The Effects of Soils from Different Forest Types on the Growth of the Invasive Plant Phytolacca americana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Material Collection and Soil Property Analysis
2.3. Forest Soil Pot Experiments
2.4. Forest Litter Leachate Bioassay and Pot Experiments
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
3.2. Effects of Forest Soils on Plant Growth
3.3. Effects of Forest Litter Leachate on Plant Performance
4. Discussion
4.1. The Effects of Soil Nutrients on Plant Growth
4.2. The Effects of Allelopathy on Plant Growth
4.3. The Effects of Soil Microbes on Plant Growth
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | F(df) | p |
|---|---|---|
| Forests | 209.966(5,90) | <0.001 |
| Treatments | 1.136(2,90) | 0.326 |
| Forests × Treatments | 18.378(10,90) | <0.001 |
| Variables | RI of Germination Rate | RI of Total Biomass | Litter Leachate pH | |||
|---|---|---|---|---|---|---|
| F(df) | p | F(df) | p | F(df) | p | |
| Forest type (F) | 2.765(4,45) | 0.039 | 14.403(4,75) | <0.001 | 446.407(4,30) | <0.001 |
| Concentration (C) | 5.051(2,45) | 0.010 | 9.462(2,75) | <0.001 | 24.066(2,30) | <0.001 |
| F × C | 0.522(8,45) | 0.834 | 1.846(8,75) | 0.083 | 0.539(8,30) | 0.817 |
References
- Courchamp, F.; Fournier, A.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Jeschke, J.M.; Russell, J.C. Invasion biology: Specific problems and possible solutions. Trends Ecol. Evol. 2017, 32, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A. Are modern biological invasions an unprecedented form of global change? Conserv. Biol. 2007, 21, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Catford, J.A.; Vesk, P.A.; Richardson, D.M.; Pyšek, P. Quantifying levels of biological invasion: Towards the objective classification of invaded and invasible ecosystems. Glob. Chang. Biol. 2012, 18, 44–62. [Google Scholar] [CrossRef]
- Bufford, J.L.; Lurie, M.H.; Daehler, C.C. Biotic resistance to tropical ornamental invasion. J. Ecol. 2016, 104, 518–530. [Google Scholar] [CrossRef]
- Byun, C.; Lee, E.J. Ecological application of biotic resistance to control the invasion of an invasive plant, Ageratina altissima. Ecol. Evol. 2017, 7, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Jagodziński, A.M. Context-dependence of urban forest vegetation invasion level and alien species’ ecological success. Forests 2019, 10, 26. [Google Scholar] [CrossRef]
- Yannelli, F.A.; Koch, C.; Jeschke, J.M.; Kollmann, J. Limiting similarity and Darwin’s naturalization hypothesis: Understanding the drivers of biotic resistance against invasive plant species. Oecologia 2017, 183, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.L.; Burns, J.H.; Liao, Z.Y.; Li, Y.P.; Yang, J.; Chen, Y.J.; Zhang, J.L.; Zheng, Y.G. Species composition, functional and phylogenetic distances correlate with success of invasive Chromolaena odorata in an experimental test. Ecol. Lett. 2018, 21, 1211–1220. [Google Scholar] [CrossRef]
- Catford, J.A.; Smith, A.L.; Wragg, P.D.; Clark, A.T.; Kosmala, M.; Cavender-Bares, J.; Reich, P.B.; Tilman, D. Traits linked with species invasiveness and community invasibility vary with time, stage and indicator of invasion in a long-term grassland experiment. Ecol. Lett. 2019, 22, 593–604. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through reassembly: Plant traits and invasion resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.E.; Eissenstat, D.M.; Hobbie, S.E.; Oleksyn, J.; Jagodzinski, A.M.; Reich, P.B.; Chadwick, O.A.; Chorover, J. Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 2012, 111, 601–614. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Bajpai, D.; Inderjit. Impact of nitrogen availability and soil communities on biomass accumulation of an invasive species. Aob Plants 2013, 5. [Google Scholar] [CrossRef]
