Polish Pony Changes Lower Layer Biodiversity in Old Growth Scots Pine Stands
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Areas
2.2. Methods
3. Results
3.1. The Herb Layer and Undergrowth (Lower 0.5 m)
3.1.1. Deciduous Forests (DF)
3.1.2. Coniferous Forests (CF)
3.2. Understory and Undergrowth Layer Higher Than 0.5 m
3.2.1. Deciduous Forests (DF)
3.2.2. Coniferous Forests (CF)
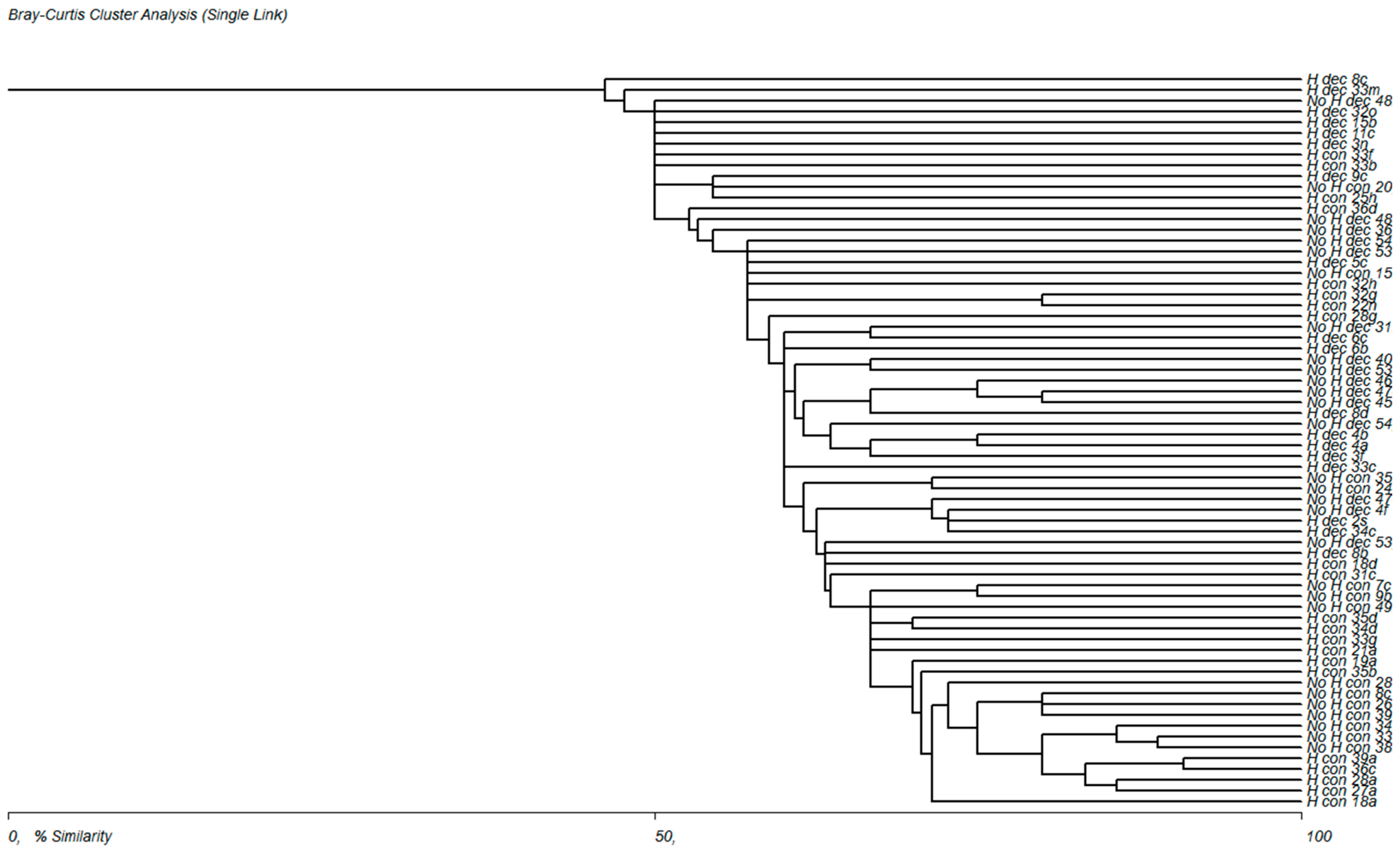
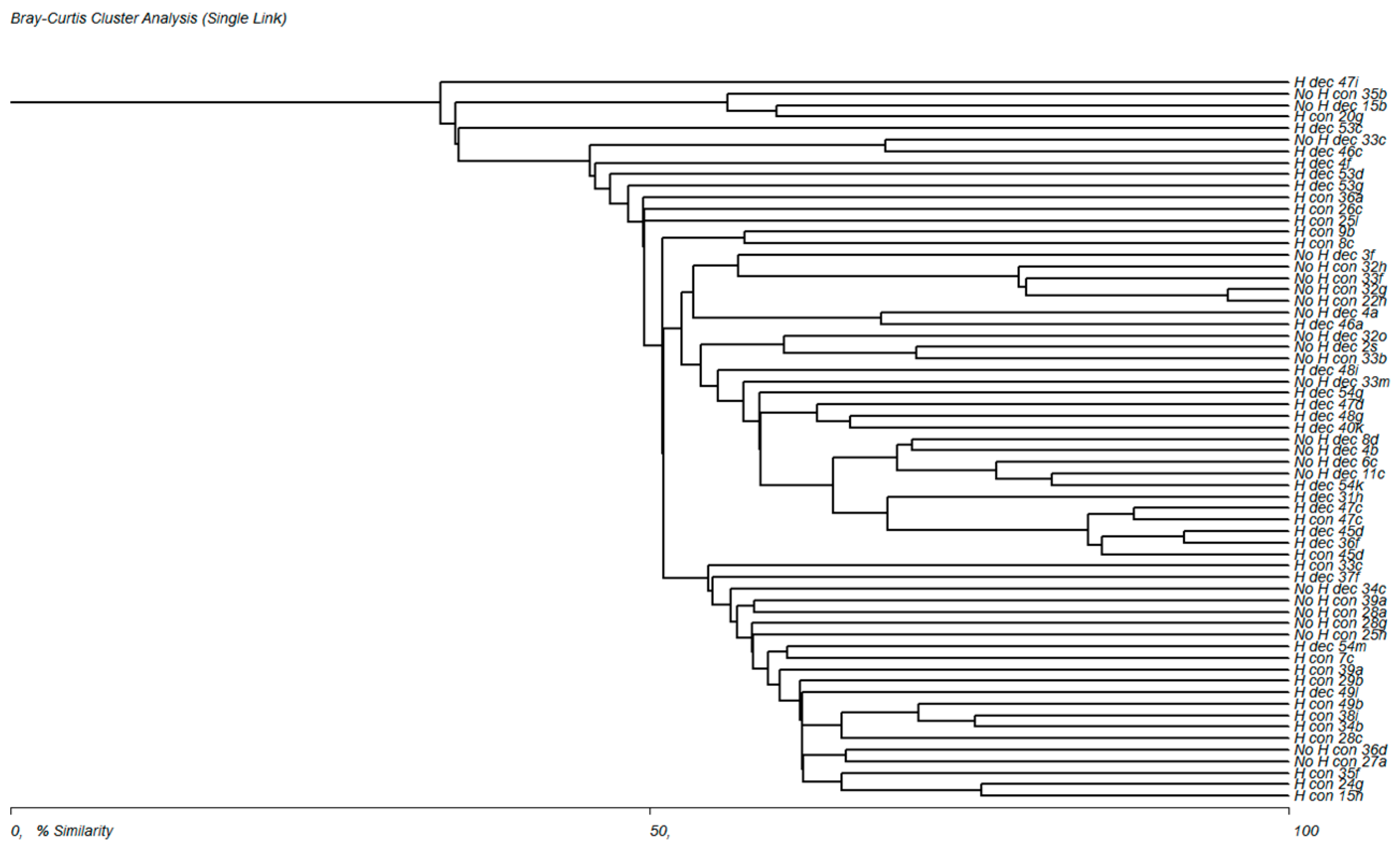
3.3. Evaluation of the Frequency of Plant Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brzeziecki, B. Ecosystem approach and close-to-nature silviculture [in context of forest multifunctionality principle]. Studia i Materiały CEPL 2008, 3, 41–54. [Google Scholar]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncčìna, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature 569 silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Douglas, J.S.; De Hart, J.R.A. Forest Farming: Towards a Solution to Problems of World Hunger and Conservation; Intermediate Technology Publications: London, UK, 1984. [Google Scholar]
