eucalyptus plantations, Brazil. Forests 2016, 7, 15. [Google Scholar] [CrossRef]
- Shen, H.; Du, H.; Wang, Z.; Huang, B. Differential responses of nutrients to heat stress in warm-season and cool-season turfgrasses. Hortic. Sci. 2009, 44, 2009–2014. [Google Scholar] [CrossRef]
- Gong, X.Y.; Chen, Q.; Lin, S.; Brueck, H.; Dittert, K.; Taube, F.; Schnyder, H. Tradeoffs between nitrogen- and water-use efficiency in dominant species of the semiarid steppe of Inner Mongolia. Plant. Soil 2011, 340, 227–238. [Google Scholar] [CrossRef]
- Xiong, D.C.; Yang, Z.J.; Chen, G.S.; Liu, X.F.; Lin, W.S.; Huang, J.X.; Bowles, F.P.; Lin, C.F.; Xie, J.S.; Li, Y.Q.; et al. Interactive effects of warming and nitrogen addition on fine root dynamics of a young subtropical plantation. Soil Biol. Biochem. 2018, 122, 180–189. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Xiong, D.C.; Xie, J.S.; Li, X.J.; You, Z.T.; Lyu, M.K.; Chen, Y.M.; Yang, Y.S. Ecophysiological process regulates the growth of Cunninghamia lanceolata to suit short-term warming and nitrogen addition in the sub-tropical regions. Trees 2018, 32, 631–643. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Qiao, M.F.; Li, D.D.; Yin, H.J.; Liu, Q. Do warming-induced changes in quantity and stoichiometry of root exudation promote soil N transformations via stimulation of soil nitrifiers, denitrifiers and ammonifiers? Eur. J. Soil. Biol. 2016, 74, 60–68. [Google Scholar] [CrossRef]
- Mei, L.; Yang, X.; Cao, H.; Zhang, T.; Guo, J. Arbuscular mycorrhizal fungi alter plant and soil C:N:P stoichiometries under warming and nitrogen input in a semiarid meadow of China. Int. J. Environ. Res. Public Health 2019, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Vanacker, H.; Gornez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: Review. Plant. Physiol. Biochem. 2002, 40, 659–668. [Google Scholar] [CrossRef]
- Kang, W.J.; Lee, H.; Lim, H.; Lee, W.Y. Identification of potential metabolic markers for the selection of a high-yield clone of Quercus acutissima in clonal seed orchard. Forests 2018, 9, 116. [Google Scholar] [CrossRef]
- Zhou, Q.; Mu, K.M.; Xu, M.; Ma, X.Y.; Ni, Z.X.; Wang, J.W.; Xu, L.A. Variation in the concentrations of major secondary metabolites in ginkgo leaves from different geographical populations. Forests 2017, 8, 266. [Google Scholar] [CrossRef]
- Winkler, D.E.; Chapin, K.J.; Kueppers, L.M. Soil moisture mediates alpine life form and community productivity responses to warming. Ecology 2016, 97, 1553–1563. [Google Scholar] [CrossRef]
- Öncel, I.; Keles, Y.; Üstün, A.S. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 2000, 107, 315–320. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larcheveque, M.; Fernandez, C.; Gallet, C.; Desrochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Balmer, D.; Flors, V.; Glauser, G.; Mauch-Mani, B. Metabolomics of cereals under biotic stress: Current knowledge and techniques. Front. Plant. Sci. 2013, 4, 82–94. [Google Scholar] [CrossRef]
- Dong, C.X.; Shen, Q.R.; Wang, G. Tomato growth and organic acid changes in response to partial replacement of NO3--N by NH4+-N. Pedosphere 2004, 14, 159–164. [Google Scholar]
- Zhang, L.H.; Shao, H.B.; Ye, G.F.; Lin, Y.M. Effects of fertilization and drought stress on tannin biosynthesis of Casuarina equisetifolia seedlings branchlets. Acta Physiol. Plant. 2012, 34, 1639–1649. [Google Scholar] [CrossRef][Green Version]