- Banerjee, S.; Baah-Acheamfour, M.; Carlyle, C.N.; Bissett, A.; Richardson, A.E.; Siddique, T.; Bork, E.W.; Chang, S.X. Determinants of bacterial communities in Canadian agroforestry systems. Environ. Microbiol. 2016, 18, 1805–1816. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Feng, Y.; Ma, K. Response of soil bacterial communities to secondary compounds released from Eupatorium adenophorum. Biol. Invasions 2017, 19, 1471–1481. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Env. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Macias, F.A.; Molinillo, J.M.; Varela, R.M.; Galindo, J.C. Allelopathy--a natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Del Fabbro, C.; Prati, D. The relative importance of immediate allelopathy and allelopathic legacy in invasive plant species. Basic Appl. Ecol. 2015, 16, 28–35. [Google Scholar] [CrossRef]
- Abgrall, C.; Forey, E.; Mignot, L.; Chauvat, M. Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol. Biochem. 2018, 127, 100–109. [Google Scholar] [CrossRef]
- Callaway, R.M.; Cipollini, D.; Barto, K.; Thelen, G.C.; Hallett, S.G.; Prati, D.; Stinson, K.; Klironomos, J. Novel weapons: Invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 2008, 89, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Inderjit; Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Inderjit. Soil: Environmental effects on allelochemical activity. Agron. J. 2001, 93, 79–84. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Li, Y.P.; Feng, Y.L.; Kang, Z.L.; Zheng, Y.L.; Zhang, J.L.; Chen, Y.J. Changes in soil microbial communities due to biological invasions can reduce allelopathic effects. J. Appl. Ecol. 2017, 54, 1281–1290. [Google Scholar] [CrossRef]
- Liu, S.; Qin, F.; Yu, S. Eucalyptus urophylla root-associated fungi can counteract the negative influence of phenolic acid allelochemicals. Appl. Soil Ecol. 2018, 127, 1–7. [Google Scholar] [CrossRef]
- Bennett, J.A.; Klironomos, J. Mechanisms of plant-soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2019, 222, 91–96. [Google Scholar] [CrossRef]
- Sun, B.; Wang, P.; Kong, C.-H. Plant-soil feedback in the interference of allelopathic rice with barnyardgrass. Plant Soil 2014, 377, 309–321. [Google Scholar] [CrossRef]
- Xia, Z.C.; Kong, C.H.; Chen, L.C.; Wang, S.L. Allelochemical-mediated soil microbial community in long-term monospecific Chinese fir forest plantations. Appl. Soil Ecol. 2015, 96, 52–59. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, D.; Ge, X.; Jin, X.; Chen, S.; Wu, F. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils 2018, 54, 363–372. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.A.; Parker, I.M.; Gilbert, G.S. Allelopathy: A tool for weed management in forest restoration. Plant Ecol. 2012, 213, 1975–1989. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Yu, F.H.; van Kleunen, M. Allelopathy of a native grassland community as a potential mechanism of resistance against invasion by introduced plants. Biol. Invasions 2016, 18, 3481–3493. [Google Scholar] [CrossRef]
- Niro, E.; Marzaioli, R.; De Crescenzo, S.; D’Abrosca, B.; Castaldi, S.; Esposito, A.; Fiorentino, A.; Rutigliano, F.A. Effects of the allelochemical coumarin on plants and soil microbial community. Soil Biol. Biochem. 2016, 95, 30–39. [Google Scholar] [CrossRef]
- Hou, Y.P.; Peng, S.L.; Chen, B.M.; Ni, G.Y. Inhibition of an invasive plant (Mikania micrantha H.B.K.) by soils of three different forests in lower subtropical China. Biol. Invasions 2011, 13, 381–391. [Google Scholar] [CrossRef]
