The Northern White-Cedar Recruitment Bottleneck: Understanding the Effects of Substrate, Competition, and Deer Browsing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field Methods
2.3. Model Selection
3. Results
3.1. White-Cedar Regeneration
3.2. Model Selection
3.3. Measures of Browsing by Deer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Potential Predictor Variable Symbol | Iron | Florence | Langlade | Marinette | Marathon |
|---|---|---|---|---|---|
| OTHERSMSEED | 19,405 | 38,452 | 25,119 | 68,810 | 19,226 |
| OTHERLGSEED | 56,310 | 31,071 | 31,607 | 34,464 | 23,095 |
| OTHERSAP | 5833 | 3217 | 2350 | 2950 | 3450 |
| CANOPY_STHA | 1154 | 979 | 925 | 2296 | 1063 |
| BSLA | 33.6 | 42.9 | 43.6 | 44.3 | 44.0 |
| DENSIOMETER | 76 | 83 | 87 | 89 | 87 |
| GRAM | 1 | 1 | 1 | 1 | 1 |
| FERN | 1 | 1 | 1 | 0 | 1 |
| FORB | 1 | 1 | 2 | 1 | 1 |
| MOSS | 3 | 3 | 2 | 3 | 2 |
| VINE | 1 | 1 | 1 | 1 | 1 |
| CEDAR_BA | 32.0 | 31.8 | 30.6 | 37.6 | 29.4 |
| CEDAR_STHA | 1021 | 421 | 671 | 2004 | 746 |
| VOL_CWD | 8.1 | 81.9 | 47.3 | 7.7 | 30.8 |
| VOL_CWD_CEDAR | 4.4 | 66.6 | 6.1 | 4.0 | 13.2 |
| VOL_CWD_3PLUS | 3.3 | 50.8 | 24.5 | 3.4 | 14.8 |
| WATER | 1 | 1 | 0 | 1 | 1 |
| L_LITTER | 2 | 2 | 3 | 2 | 3 |
| LITTER_DEPTH | 2.5 | 2.4 | 2.8 | 2.5 | 3.0 |
| CEC | 54 | 98 | 103 | 115 | 102 |
| CA | 1897 | 3213 | 4350 | 3477 | 4338 |
| pH | 5.4 | 6.2 | 6.4 | 6.4 | 6.5 |
| OM | 61 | 71 | 64 | 71 | 58 |
| P | 5 | 6 | 5 | 6 | 10 |
| K | 46 | 92 | 43 | 40 | 52 |
| MG | 371 | 773 | 1114 | 798 | 873 |
| CTY_DEER | 2 | 7 | 7 | 9 | 12 |
| BUFF_AG.GRASS | 0.5 | 0 | 203.2 | 25.8 | 91.6 |
| DEER_BROWSE | 4 | 5 | 3 | 4 | 4 |
References
- Epstein, E.; Smith, W.; Dobberpuhl, J.; Galvin, A. Biotic Inventory and Analysis of the Northern Highland- American Legion State Forest; Wisconsin Natural Heritage Inventory Program, Bureau of Endangered Resources, Department of Natural Resources: Madison, WI, USA, 1999; p. 83.
- Johnston, W.F. Northern White-Cedar. In Silvics of North America: 1; Burns, R.M., Honkala, B.H., Eds.; Agriculture Handbook 654; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Pregitzer, K.S. The ecology of northern white-cedar. In Proceedings of the Northern White Cedar in Michigan, Sault Ste. Marie, MI, USA, 21–22 February 1990; Lantagne, D.O., Ed.; Michigan State University Agricultural Experiment Station: East Lansing, MI, USA, 1991; pp. 7–12. [Google Scholar]
- Cornett, M.W.; Frelich, L.E.; Puettmann, K.J.; Reich, P.B. Conservation implications of browsing by Odocoileus virginianus in remnant upland Thuja occidentalis forests. Biol. Conserv. 2000, 93, 359–369. [Google Scholar] [CrossRef]
- Larouche, C.; Kenefic, L.S.; Ruel, J.C. Northern White-Cedar Regeneration Dynamics on the Penobscot Experimental Forest in Maine: 40-Year Results. North. J. Appl. For. 2010, 27, 5–12. [Google Scholar]
- Dupuis, S.; Arseneault, D.; Sirois, L. Change from pre-settlement to present-day forest composition reconstructed from early land survey records in eastern Québec, Canada. J. Veg. Sci. 2011, 22, 564–575. [Google Scholar] [CrossRef]
- Larouche, C.; Ruel, J.C. Development of northern white-cedar regeneration following partial cutting, with and without deer browsing. Forests 2015, 6, 344–359. [Google Scholar] [CrossRef]
- Chimner, R.A.; Hart, J.B. Hydrology and microtopography effects on northern white-cedar regeneration in Michigan’s Upper Peninsula. Can. J. For. Res. 1996, 26, 389–393. [Google Scholar] [CrossRef]
- Davis, A.; Puettmann, K.; Perala, D. Site preparation Treatments and Browse Protection Affect Establishment and Growth of Northern White-Cedar; Paper NC-330; USDA Forest Service Research: St. Paul, MN, USA, 1998; p. 9.