- Kownacki, M. Polish Horses. Polish Academy of Sciences Scientific; Polish Scientific Publisher: Warszawa, Poland, 1984; p. 78. [Google Scholar]
- Pasicka, E. Polish Konik horse–characteristics and historical background of native descendants of tarpan. Acta Sci. Pol. Med. Vet. 2013, 12, 25–38. [Google Scholar]
- Żurkowska, T.; Żurkowski, M. Available online: http://popielno.pl/las-doswiadczalny/ (accessed on 8 May 2019).
- Vera, F.W.M. Grazing Ecology and Forest History; CABI Publishing: New York, NY, USA, 2000; 506p. [Google Scholar]
- Bakker, E.S.; Olff, H.; Vandenberghe, C.; De Maeyer, K.; Smit, R.; Gleichman, J.; Vera, F.W.M. Ecological anachronisms the recruitment of temperate light-demanding tree species in wooded pastures. J. Appl. Ecol. 2004, 41, 571–582. [Google Scholar] [CrossRef]
- Weisberg, P.J.; Bugmann, H. Forest dynamics and ungulate herbivory: From leaf to landscape. Forest Ecol. Manag. 2003, 181, 1–12. [Google Scholar] [CrossRef]
- Klich, D. Analysis of bark damage caused by Polish horse in farm conditions in Bieszczady. Bieszczady Yearbooks 2009, 17, 307–317. [Google Scholar]