- Peng, S.L.; Chen, Z.Q.; Wen, J.; Shao, H. Is allelopathy a driving force in forest succession? Allelopath. J. 2005, 14, 197–204. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, S.; Chen, Z.; Wu, Z.; Zhou, G.; Wang, X.; Qiu, Z. Abscisic acid in soil facilitates community succession in three forests in China. J. Chem. Ecol. 2011, 37, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Hervé, M.R.; Carrillo, J.; Ding, J.; Huang, W. Latitudinal trends in growth, reproduction and defense of an invasive plant. Biol. Invasions 2019, 21, 189–201. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, X.; Xiao, Y.; Zhang, Z.; Li, X. Traits of reproductive biology associated with invasiveness in alien invasive plant Phytolacca americana. Ecol. Environ. Sci. 2013, 22, 567–574. (In Chinese) [Google Scholar]
- Huang, W.; Ding, J. Effects of generalist herbivory on resistance and resource allocation by the invasive plant, Phytolacca americana. Insect Sci. 2016, 23, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, X.; Hou, Y.; Dong, Z.; Bu, Q. Comparison on the competitiveness of the invaded pokeweed with its accompanying species in the coastal protection forest of Shandong Peninsula. Sci. Silvae Sin. 2016, 52, 23–29. (In Chinese) [Google Scholar]
- Fu, J.P.; Li, C.R.; Xu, J.W.; Cheng, W.L.; Song, R.F.; Liu, Y. Prevention and control of invaded plant Phytolacca americana in sandy coastal shelter forests. Chin. J. Appl. Ecol. 2012, 23, 991–997. (In Chinese) [Google Scholar]
- Wang, R.; Zhou, G. The Vegetation of Shandong Province; Shandong Science Technology Publish: Jinan, China, 2000. (In Chinese) [Google Scholar]
- Vitkova, M.; Mullerova, J.; Sadlo, J.; Pergl, J.; Pysek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Lazzaro, L.; Mazza, G.; d’Errico, G.; Fabiani, A.; Giuliani, C.; Inghilesi, A.F.; Lagomarsino, A.; Landi, S.; Lastrucci, L.; Pastorelli, R.; et al. How ecosystems change following invasion by Robinia pseudoacacia: Insights from soil chemical properties and soil microbial, nematode, microarthropod and plant communities. Sci. Total Environ. 2018, 622–623, 1509–1518. [Google Scholar] [CrossRef]
- Rice, S.K.; Westerman, B.; Federici, R. Impacts of the exotic, nitrogen-fixing black locust (Robinia pseudoacacia) on nitrogen-cycling in a pine–oak ecosystem. Plant Ecol. 2004, 174, 97–107. [Google Scholar] [CrossRef]
- Baribault, T.W.; Kobe, R.K.; Finley, A.O. Tropical tree growth is correlated with soil phosphorus, potassium, and calcium, though not for legumes. Ecol. Monogr. 2012, 82, 189–203. [Google Scholar] [CrossRef]
- Qiu, S.; Xie, J.; Zhao, S.; Xu, X.; Hou, Y.; Wang, X.; Zhou, W.; He, P.; Johnston, A.M.; Christie, P.; et al. Long-term effects of potassium fertilization on yield, efficiency, and soil fertility status in a rain-fed maize system in northeast China. Field Crop. Res. 2014, 163, 1–9. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; de Almeida Lobo, F.; Biudes, M.S.; Rodríguez Ortíz, C.E.; de Souza Nogueira, J. Spatial variations in soil chemistry and organic matter content across a invasion front in the brazilian pantanal. Soil Sci. Soc. Am. J. 2011, 75, 1554. [Google Scholar] [CrossRef]
- Perkins, L.B.; Nowak, R.S. Native and non-native grasses generate common types of plant–soil feedbacks by altering soil nutrients and microbial communities. Oikos 2013, 122, 199–208. [Google Scholar] [CrossRef]
- Chen, B.M.; Peng, S.L.; Ni, G.Y. Effects of the invasive plant Mikania micrantha H.B.K. on soil nitrogen availability through allelopathy in South China. Biol. Invasions 2009, 11, 1291–1299. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, R.; Huang, Y.; Feng, S.; Ma, X.; Zhang, Y.; Sikdar, A.; Roy, R. Allelopathic effects of aqueous leaf extracts from four shrub species on seed germination and initial growth of amygdalus pedunculata pall. Forests 2018, 9, 711. [Google Scholar] [CrossRef]