- Cornett, M.W.; Reich, P.B.; Puettmann, K.J.; Frelich, L.E. Seedbed and moisture availability determine safe sites for early Thuja occidentalis (Cupressaceae) regeneration. Am. J. Bot. 2000, 87, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Substrate and litterfall effects on conifer seedling survivorship in southern boreal stands of Canada. Can. J. For. Res. 2003, 681, 672–681. [Google Scholar] [CrossRef]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Conifer seedling recruitment in a southeastern Canadian boreal forest: The importance of substrate. J. Veg. Sci. 1998, 9, 575–582. [Google Scholar] [CrossRef]
- Larouche, C.; Ruel, J.-C.; Lussier, J.-M. Factors affecting northern white-cedar (Thuja occidentalis) seedling establishment and early growth in mixedwood stands. Can. J. For. Res. 2011, 41, 568–582. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Ecological factors affecting the abundance of advance regeneration in Quebec’s southwestern boreal forest. Can. J. For. Res. 1996, 26, 888–898. [Google Scholar] [CrossRef]
- Rolley, R. Wisconsin deer status report, 2015. In Proceedings of the Midwest Deer & Wild Turkey Study Group Meeting, Carrollton, KY, USA, 22–25 August 2016; Kentucky Department of Fish and Wildlife Resources: Frankfort, KY, USA, 2016; pp. 123–133. [Google Scholar]
- Rooney, T.P. Deer impacts on forest ecosystems: A North American perspective. Forestry 2001, 74, 201–208. [Google Scholar] [CrossRef]
- Bradshaw, L.; Waller, D.M. Impacts of white-tailed deer on regional patterns of forest tree recruitment. For. Ecol. Manag. 2016, 375, 1–11. [Google Scholar] [CrossRef]
- Rooney, T.P.; Solheim, S.L.; Waller, D.M. Factors affecting the regeneration of northern white cedar in lowland forests of the Upper Great Lakes region, USA. For. Ecol. Manag. 2002, 163, 119–130. [Google Scholar] [CrossRef]
- Frelich, L.E.; Lorimer, C.G. Current and predicted long-term effects of deer browsing in hemlock forests in Michigan, USA. Biol. Conserv. 1985, 34, 99–120. [Google Scholar] [CrossRef]
- Frerker, K.; Sabo, A.; Waller, D. Long-term regional shifts in plant community composition are largely explained by local deer impact experiments. PLoS ONE 2014, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C. Height of White-Flowered Trillium (Trillium grandiflorum) as an Index of Deer Browsing Intensity. Ecol. Appl. 1994, 4, 104–109. [Google Scholar] [CrossRef]
- Balgooyen, C.P.; Waller, D.M. The Use of Clintonia borealis and Other Indicators to Gauge Impacts of White-Tailed Deer on Plant Communities in Northern Wisconsin, USA. Nat. Areas J. 1995, 15, 308–318. [Google Scholar]
- Kirschbaum, C.D.; Anacker, B.L. The Utility of Trillium and Maianthemum as Phyto-Indicators of Deer Impact in Northwestern Pennsylvania, USA. For. Ecol. Manag. 2005, 217, 54–66. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resource Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: https://websoilsurvey.sc.egov.udsa.gov/ (accessed on 12 September 2016).
- Wilson, B.T.; Lister, A.J.; Riemann, R.I.; Griffith, D.M. Live Tree Species Basal Area of the Contiguous United States (2000–2009); USDA Forest Service, Northern Research Station: Newtown Square, PA, USA, 2013.
- Midwestern Regional Climate Center Historical Climate Summaries 1981–2010. Available online: https://mrcc.illinois.edu/mw_climate/climateSummaries/climSumm.jsp (accessed on 6 August 2018).
- Wisconsin Department of Natural Resources Fall Deer Population Density 2015. Available online: https://dnr.wi.gov/topic/wildlifeHabitat/reports.html (accessed on 4 April 2016).
- National Operational Hydrologic Remote Sensing Center Northern Great Lakes Snow Depth Observations for Florence and Antigo, Wisconsin. Available online: https://www.nohrsc.noaa.gov/nsa/ (accessed on 6 August 2018).
- Dahlberg, B.L.; Guettinger, R.C. The White-Tailed Deer in Wisconsin; Technical Wildlife Bulletin 14; Wisconsin Conservation Department: Madison, WI, USA, 1956; p. 282.