- Rivas-Ubach, A.; Sardans, J.; Pérez-Trujillo, M.; Estiarte, M.; Peñuelas, J. Strong relationship between elemental stoichiometry and metabolome in plants. PNAS 2012, 109, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BCM Plant. Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gòmes, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Gypsy moth caterpillar feeding has only a marginal impact on phenolic compounds in old-growth black poplar. J. Chem. Ecol. 2013, 39, 1301–1312. [Google Scholar] [CrossRef]



| Time (min) | Flow Rate (mL min−1) | Pressure Limit (bar) | Solv Ratio B (%) |
|---|---|---|---|
| 0 | 0.35 | 800 | 5 |
| 1 | 0.35 | 800 | 5 |
| 6 | 0.35 | 800 | 20 |
| 9 | 0.35 | 800 | 50 |
| 13 | 0.35 | 800 | 95 |
| 15 | 0.35 | 800 | 95 |
| Index | Control | Warming |
|---|---|---|
| Tree height (m) | 4.28 ± 0.15 a | 4.47 ± 0.10 a |
| Leaf C concentration (mg g−1) | 457.49 ± 4.46 a | 449.86 ± 3.23 b |
| Leaf N concentration (mg g−1) | 6.21 ± 0.99 a | 5.47 ± 0.47 a |
| Leaf P concentration (mg g−1) | 0.95 ± 0.17 a | 0.70 ± 0.12 b |
| Leaf C:N ratio | 74.97 ± 10.35 a | 82.78 ± 7.01 a |
| Root C concentration (mg g−1) | 462.30 ± 7.20 a | 432.76 ± 10.45 b |
| Root N concentration (mg g−1) | 8.22 ± 1.26 a | 5.90 ± 0.66 b |
| Root P concentration (mg g−1) | 0.35 ± 0.03 a | 0.26 ± 0.04 b |
| Root C:N ratio | 57.28 ± 8.38 b | 74.00 ± 6.87 a |
| No | Compound | Abbreviation | RT (min) | Molecular Weight | VIP | t test | Fold Change (CT/W) | Mode |
|---|---|---|---|---|---|---|---|---|
| Phospholipid | ||||||||
| 1 | DG(14:0/15:0/0:0) | DG1 | 16.65 | 526.4597 | 1.89 | 0.031 | −0.97 | ESI+ |
| 2 | DG(14:1(9Z)/15:0/0:0) | DG2 | 17.60 | 524.4441 | 1.99 | 0.020 | −0.43 | ESI+ |
| 3 | DG(14:1(9Z)/22:2(13Z,16Z)/0:0) | DG3 | 16.64 | 618.5223 | 1.87 | 0.034 | −0.52 | ESI+ |
| 4 | DG(18:3(6Z,9Z,12Z)/22:5(4Z,7Z,10Z,13Z,16Z)/0:0) | DG4 | 16.10 | 664.5067 | 2.06 | 0.014 | −0.53 | ESI+ |
| 5 | DG(18:3(6Z,9Z,12Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | DG5 | 16.09 | 662.4910 | 2.29 | 0.004 | −0.87 | ESI+ |
| 6 | PC(18:2(9Z,12Z)/P-18:1(11Z)) | PC1 | 13.59 | 767.5829 | 2.20 | 0.006 | 1.22 | ESI+ |
| 7 | PC(18:3(9Z,12Z,15Z)/22:5(4Z,7Z,10Z,13Z,16Z)) | PC2 | 15.67 | 829.5622 | 2.27 | 0.004 | −0.75 | ESI+ |
| 8 | PC(22:4(7Z,10Z,13Z,16Z)/24:1(15Z)) | PC3 | 15.84 | 919.7030 | 1.96 | 0.023 | −0.99 | ESI+ |
| 9 | lysoPC(20:2(11Z,14Z)) | PC4 | 15.13 | 547.3638 | 2.14 | 0.009 | −0.24 | ESI+ |
| 10 | PC(15:0/18:1(11Z)) | PC5 | 10.83 | 745.5622 | 3.44 | 0.000 | 2.07 | ESI− |
| 11 | PE(20:3(5Z,8Z,11Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | PE1 | 15.69 | 813.5309 | 2.50 | 0.000 | −0.71 | ESI+ |
| 12 | PE(22:2(13Z,16Z)/P-18:1(11Z)) | PE2 | 13.59 | 781.5985 | 2.54 | 0.000 | −1.11 | ESI+ |
| 13 | PE(22:5(4Z,7Z,10Z,13Z,16Z)/P-18:1(11Z)) | PE3 | 15.64 | 775.5516 | 2.51 | 0.000 | −0.65 | ESI+ |
| 14 | PG(16:0/22:5(4Z,7Z,10Z,13Z,16Z)) | PG1 | 15.64 | 796.5254 | 2.51 | 0.000 | −0.64 | ESI+ |
| 15 | PG(16:0/18:3(6Z,9Z,12Z)) | PG2 | 10.83 | 744.4941 | 3.31 | 0.001 | 1.80 | ESI− |
| Carbohydrate | ||||||||
| 16 | melibiitol | Mel | 4.10 | 344.1319 | 1.89 | 0.030 | 0.29 | ESI+ |
| 17 | physcion 8-gentiobioside | Phy | 16.35 | 608.1741 | 2.05 | 0.015 | 1.76 | ESI+ |