- Li, Y.P.; Feng, Y.L.; Chen, Y.J.; Tian, Y.H. Soil microbes alleviate allelopathy of invasive plants. Sci. Bull. 2015, 60, 1083–1091. [Google Scholar] [CrossRef]
- Eskelinen, A.; Kaarlejärvi, E.; Olofsson, J. Herbivory and nutrient limitation protect warming tundra from lowland species’ invasion and diversity loss. Glob. Chang. Biol. 2017, 23, 245–255. [Google Scholar] [CrossRef]
- Suding, K.N.; LeJeune, K.D.; Seastedt, T.R. Competitive impacts and responses of an invasive weed: Dependencies on nitrogen and phosphorus availability. Oecologia 2004, 141, 526–535. [Google Scholar] [CrossRef]
- Devau, N.; Cadre, E.L.; Hinsinger, P.; Jaillard, B.; Gérard, F. Soil pH controls the environmental availability of phosphorus: Experimental and mechanistic modelling approaches. Appl. Geochem. 2009, 24, 2163–2174. [Google Scholar] [CrossRef]
- Yang, T.; Han, G.; Yang, Q.; Friman, V.P.; Gu, S.; Wei, Z.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Resource stoichiometry shapes community invasion resistance via productivity-mediated species identity effects. Proc. Soc. B 2018, 285, 20182035. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Chang. Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.S.; Cralle, S.P. Phosphorus addition reduces invasion of a longleaf pine savanna (Southeastern USA) by a non-indigenous grass (Imperata cylindrica). Plant Ecol. 2003, 167, 237–245. [Google Scholar] [CrossRef]
- Lee, Y.C.; Nam, J.M.; Kim, J.G. The influence of black locust (Robinia pseudoacacia) flower and leaf fall on soil phosphate. Plant Soil 2011, 341, 269–277. [Google Scholar] [CrossRef]
- Medina-Villar, S.; Alonso, Á.; Castro-Díez, P.; Pérez-Corona, M.E. Allelopathic potentials of exotic invasive and native trees over coexisting understory species: The soil as modulator. Plant Ecol. 2017, 218, 579–594. [Google Scholar] [CrossRef]
- Torres, A.; Alarcón, P.A.E.; Rodríguez-Cabal, M.A.; Nuñez, M.A. Secondary invasions hinder the recovery of native communities after the removal of nonnative pines along a precipitation gradient in patagonia. Forests 2018, 9, 394. [Google Scholar] [CrossRef]
- Green, P.T.; O’Dowd, D.J.; Abbott, K.L.; Jeffery, M.; Retallick, K.; Mac Nally, R. Invasional meltdown: Invader-invader mutualism facilitates a secondary invasion. Ecology 2011, 92, 1758–1768. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Kabouw, P.; Nab, M.; van Dam, N.M. Activated carbon addition affects substrate pH and germination of six plant species. Soil Biol. Biochem. 2010, 42, 1165–1167. [Google Scholar] [CrossRef]
- Wurst, S.; van Beersum, S. The impact of soil organism composition and activated carbon on grass-legume competition. Plant Soil 2009, 314, 1–9. [Google Scholar] [CrossRef]
- Dostál, P. Plant competitive interactions and invasiveness: Searching for the effects of phylogenetic relatedness and origin on competition intensity. Am. Nat. 2011, 177, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Nilsen, E.T. Bioassays and field studies for allelopathy in terrestrial plants: Progress and problems. Crit. Rev. Plant Sci. 2003, 22, 221–238. [Google Scholar] [CrossRef]
- Inderjit. Soil microorganisms: An important determinant of allelopathic activity. Plant Soil 2005, 274, 227–236. [Google Scholar] [CrossRef]
- Dickie, I.A.; Alexander, I.; Lennon, S.; Öpik, M.; Selosse, M.-A.; van der Heijden, M.G.A.; Martin, F.M. Evolving insights to understanding mycorrhizas. New Phytol. 2015, 205, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Dickie, I.A.; Davis, M.; Carswell, F.E. Quantification of mycorrhizal limitation in beech spread. N. Z. J. Ecol. 2012, 36, 210–215. Available online: https://newzealandecology.org/system/files/articles/NZJEcol36_2_210.pdf (accessed on 16 April 2019).