- Anderson, R.C.; Corbett, E.A.; Anderson, M.R.; Corbett, G.A.; Kelley, T.M. High White-Tailed Deer Density Has Negative Impact on Tallgrass Prairie Forbs. J. Torrey Bot. Soc. 2001, 128, 381–392. [Google Scholar] [CrossRef]
- McWilliams, W.H.; Westfall, J.A.; Brose, P.H.; Dey, D.C.; Hatfield, M.; Johnson, K.; Laustsen, K.M.; Lehman, S.L.; Morin, R.S.; Nelson, M.D.; et al. A Regeneration Indicator for Forest Inventory and Analysis: History, Sampling, Estimation, Analytics, and Potential Use in the Midwest and Northeast United States; Techinical Report NRS-148; USDA Forest Service General: Newtown Square, PA, USA, 2015; 74p.
- Wisconsin Department of Natural Resources Wiscland 2 Land Cover Data. Available online: ftp://dnrftp01.wi.gov/geodata/landcover/wiscland2/ (accessed on 10 April 2017).
- Larson, T.J.; Rongstad, O.J.; Terbilcox, F.W. Movement and Habitat Use of White-Tailed Deer in Southcentral Wisconsin. J. Wildl. Manag. 1978, 42, 113. [Google Scholar] [CrossRef]
- Strickler, G. Use of the Densiometer to Estimate Density of Forest Canopy on Permanent Sample Plots; USDA Forest Service Research Note 180: Portland, OR, USA, 1959; p. 5.
- USDA Forest Service. Forest Inventory and Analysis National Core Field Guide. Volume II: Field Data Collection Procedures for Phase 3 Plots. Version 5.1; U.S. Department of Agriculture Forest Service, Forest Inventory and Analysis Program: Washington, DC, USA, 2011; Section 25; p. 13.
- Marshall, P.L.; Davis, G.; LeMay, V.M. Using Line Intersect Sampling for Coarse Woody Debris; Techinical Report 003; British Columbia Forest Service: Nanaimo, BC, Canada, 2000; p. 37.
- Genuer, R.; Poggi, J.-M.; Tuleau-Malot, C. VSURF: An R Package for Variable Selection Using Random Forests. R J. 2015, 7, 19–33. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Conover, M.R.; Kania, G.S. Annual variation in white-tailed deer damage in commercial nurseries. Agric. Ecosyst. Environ. 1995, 55, 213–217. [Google Scholar] [CrossRef]
- Van Deelen, T.R.; Campa, H., II; Hamady, M.; Haufler, J.B. Migration and Seasonal Range Dynamics of Deer Using Adjacent Deeryards in Northern Michigan. J. Wildl. Manag. 1998, 62, 205–213. [Google Scholar] [CrossRef]
- Sabine, D.L.; Morrison, S.F.; Whitlaw, H.A.; Ballard, W.B.; Forbes, G.J.; Bowman, J. Migration Behavior of White-Tailed Deer under Varying Winter Climate Regimes in New Brunswick. J. Wildl. Manag. 2002, 66, 718–728. [Google Scholar] [CrossRef]
- Verme, L.J.; Johnston, W.F. Regeneration of Northern White Cedar Deeryards in Upper Michigan. J. Wildl. Manag. 1986, 50, 307–313. [Google Scholar] [CrossRef]
- Johnston, W.F. Managers Handbook for Northern White-Cedar in the North Central States; Techinical Report NC-35; USDA Forest Service General: St. Paul, MN, USA, 1977; p. 18.
- Forester, J.D.; Anderson, D.P.; Turner, M.G. Landscape and Local Factors Affecting Northern White Cedar (Thuja Occidentalis) Recruitment in The Chequamegon-Nicolet National Forest, Wisconsin (U.S.A.). Am. Midl. Nat. 2008, 160, 438–453. [Google Scholar] [CrossRef]
- Nelson, T.C. A Reproduction Study of Northern White Cedar Including Results of Investigations under Federal Aid in Wildlife Restoration Project Michigan 49-R; Department of Conservation Game Division: Lansing, MI, USA, 1951; 100p.