| Amino acids | ||||||||
| 18 | L-tryptophan | Try | 12.69 | 204.0899 | 1.93 | 0.026 | 0.20 | ESI+ |
| 19 | hydroxylysine | Hyd | 6.03 | 162.1004 | 1.83 | 0.039 | 0.77 | ESI+ |
| Organic acids | ||||||||
| 20 | tetradecanedioic acid | Tet | 12.66 | 258.1831 | 1.83 | 0.038 | 0.09 | ESI+ |
| 21 | gibberellin A3 | Gib | 1.20 | 346.1416 | 1.77 | 0.048 | −0.80 | ESI+ |
| 22 | pisumic acid | Pis | 3.75 | 282.1467 | 2.45 | 0.001 | 1.02 | ESI+ |
| Flavonoid | ||||||||
| 23 | flavone | Fla | 12.66 | 222.0681 | 2.03 | 0.016 | 0.25 | ESI+ |
| Phenolic compound | ||||||||
| 24 | homovanillic acid | Hom | 6.54 | 182.0579 | 2.36 | 0.002 | 0.35 | ESI+ |
| 25 | peonidin 3-ferulyldiglucoside-5-glucoside | Peo | 13.50 | 962.2692 | 2.98 | 0.006 | −0.27 | ESI− |
| Terpene compound | ||||||||
| 26 | isogermafurene | Iso | 3.32 | 216.1514 | 2.46 | 0.001 | 1.21 | ESI+ |
| 27 | barringtogenol C | Bar | 17.32 | 490.3658 | 1.99 | 0.020 | −0.60 | ESI+ |
| N compound | ||||||||
| 28 | indicine | Ind | 3.75 | 299.1733 | 2.53 | 0.000 | 1.21 | ESI+ |
| 29 | anabasine | Ana | 6.06 | 162.1157 | 2.36 | 0.002 | 0.67 | ESI+ |
| 30 | 3-acetyl-2, 7-naphthyridine | 3-Ace | 1.49 | 172.0637 | 2.34 | 0.002 | 1.52 | ESI+ |
| 31 | acetylintermedine | Ace | 1.12 | 341.1838 | 1.93 | 0.026 | −0.86 | ESI+ |
| Acylamide compound | ||||||||
| 32 | adrenoyl ethanolamide | Adr | 15.78 | 375.3137 | 2.64 | 0.000 | −0.85 | ESI+ |
| 33 | palmitic amide | Pal | 15.22 | 255.2562 | 2.08 | 0.013 | −0.35 | ESI+ |
| Pigment | ||||||||
| 34 | xanthophyll | Xan | 17.63 | 568.4280 | 2.03 | 0.016 | −0.47 | ESI+ |
| Vitamin | ||||||||
| 35 | vitamin D3 | Vit | 16.49 | 384.3392 | 2.45 | 0.001 | −0.73 | ESI+ |
| S compound | ||||||||
| 36 | allicin | All | 8.15 | 162.0173 | 2.34 | 0.002 | 1.01 | ESI+ |
| Other | ||||||||
| 37 | phaseolotoxin | Pha | 15.21 | 531.1989 | 1.96 | 0.023 | 1.62 | ESI+ |
| 38 | fraxin | Fra | 15.23 | 370.0900 | 2.01 | 0.018 | 1.77 | ESI+ |
| 39 | dibutyl phthalate | Dib | 12.68 | 278.1518 | 1.91 | 0.028 | 0.17 | ESI+ |
| 40 | absinthin | Abs | 7.40 | 496.2825 | 1.89 | 0.030 | 0.11 | ESI+ |
| 41 | cis, cis-3, 6-dodecadienoyl-CoA | Cis | 13.58 | 945.2510 | 2.44 | 0.041 | −0.27 | ESI− |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Yang, Z.; Chen, T.; Gong, X.; Xiong, D.; Ye, W.; Chen, Y.; Yang, Y. Warming Alters Plant Chemical and Nutrient Compositions by Affecting Metabolites in Cunninghamia lanceolata (Lamb.) Hook. Forests 2019, 10, 553. https://doi.org/10.3390/f10070553
Zhang Q, Yang Z, Chen T, Gong X, Xiong D, Ye W, Chen Y, Yang Y. Warming Alters Plant Chemical and Nutrient Compositions by Affecting Metabolites in Cunninghamia lanceolata (Lamb.) Hook. Forests. 2019; 10(7):553. https://doi.org/10.3390/f10070553
Chicago/Turabian StyleZhang, Qiufang, Zhijie Yang, Tingting Chen, Xiaoying Gong, Decheng Xiong, Wangmin Ye, Yuehmin Chen, and Yusheng Yang. 2019. "Warming Alters Plant Chemical and Nutrient Compositions by Affecting Metabolites in Cunninghamia lanceolata (Lamb.) Hook" Forests 10, no. 7: 553. https://doi.org/10.3390/f10070553
APA StyleZhang, Q., Yang, Z., Chen, T., Gong, X., Xiong, D., Ye, W., Chen, Y., & Yang, Y. (2019). Warming Alters Plant Chemical and Nutrient Compositions by Affecting Metabolites in Cunninghamia lanceolata (Lamb.) Hook. Forests, 10(7), 553. https://doi.org/10.3390/f10070553