- Borkowski, J. What did European primeval forests under high herbivore pressure look like? Leśne Prace Badawcze 2011, 72, 183–190. [Google Scholar]
- Jaworski, Z. Evaluation of the ethological and breeding conditions of Polish ponies kept in the reserve system. Dissertations and Monographs UWM, Olsztyn. 2003; 7989p. [Google Scholar]
- Rykowski, K. Ecological and Economic Aspects of the Ecosystem Approach (EA) and Sustainable Forest Management (SFM) on the Example of the Tuszyma Forest District (RDLP Krosno); Information Center of State Forests: Warsaw, Poland, 2008. [Google Scholar]
- Chodkiewicz, A.; Stypiński, P. Food preferences of Konik horses grazing in the Biebrza National Park. Water Environ. Rural Areas 2011, 2, 33–42. [Google Scholar]
- Koniki polskie. Available online: http://popielno.pl/koniki-polskie/ (accessed on 2 March 2018).
- Debiec, T. Trimer with hooves. Polish horses in Tuszyma Forest District. Las Polski. 2010, 22, 20–21. [Google Scholar]
- Hoffmann, M. Experiences with grazing in Flemish nature reserves (N. Belgium). Available online: https://purews.inbo.be/ws/files/6809702/ReportGrazingPeatland2002.pdf (accessed on 10 May 2019).