- Nuñez, M.A.; Horton, T.R.; Simberloff, D. Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 2009, 90, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
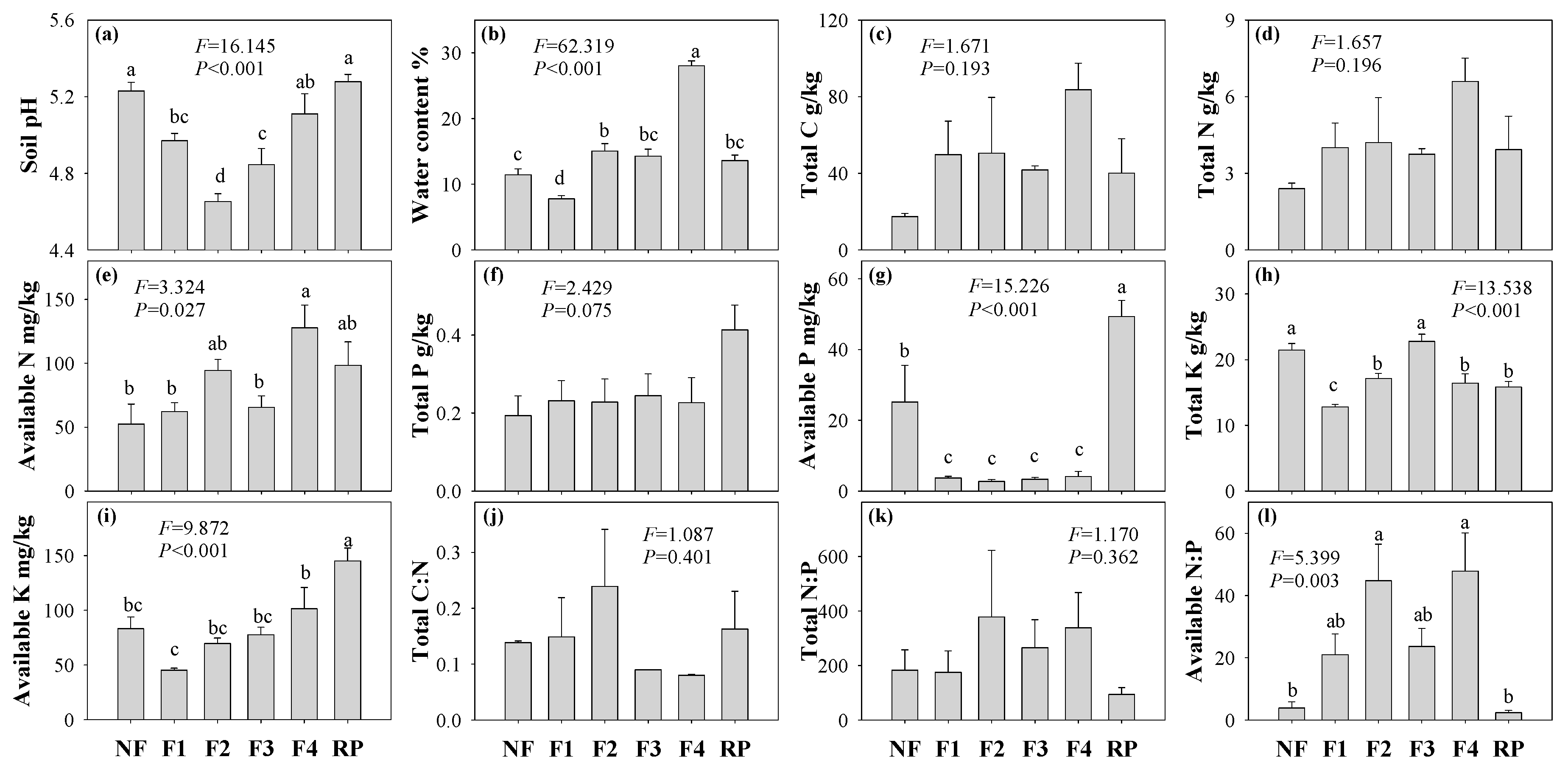
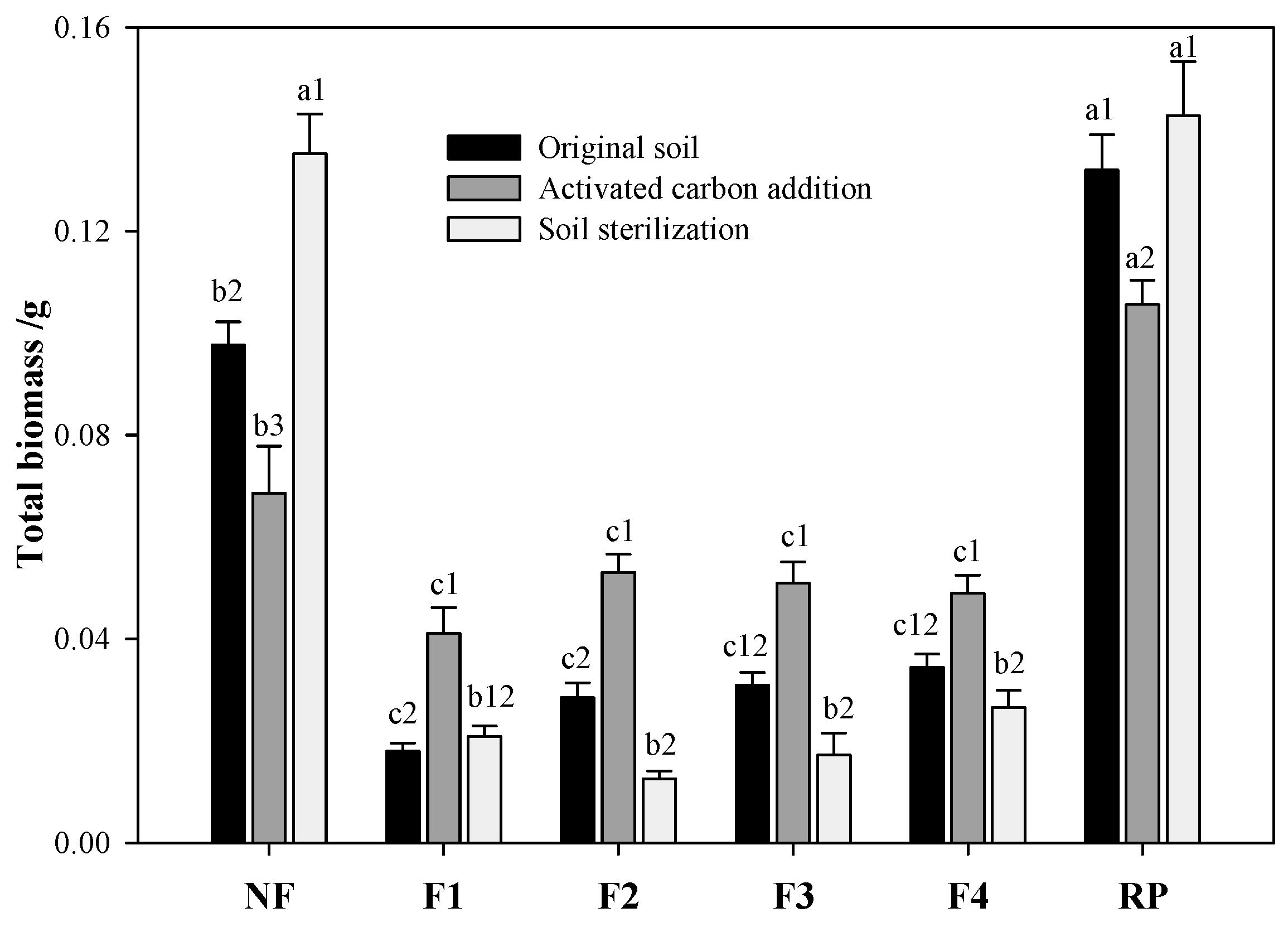
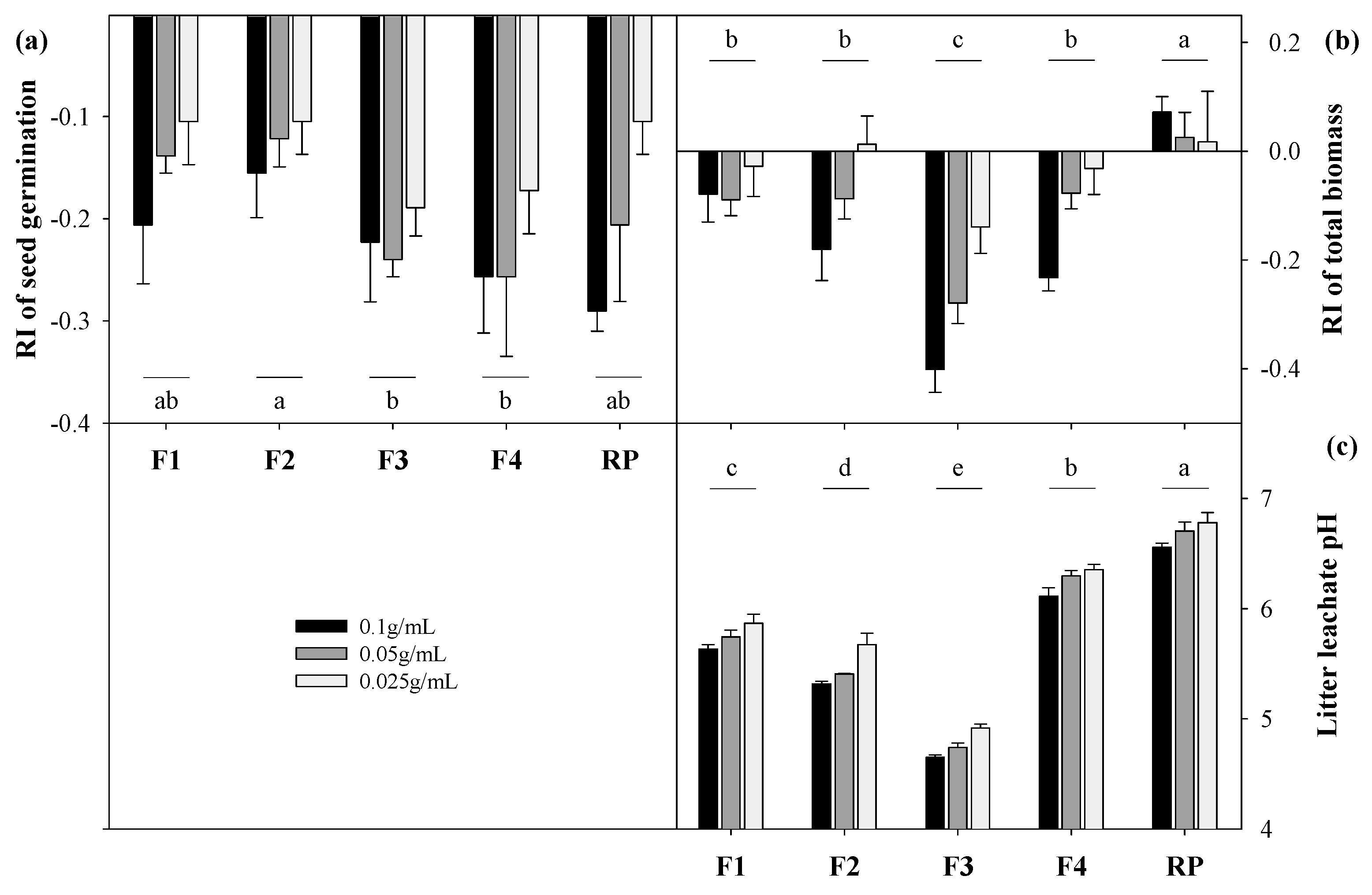
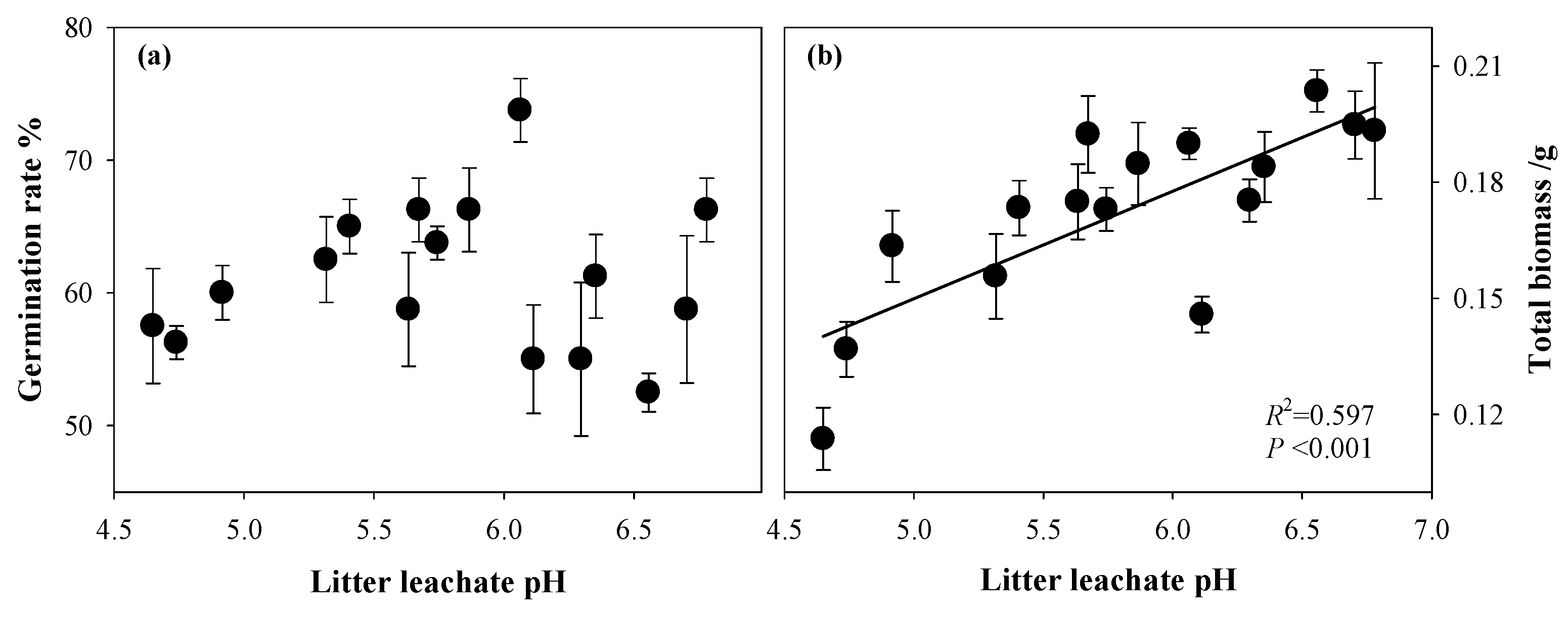
| Forest Name | Forest Type | Abbreviation | Dominant Trees | Distribution |
|---|---|---|---|---|
| Pinus densiflora forest | Natural pine forest | F1 | Pinus densiflora | Major distribution forest |