- Kell, J. Soil-Site Influences on Northern White-Cedar (Thuja Occidentalis, L.) Stem Quality and Growth. Masters’s Thesis, University of Maine, Orono, ME, USA, 2009. [Google Scholar]
- Curtis, J.D. Preliminary Observations on Northern White Cedar in Maine. Ecology 1946, 27, 23–36. [Google Scholar] [CrossRef]
- Cornett, M.W.; Reich, P.B.; Puettmann, K.J. Canopy feedbacks and microtopography regulate conifer seedling distribution in two Minnesota conifer-deciduous forests. Ecoscience 1997, 4, 353–364. [Google Scholar] [CrossRef]
- Hofmeyer, P.V.; Kenefic, L.S.; Seymour, R.S. Historical Stem Development of Northern White-Cedar (Thuja occidentalis L.) in Maine. North. J. Appl. For. 2010, 27, 92–96. [Google Scholar]
- Herfindal, I.; Tremblay, J.-P.; Hester, A.J.; Lande, U.S.; Wam, H.K. Associational relationships at multiple spatial scales affect forest damage by moose. For. Ecol. Manag. 2015, 348, 97–107. [Google Scholar] [CrossRef]
- Kangas, L.C.; Schwartz, R.; Pennington, M.R.; Webster, C.R.; Chimner, R.A. Artificial microtopography and herbivory protection facilitates wetland tree (Thuja occidentalis L.) survival and growth in created wetlands. New For. 2016, 47, 73–86. [Google Scholar] [CrossRef]
- Scott, M.L.; Murphy, P.G. Regeneration Patterns of Northern White Cedar, an Old-Growth Forest Dominant. Am. Midl. Nat. 1987, 117, 10–16. [Google Scholar] [CrossRef]