- Cosyns, E.; Degezelle, T.; Demeulenaere, E.; Hoffmann, M. Feeding ecology of Konik horses and donkeys in Belgian coastal dunes and its implications for nature management. Belg. J. Zool. 2001, 131, 111–118. [Google Scholar]
- Lamoot, I.; Meert, C.; Hoffmann, M. Habitat use of ponies and cattle foraging together in a coastal dune area. Biol. Conserv. 2005, 122, 523–536. [Google Scholar] [CrossRef]
- Gebert, C.; Verheyden-Tixier, H. Variations of diet composition of Red Deer (Cervus elaphus L.) in Europe. Mammal. Rev. 2001, 31, 189–201. [Google Scholar] [CrossRef]
- Duncan, P. Horses and Grasses: The Nutritional Ecology of Equids and Their Impact on the Camargue; (Ecological studies 87); Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1992; p. 287. [Google Scholar]
- Vulink, J.T. Hungry herds: Management of temperate lowland wetlands by grazing. Available online: https://core.ac.uk/download/pdf/29298047.pdf#page=57 (accessed on 10 May 2019).
- Ralston, S.L. Controls of feeding in horses. J. Anim. Sci. 1984, 59, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Hintz, H.F. Equine Nutrition Update; AAEP Proceedings 46: San Antonio, TX, USA, 2000; pp. 62–79. [Google Scholar]
- King, S.R.B. Home range and habitat use of free-ranging Przewalski horses at Hustai National Park, Mongolia. Appl. Anim. Behav. Sci. 2002, 78, 103–113. [Google Scholar] [CrossRef]
- Jorritsma, I.T.M.; Van Hees, A.F.M.; Mohren, G.M.J. Forest development in relation to ungulate grazing: A modeling approach. Forest Ecol. Manag. 1999, 120, 23–34. [Google Scholar] [CrossRef]
- Kuiters, A.T.; Van Der Sluijs, L.A.M.; Wytema, G.A. Selective bark-stripping of beech, Fagus silvatica, by free-ranging horses. Forest Ecol. Manag. 2006, 222, 1–8. [Google Scholar] [CrossRef]
- Jezierski, T.; Jaworski, Z. Defense reactions against insects of Polish horse in the conditions of a pasture and a forest reserve. In Conservative Biology and Breeding of a Polish Horse; Jaczewski, Z., Żurkowski, M., Jaworski, Z., Eds.; Research Station of Organic Farming and Animal Conservation Breeding of the Polish Academy of Sciences in Popielno: Ruciane-Nida, Poland, 1995; pp. 29–34. [Google Scholar]
- Westhoff, V. Nature management in coastal areas of Western Europe. Vegetatio 1985, 62, 523–532. [Google Scholar] [CrossRef]
- Hewett, D.G. Grazing and mowing as management tools on dunes. Vegetatio 1985, 62, 441–447. [Google Scholar] [CrossRef]
- Van Deursen, M.; Cornelissen, P.; Vulink, J.T.; Esselink, P. Yearlong grazing in the Lauwers Sea Polder: Performance of grazing animals and their impact on the vegetation. De Levende Natuur. 1993, 94, 196–204. [Google Scholar]
- Ebrahimi, A.; Milotić, T.; Hoffmann, M. A herbivore specific grazing capacity model accounting for spatio-temporal environmental variation: A tool for a more sustainable nature conservation and rangeland Management. Ecol. Model. 2010, 221, 900–910. [Google Scholar] [CrossRef]