| Pinus densiflora+Quercus acutissima mixed forest | Natural pine-broadleaf mixed forest | F2 | Pinus densiflora and Quercus acutissima | Major distribution forest |
| Quercus acutissima forest | Natural broadleaved forest | F3 | Quercus acutissima | Major distribution forest |
| Deciduous broadleaved mixed forest | Natural secondary forest | F4 | No obvious dominant species; common tree species: Sorbus alnifolia (Sieb. et Zucc.) K. Koch, Ailanthus altissima (Mill.) Swingle, Kalopanax septemlobus (Thunb.) Koidz., etc. | Distribution is relatively small |
| Robinia pseudoacacia plantation | Plantation | RP | Robinia pseudoacacia | Fragmented distribution on both sides of the roads |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-D.; Hou, Y.-P.; Zhuge, Y.-H.; Wei, W.; Huang, Q.-Q. The Effects of Soils from Different Forest Types on the Growth of the Invasive Plant Phytolacca americana. Forests 2019, 10, 492. https://doi.org/10.3390/f10060492
Chen P-D, Hou Y-P, Zhuge Y-H, Wei W, Huang Q-Q. The Effects of Soils from Different Forest Types on the Growth of the Invasive Plant Phytolacca americana. Forests. 2019; 10(6):492. https://doi.org/10.3390/f10060492
Chicago/Turabian StyleChen, Peng-Dong, Yu-Ping Hou, Yan-Hui Zhuge, Wei Wei, and Qiao-Qiao Huang. 2019. "The Effects of Soils from Different Forest Types on the Growth of the Invasive Plant Phytolacca americana" Forests 10, no. 6: 492. https://doi.org/10.3390/f10060492
APA StyleChen, P.-D., Hou, Y.-P., Zhuge, Y.-H., Wei, W., & Huang, Q.-Q. (2019). The Effects of Soils from Different Forest Types on the Growth of the Invasive Plant Phytolacca americana. Forests, 10(6), 492. https://doi.org/10.3390/f10060492