- Cornett, M.W.; Puettmann, K.J.; Frelich, L.E.; Reich, P.B. Comparing the importance of seedbed and canopy type in the restoration of upland Thuja occidentalis forests of Northeastern Minnesota. Restor. Ecol. 2001, 9, 386–396. [Google Scholar] [CrossRef]
- Johnston, W.M. Balsam fir Dominant Species under Rethinned Northern White-Cedar; Research Note NC-133; USDA Forest Service North Central Forest Experiment Station: St. Paul, Minnesota, USA, 1972; p. 4.
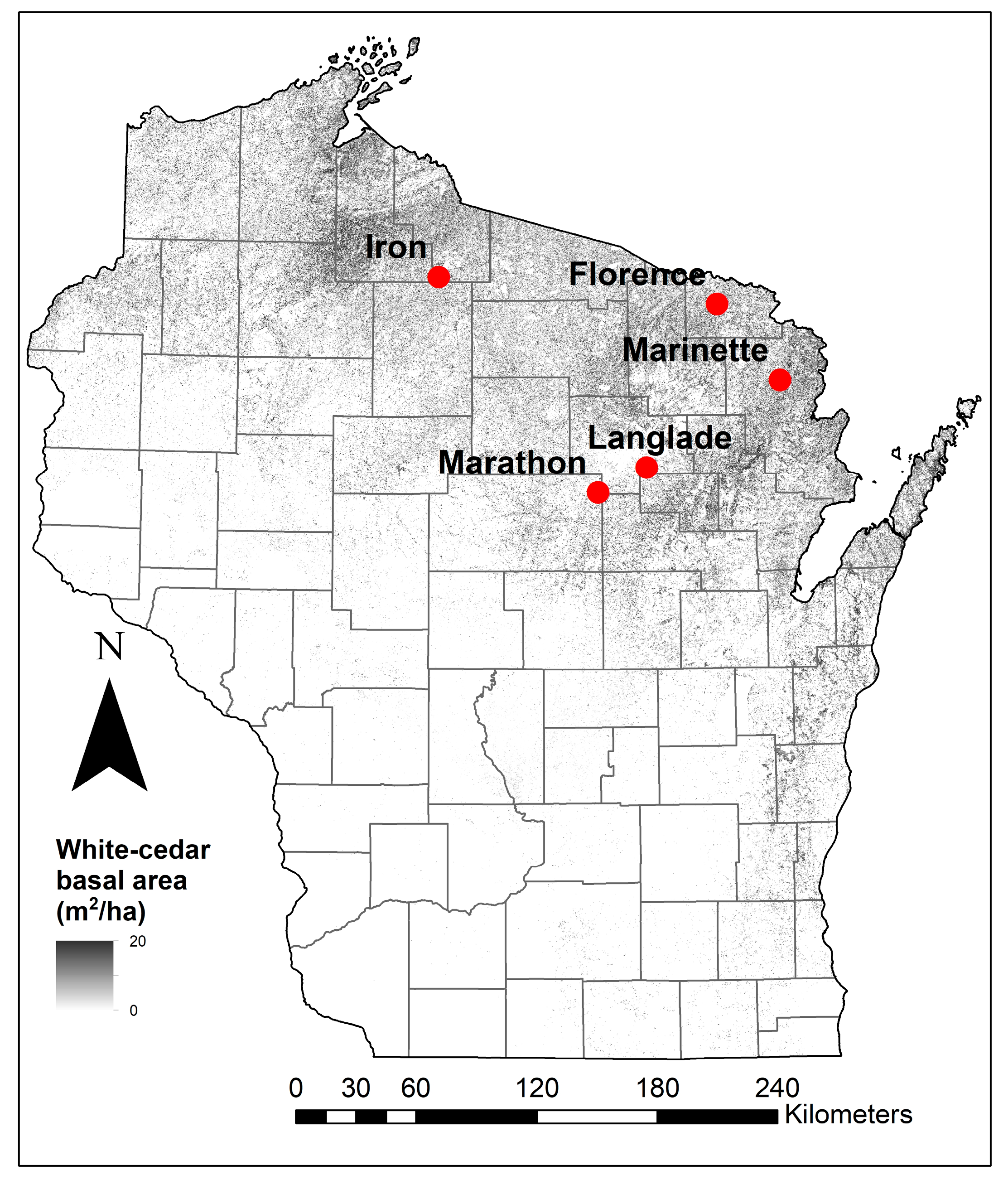
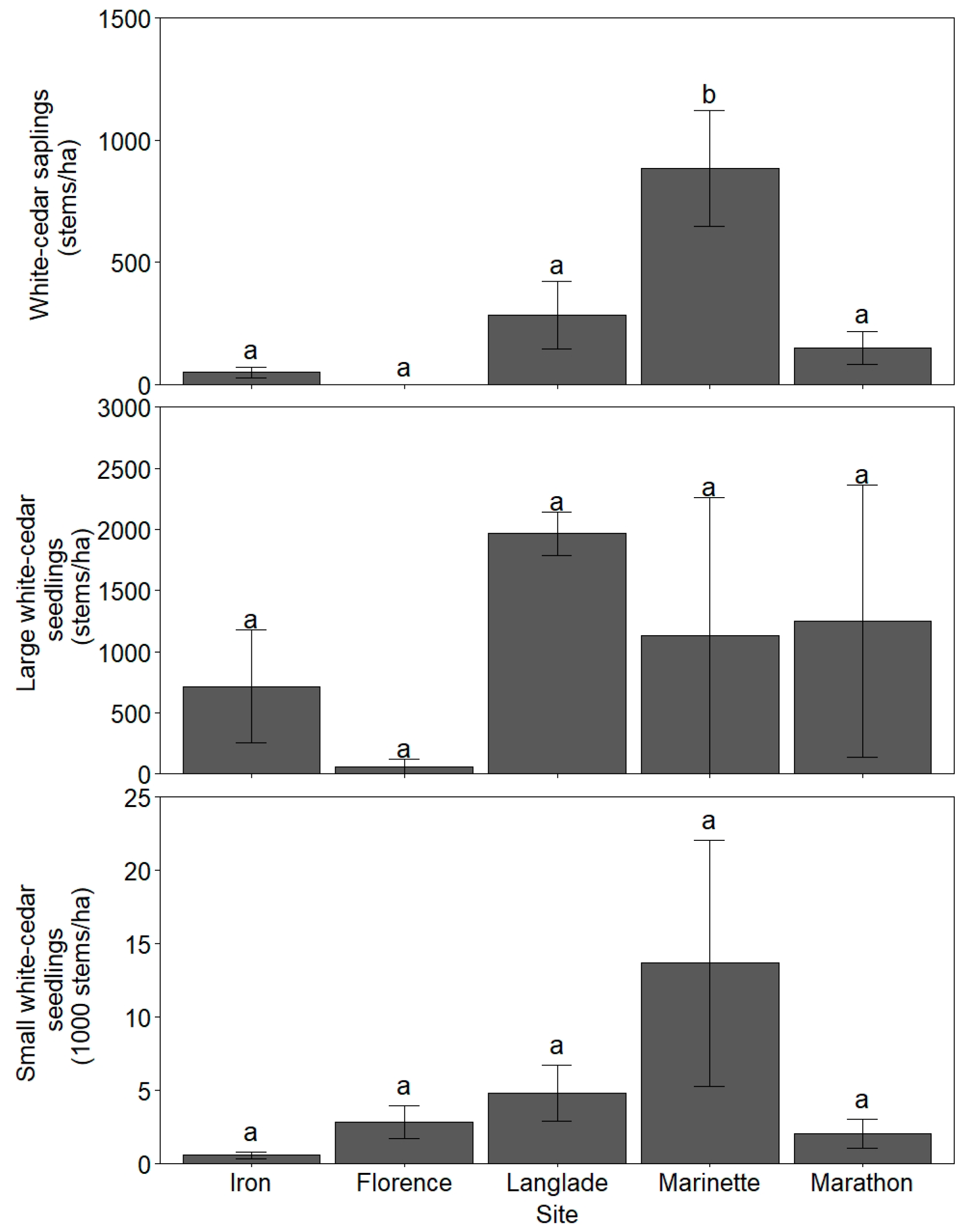
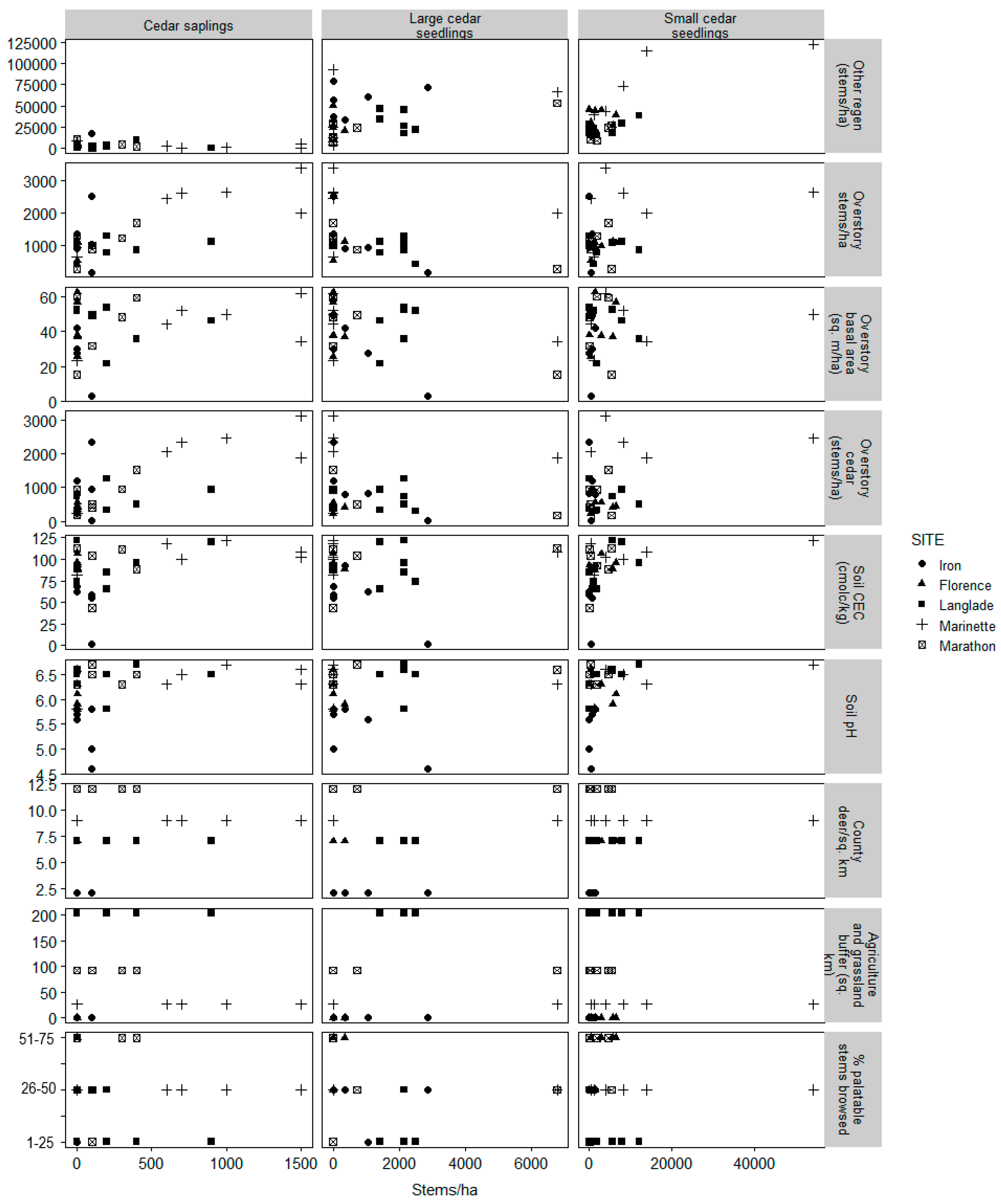
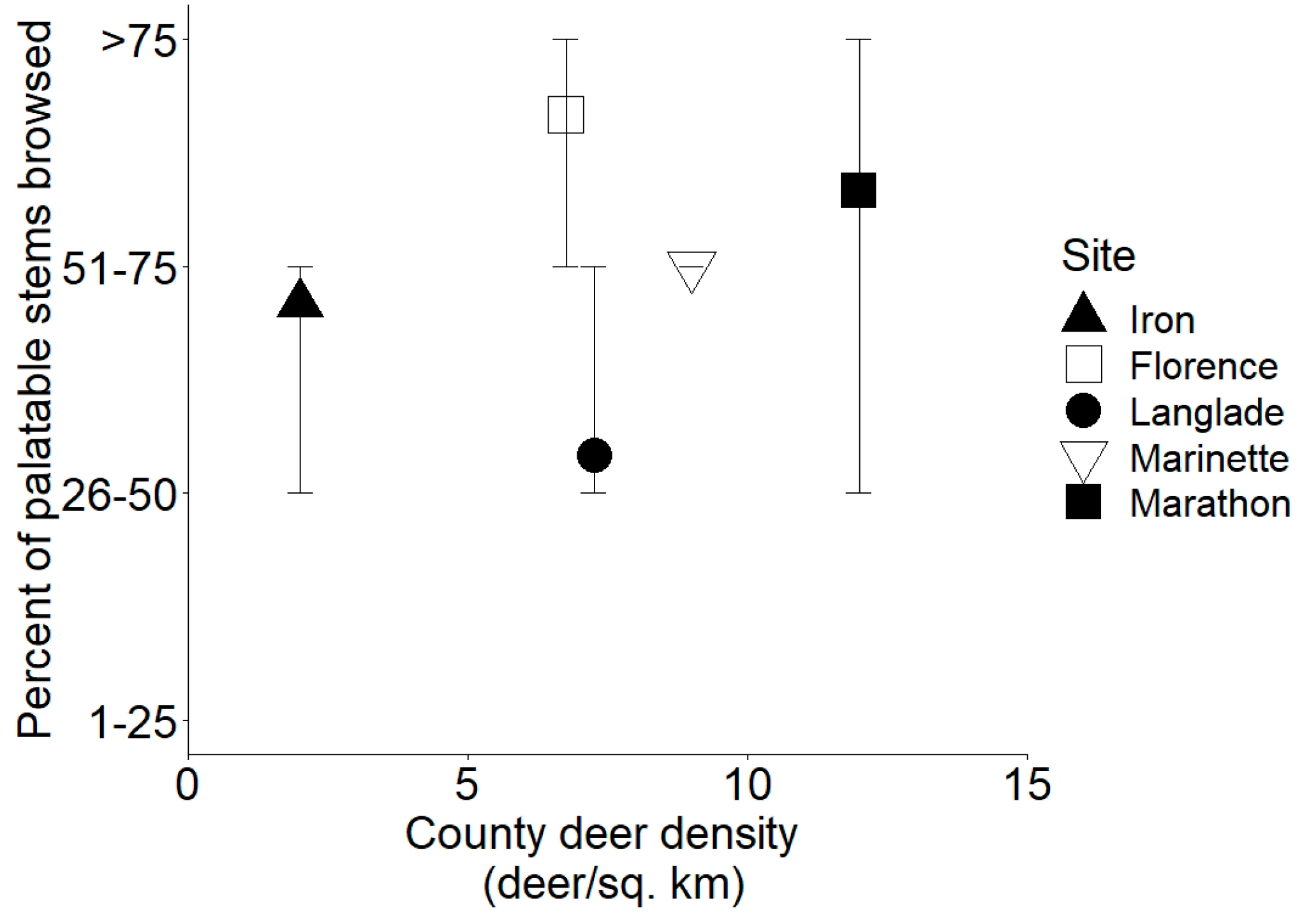
| Site | Annual Temperature (°C) | Annual Precipitation (mm) | Annual Snowfall (cm) | Stand Basal Area (m2 ha−1) | White-Cedar Basal Area (m2 ha−1) | County Deer Density (deer km−2) |
|---|---|---|---|---|---|---|
| Iron | 4.4 | 810 | 281 | 33.6 | 32.0 | 2 |
| Florence | 5.7 | 750 | 175 | 42.9 | 31.8 | 7 |
| Langlade | 5.5 | 820 | 151 | 43.6 | 30.6 | 7 |
| Marinette | 5.7 | 750 | 189 | 44.3 | 37.6 | 9 |
| Marathon | 6.4 | 820 | 151 | 44.0 | 29.4 | 12 |
| Factor | Symbol | Description |
|---|---|---|
| CE | OTHERSMSEED | Stem density of small seedlings of other species (stems ha−1, <20 cm tall) |
| CE | OTHERLGSEED | Stem density of large seedlings of other species (stems ha−1, 0 cm ≤ height < 1.83 m) |
| CE | OTHERSAP | Stem density of saplings of other species (stems ha−1, 1.83 m tall to <10 cm dbh) |
| CE | CANOPY_STHA | Stem density of all trees ≥ 10 cm dbh (stems ha−1) |
| CE | BSLA | Basal area (m2 ha−1) of all trees ≥ 10 cm dbh |
| CE | DENSIOMETER | Percent canopy closure as measured with densiometer |