- Helmer, W. Natural grazing versus seasonal grazing. Vakblad Naturbeheer; In Special Issue Grazing and Grazing Animals; Ponsen and Looijen BV: Wageningen, The Netherlands, 2002; pp. 31–33. [Google Scholar]
- Josten, D. Large grazing animals in Flanders. Vakblad Naturbeheer; In Special Issue Grazing and Grazing Animals; Ponsen and Looijen BV: Wageningen, The Netherlands, 2002; pp. 16–18. [Google Scholar]
- Warda, M.; Rogalski, M. Grazing animals as an element of natural landscape. Annales UMCS. Sect. E. 2004, 59, 1985–1991. [Google Scholar]
- Pławska-Olejniczak, J.; Żywiczka, A. The effect of Polish Koniks and Scottish Highland Cattle grazing on the vascular flora of extensively used Skoszewskie Meadows. Łąkarstwo w Polsce 2009, 12, 131–140. [Google Scholar]
- Putman, R.J. Foraging by roe deer in agricultural areas and impact on arable crops. J. Appl. Ecol. 1986, 23, 91–99. [Google Scholar] [CrossRef]
- Nieppola, J. Long term vegetation changes in stands of Pinus sylvestris in southern Finland. J. Veg. Sci. 1992, 3, 475–484. [Google Scholar] [CrossRef]
- Graae, B.J.; Heskær, V.S. A comparison of understory vegetation between untouched and managed deciduous forest in Denmark. For. Ecol. Manag. 1997, 96, 111–123. [Google Scholar] [CrossRef]
- Van Oene, H.; Van Deursen, M.; Berendse, F. Plant–herbivore interaction and its consequences for succession in wetland ecosystems: A modeling approach. Ecosystems 1999, 2, 122–138. [Google Scholar] [CrossRef]
- Courchamp, F.; Chapuis, J.L.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef]
- Czerepko, J. The role of Scots pine stand in the development of the phytocoenosis in an oak-lime-hornbeam forest habitat. Leśne Prace Badawcze 2004, 4, 77–102. [Google Scholar]
- Borkowski, M. Limiting bush encroachment at Biebrza marsh by Konik/Tarpan grazing. Available online: https://core.ac.uk/download/pdf/29298048.pdf#page=101 (accessed on 10 May 2019).
- Crassous, C.; Karas, F. Guide de Gestion des Tourbières et Marais Alcalins des Vallées Alluviales de France Septentrionale; Pôle-Relais Tourbières: Besançon, France, 2007; p. 203. [Google Scholar]
- Kondracki, J. Regional Geography of Poland; Polish Scientific Publisher: Warszawa, Poland, 2000; p. 440. [Google Scholar]
- Begossi, A. Use of ecological methods in ethnobotany: Diversity indices. Econ. Bot. 1996, 50, 280–289. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Skiwski, M.; Klich, D. Spring and summer browsing by Polish konik in enclosures and free ranging conditions in the Bieszczady Mts. Sylwan 2012, 156, 792–800. [Google Scholar]
- Pépin, D.; Lamerenx, F.; Chadelaud, H.J.-M. Human-related disturbance risk and distance to cover affect use of montane pastures by Pyrenean chamois. Appl. Anim. Behav. Sci. 1996, 46, 217–228. [Google Scholar]
- Hanggi, E.B.; Ingersoll, J.F. Long-term memory for categories and concepts in horses (Equus caballus). Anim. Cogn. 2009, 12, 451–462. [Google Scholar] [CrossRef]
- Taraborelli, P.; Gregorio, P.; Moreno, P.; Novaro, A.; Carmanchahi, P. Cooperative vigilance: The guanaco’s (Lama guanicoe) key antipredator mechanism. Behav. Process. 2012, 91, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, Z.; Andrzejewski, A.; Nowakowska, A.; Rebkowska, A. Research on the coverage of nutritional needs by Polish ponies tucked on a natural meadow. Sci. Papers Univ. Agric. Wroc. 1998, 30, 179–188. [Google Scholar]
- Dynowski, P. Vegetation of the Popielno Peninsula as a food base for the Polish horse. Ph.D. Thesis, Botany and Nature Protection Department, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland, 2006. [Google Scholar]
- Shibata, E.I.; Torazawa, Y. Effects of bark stripping by sika deer, Cervus nippon, on wind damage to coniferous trees in subalpine forest of central Japan. J. For. Res. 2008, 13, 296–301. [Google Scholar] [CrossRef]
- Jamrozy, G. Winter food resources and food preferences of red deer in Carpathian forests. Acta Theriol. 1980, 25, 221–238. [Google Scholar] [CrossRef]
- Mátrai, K.; Kabai, P. Winter plant selection by red and roe deer in a forest habitat in Hungary. Acta Theriol. 1989, 34, 227–234. [Google Scholar] [CrossRef]
- Vera, F.W.M. A park-like landscape rather than closed forest. In Grazing And Grazing Animals; Ponsen and Looijen BV: Wageningen, The Netherlands, 2002; pp. 13–14. [Google Scholar]
- Rigueiro-Rodríguez, A.; Mouhbi, R.; Santiago-Freijanes, J.J.; González-Hernández, M.; Mosquera-Losada, M.R. Horse grazing systems: Understory biomass and plant biodiversity of a Pinus radaiata stands. Sci. Agric. 2012, 69, 38–46. [Google Scholar] [CrossRef]
- Zawiślak, J.; Ogińska, M.; Drewka, M.; Święcicka, N. Utilization of horses in forestry economy. Wiadomości Zootechniczne 2014, 52, 61–65. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity, 2nd ed.; Blackwell Science Ltd.: Oxford, UK, 2004. [Google Scholar]
- Stefańska-Krzaczek, E. Species diversity across the successional gradient of managed Scots pine stands in oligotrophic sites (SW Poland). J. For. Sci. 2012, 58, 345–356. [Google Scholar]

| Species | Deciduous Forest (DF) | Coniferous Forest (CF) | ||
|---|---|---|---|---|
| No Horse | Horse | No Horse | Horse | |
| Acer campestre L. | + | |||
| Acer platanoides L. | + | ++ | ||
| Acer pseudoplatanus L. | + | |||
| Aegopodium podagraria L. | + | + | ||
| Anthericum ramosum L. | + | + | ||
| Asarum europaeum L. | + | |||
| Athyrium filix-femina (L.) Roth | + | |||
| Betula pendula Roth. | + | + | + | + |
| Calamagrostis arundinacea L. Roth | + | |||
| Calluna vulgaris L. | + | + | ||
| Campanula rotundifolia L. | + | + | ||
| Carex digitata L. | + | + | + | ++ |
| Carpinus betulus L. | +++ | ++ | + | |
| Convallaria majalis L. | + | ++ | + | ++ |
| Corylus avellana L. | + | |||