| CE | GRAM | Percent cover of plot by graminoids |
| CE | FERN | Percent cover of plot by ferns and fern allies |
| CE | FORB | Percent cover of plot by other forbs (not including ferns or graminoids) |
| CE | MOSS | Percent cover of plot by moss |
| CE | VINE | Percent cover of plot by woody vines |
| SS | CEDAR_BA | Basal area (m2 ha−1) of all white-cedar ≥ 10 cm dbh |
| SS | CEDAR_STHA | Stem density of all white-cedar ≥ 10 cm dbh (stems ha−1) |
| Sbed | VOL_CWD | Volume of all coarse woody debris (m3 ha−1) |
| Sbed | VOL_CWD_CEDAR | Volume of white-cedar coarse woody debris (m3 ha−1) |
| Sbed | VOL_CWD_3PLUS | Volume of coarse woody debris in decay classes 3–5 (m3 ha−1) |
| Sbed | WATER | Percent of the subplot regularly covered by standing water |
| Sbed | L_LITTER | Percent cover of subplots by undecomposed leaf litter |
| Sbed | LITTER_TYPE | Main type of leaf litter on the plot: Deciduous, Conifer, Mixed |
| Sbed | LITTER_DEPTH | Mean depth of litter layer in centimeters |
| Sbed | CEC | Soil cation exchange capacity (cmol kg−1) |
| Sbed | CA | Soil calcium (ppm) |
| Sbed | pH | Soil pH |
| Sbed | OM | Percent organic matter in top 30 cm of soil |
| Sbed | P | Soil phosphorus (ppm) |
| Sbed | K | Soil potassium (ppm) |
| Sbed | MG | Soil magnesium (ppm) |
| Br | CTY_DEER | Estimated deer population density (deer km−2) in county where stand occurs |
| Br | BUFF_AG.GRASS | Total hectares of agricultural land and grassland in a 1.5 km buffer around the stand |