| Crataegus monogyna Jacq. | + | |||
| Dactylis polygama Horv. | ++ | + | ||
| Daphne mezereum L. | + | + | ||
| Deschampsia flexuosa L. | + | + | +++ | +++ |
| Dicranum polysetum Sw. | + | +++ | ||
| Dicranum scoparium (L.) Hedw. | + | + | ++ | + |
| Dryopteris carthusiana (Vill.) H. P. Fuchs | + | +++ | ++ | |
| Dryopteris filix-mas (L.) Schott | + | + | ||
| Equisetum sylvaticum L. | + | |||
| Fagus sylvatica L. | + | + | ||
| Ficaria verna Huds. | + | + | ||
| Fragaria vesca L. | + | + | + | + |
| Fraxinus excelsior L. | + | |||
| Geranium robertianum L. | + | + | + | |
| Hepatica nobilis Mill. | ++ | ++ | + | |
| Hylocomium splendens (Hedw.) Schin Bruch, Schimp. & W.Gümbel | + | +++ | ||
| Impatiens noli-tangere L. | ++ | + | ||
| Juniperus communis L. | + | |||
| Larix decidua Mill. | + | |||
| Luzula pilosa (L.) Willd. | + | ++ | ||
| Lycopodium clavatum L. | + | |||
| Maianthemum bifolium (L.) F. W. Schmidt | +++ | ++ | + | + |
| Melampyrum pratense L. | + | + | + | + |
| Milium effusum L. | + | + | ||
| Mnium undulatum (L.) Hedw. | + | ++ | ||
| Mycelis muralis L. | +++ | +++ | + | |
| Oxalis acetosella L. | +++ | +++ | +++ | ++ |
| Paris quadrifolia L. | + | + | ||
| Picea abies L. | + | |||
| Pinus sylvestris L. | + | + | + | |
| Pleurozium schreberi (Willd.) Mitten. | +++ | ++ | +++ | +++ |
| Poa nemoralis L. | + | + | ||
| Polygonatum multiflorum (L.) All. | + | |||
| Polygonatum odoratum (Mill) Druce | + | + | + | |
| Polypodium vulgare L. | + | |||
| Polytrichum commune Hedw. | + | + | ||
| Populus tremula L. | + | |||
| Potentilla erecta (L.) Raeusch | + | |||
| Prunus serotina (Ehrh.) Borkh. | + | |||
| Pteridium aquilinum (L.) Kuhn | + | + | + | |
| Quercus robur L. | + | + | +++ | + |
| Quercus rubra L. | + | + | ||
| Rubus caesius L. | + | |||
| Rubus chamaemorus L. | + | |||
| Rubus idaeus L. | ++ | + | +++ | + |
| Rubus saxatilis L. | ++ | + | ++ | |
| Sorbus aucuparia L. | + | + | + | + |
| Tilia cordata Mill. | + | + | + | |
| Tilia platyphyllos L. | + | |||
| Trientalis europaea L. | + | + | ++ | + |
| Ulmus minor Mill. | + | + | ||
| Urtica dioica L. | + | + | + | |
| Vaccinium myrtillus L. | ++ | +++ | +++ | +++ |
| Vaccinium vitis-idaea L. | + | +++ | ++ | |
| Veronica officinalis L. | + | |||
| Vinca minor L. | + | |||
| Viola hirta L. | + | |||
| Viola reichenbachiana Jordan ex Bor. | +++ | |||
| Total | 42 | 51 | 30 | 40 |
| Effect | N | Shannon-Wiener Index (±SE) | Average Height of the Layer (±SE) [cm] |
|---|---|---|---|
| The herb layer and undergrowth (lower 0.5 m) | |||
| Horse effect | |||
| No H | 29 | 0.823 ± 0.038ns | 14.28 ± 0.98ns |
| H | 39 | 0.855 ± 0.031ns | 17.07 ± 1.19ns |
| Habitat effect | |||
| DF | 34 | 0.878 ± 0.035ns | 13.95 ± 0.94b |
| CF | 34 | 0.805 ± 0.033ns | 17.70 ± 1.24a |
| Interaction horse × habitat | |||
| No H × DF | 18 | 0.879 ± 0.043ns | 12.46 ± 1.26ns |
| H × DF | 15 | 0.865 ± 0.058ns | 15.18 ± 1.32ns |