| Br | DEER_BROWSE | Categorical browse assessment, percent of stems browsed on palatable species |
| Regeneration Height Class | Model | Model Parameters (Fixed Effects) | k | AICc | ΔAICc |
|---|---|---|---|---|---|
| Saplings | Lowest AICc | CEDAR_STHA + CEC + CEDAR_STHA*CEC | 6 | 218.62 | |
| Null | Intercept only | 3 | 235.11 | 16.49 | |
| County deer | CTY_DEER | 4 | 236.91 | 18.30 | |
| Habitat buffer | BUFF_AG.GRASS | 4 | 237.56 | 18.95 | |
| Deer browse | DEER_BROWSE | 4 | 237.75 | 19.13 | |
| Selected model | CEDAR_STHA | 4 | 221.75 | 3.13 | |
| Large seedlings | Lowest AICc | BSLA + CEC + OTHERLGSEED + BSLA∙CEC + BSLA∙OTHERLGSEED + CEC∙OTHERLGSEED + BSLA∙CEC∙OTHERLGSEED | 11 | 1122.02 | |
| Null | Intercept only | 4 | 1152.91 | 30.89 | |
| County deer | CTY_DEER | 5 | 1155.08 | 33.06 | |
| Habitat buffer | BUFF_AG.GRASS | 5 | 1148.58 | 26.56 | |
| Deer browse | DEER_BROWSE | 5 | 1150.09 | 28.07 | |
| Selected model | OTHERLGSEED | 5 | 1128.31 | 6.29 | |
| Small seedlings | Lowest AICc | pH + OTHERSMSEED + CANOPY_STHA + OTHERSMSEED∙CANOPY_STHA | 8 | 1284.09 | |
| Null | Intercept only | 4 | 1303.08 | 18.99 | |
| County deer | CTY_DEER | 5 | 1303.93 | 19.84 | |
| Habitat buffer | BUFF_AG.GRASS | 5 | 1305.24 | 21.15 | |
| Deer browse | DEER_BROWSE | 5 | 1305.19 | 21.11 | |
| Selected model | pH + OTHERSMSEED | 6 | 1289.90 | 5.82 |
| Response Variable (White-Cedar Density) | Fixed Effects | β | SE | Lower CI | Upper CI | t Value |
|---|---|---|---|---|---|---|