| No H × CF | 21 | 0.835 ± 0.045ns | 16.22 ± 1.35ns |
| H × CF | 14 | 0.779 ± 0.047ns | 18.69 ± 1.86ns |
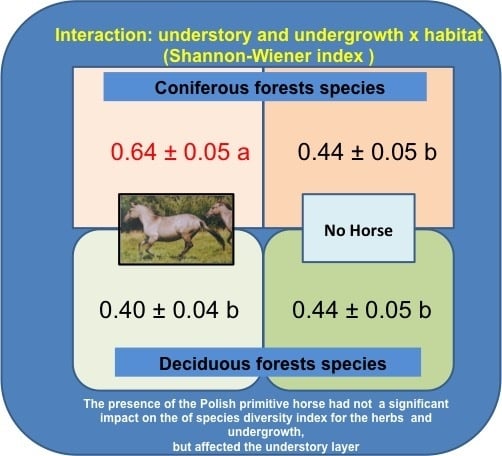
| Understory and undergrowth (higher 0.5 m) layer | |||
| Horse effect | |||
| No H | 25 | 0.441 ± 0.034ns | 80.02 ± 4.49a |
| H | 39 | 0.517 ± 0.036ns | 56.35 ± 7.21b |
| Habitat effect | |||
| DF | 32 | 0.414 ± 0.033a | 68.96 ± 6.40ns |
| CF | 32 | 0.561 ± 0.036b | 62.23 ± 5.33ns |
| Interaction horse × habitat | |||
| No H × DF | 12 | 0.441 ± 0.053b | 75.72 ± 12.19ns |
| H × DF | 19 | 0.397 ± 0.043b | 64.91 ± 7.29ns |
| No H × CF | 12 | 0.441 ± 0.045b | 83.99 ± 8.46ns |
| H × CF | 19 | 0.640 ± 0.045a | 47.34 ± 4.41ns |
| Species | Deciduous Forest (DF) | Coniferous Forest (CF) | ||
|---|---|---|---|---|
| No Horse | Horse | No Horse | Horse | |
| Acer campestre L. | + | |||
| Acer platanoides L. | ++ | ++ | + | + |
| Acer pseudoplatanus L. | + | |||
| Alnus glutinosa [L.) Gaertn. | + | |||
| Betula pendula Roth. | ++ | ++ | +++ | |
| Carpinus betulus L. | +++ | +++ | +++ | ++ |
| Corylus avellana L. | +++ | +++ | +++ | +++ |
| Crataegus monogyna Jacq. | + | |||
| Daphne mezereum L. | + | |||
| Euonymus verrucosus Scop. | + | |||
| Fagus sylvatica L. | + | ++ | ++ | |
| Frangula alnus Mill. | + | + | + | + |
| Juniperus communis L. | + | + | ||
| Larix decidua Mill. | + | + | + | |
| Picea abies [L.) H. Karst | ++ | + | +++ | +++ |
| Pinus sylvestris L. | + | + | + | ++ |
| Populus tremula L. | + | + | + | |
| Prunus cerasifera Ehrh. | + | + | ||
| Prunus domestica L. | + | |||
| Quercus robur L. | ++ | ++ | +++ | +++ |
| Quercus rubra L. | + | + | + | |
| Robinia pseudoacacia L. | + | + | ||
| Rubus idaeus L. | + | + | + | + |
| Sorbus aucuparia L. | ++ | ++ | ++ | +++ |
| Tilia cordata Mill. | ++ | +++ | ++ | + |
| Ulmus glabra Huds. | + | |||
| Total | 17 | 21 | 13 | 18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiko, S.; Bielinis, E.; Sierota, Z.; Zawadzka, A.; Słupska, A.; Nasiadko, M.; Borkowski, J. Polish Pony Changes Lower Layer Biodiversity in Old Growth Scots Pine Stands. Forests 2019, 10, 417. https://doi.org/10.3390/f10050417
Boiko S, Bielinis E, Sierota Z, Zawadzka A, Słupska A, Nasiadko M, Borkowski J. Polish Pony Changes Lower Layer Biodiversity in Old Growth Scots Pine Stands. Forests. 2019; 10(5):417. https://doi.org/10.3390/f10050417
Chicago/Turabian StyleBoiko, Sergii, Ernest Bielinis, Zbigniew Sierota, Anna Zawadzka, Alicja Słupska, Maciej Nasiadko, and Jakub Borkowski. 2019. "Polish Pony Changes Lower Layer Biodiversity in Old Growth Scots Pine Stands" Forests 10, no. 5: 417. https://doi.org/10.3390/f10050417
APA StyleBoiko, S., Bielinis, E., Sierota, Z., Zawadzka, A., Słupska, A., Nasiadko, M., & Borkowski, J. (2019). Polish Pony Changes Lower Layer Biodiversity in Old Growth Scots Pine Stands. Forests, 10(5), 417. https://doi.org/10.3390/f10050417