| Saplings 1 | Intercept | 0.718 | 3.023 | −5.328 | 6.765 | 0.238 |
| CEDAR_STHA | 0.010 | 0.002 | 0.006 | 0.015 | 4.905 | |
| Large seedlings 1 | Intercept | −2.418 | 4.895 | −12.208 | 7.373 | −0.494 |
| OTHERLGSEED | 0.000412 | 0.0000747 | 0.000263 | 0.000561 | 5.515 | |
| Small seedlings 1 | Intercept | −151.15 | 64.82 | −280.80 | −21.50 | −2.332 |
| pH | 26.56 | 10.59 | 5.36 | 47.74 | 2.507 | |
| OTHERSMSEED | 0.000706 | 0.000158 | 0.000391 | 0.001022 | 4.480 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reuling, L.F.; Kern, C.C.; Kenefic, L.S.; Bronson, D.R. The Northern White-Cedar Recruitment Bottleneck: Understanding the Effects of Substrate, Competition, and Deer Browsing. Forests 2019, 10, 501. https://doi.org/10.3390/f10060501
Reuling LF, Kern CC, Kenefic LS, Bronson DR. The Northern White-Cedar Recruitment Bottleneck: Understanding the Effects of Substrate, Competition, and Deer Browsing. Forests. 2019; 10(6):501. https://doi.org/10.3390/f10060501
Chicago/Turabian StyleReuling, Laura F., Christel C. Kern, Laura S. Kenefic, and Dustin R. Bronson. 2019. "The Northern White-Cedar Recruitment Bottleneck: Understanding the Effects of Substrate, Competition, and Deer Browsing" Forests 10, no. 6: 501. https://doi.org/10.3390/f10060501
APA StyleReuling, L. F., Kern, C. C., Kenefic, L. S., & Bronson, D. R. (2019). The Northern White-Cedar Recruitment Bottleneck: Understanding the Effects of Substrate, Competition, and Deer Browsing. Forests, 10(6), 501. https://doi.org/10.3390/f10060501







