Microsite Effects on Physiological Performance of Betula ermanii at and Beyond an Alpine Treeline Site on Changbai Mountain in Northeast China
Abstract
1. Introduction
2. Materials and Methods
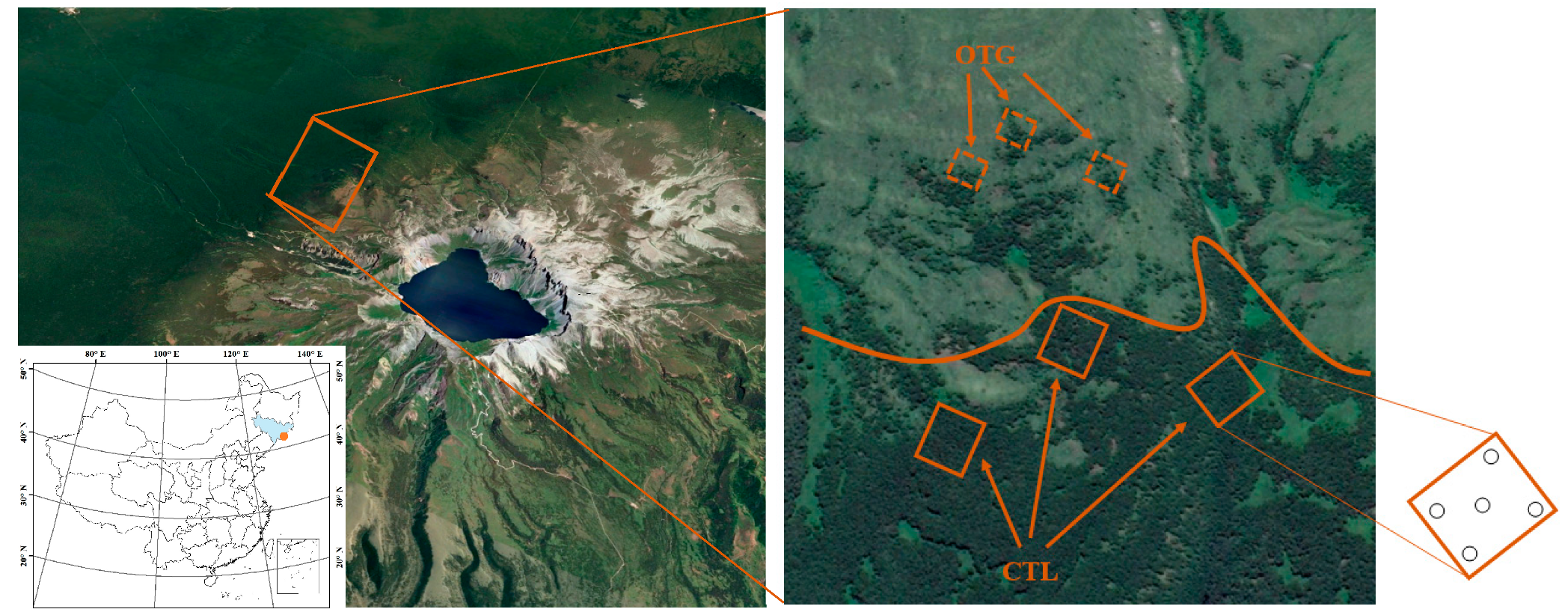
2.1. Study Site Description
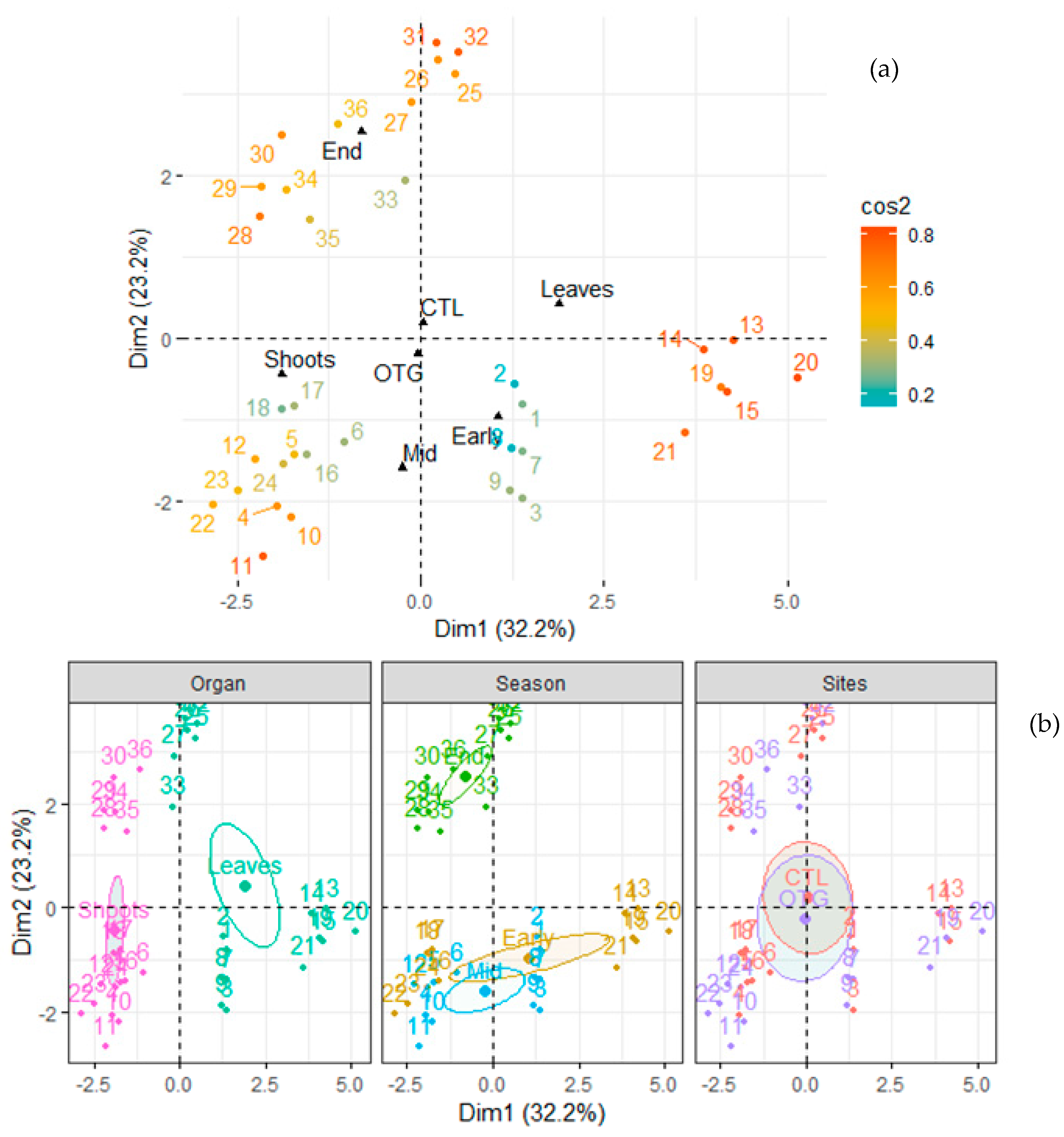
2.2. Plant Sampling and Chemical Analyses
2.3. Soil Sampling and Chemical Analyses
2.4. Climate Data Collection
2.5. Data Analyses
3. Results
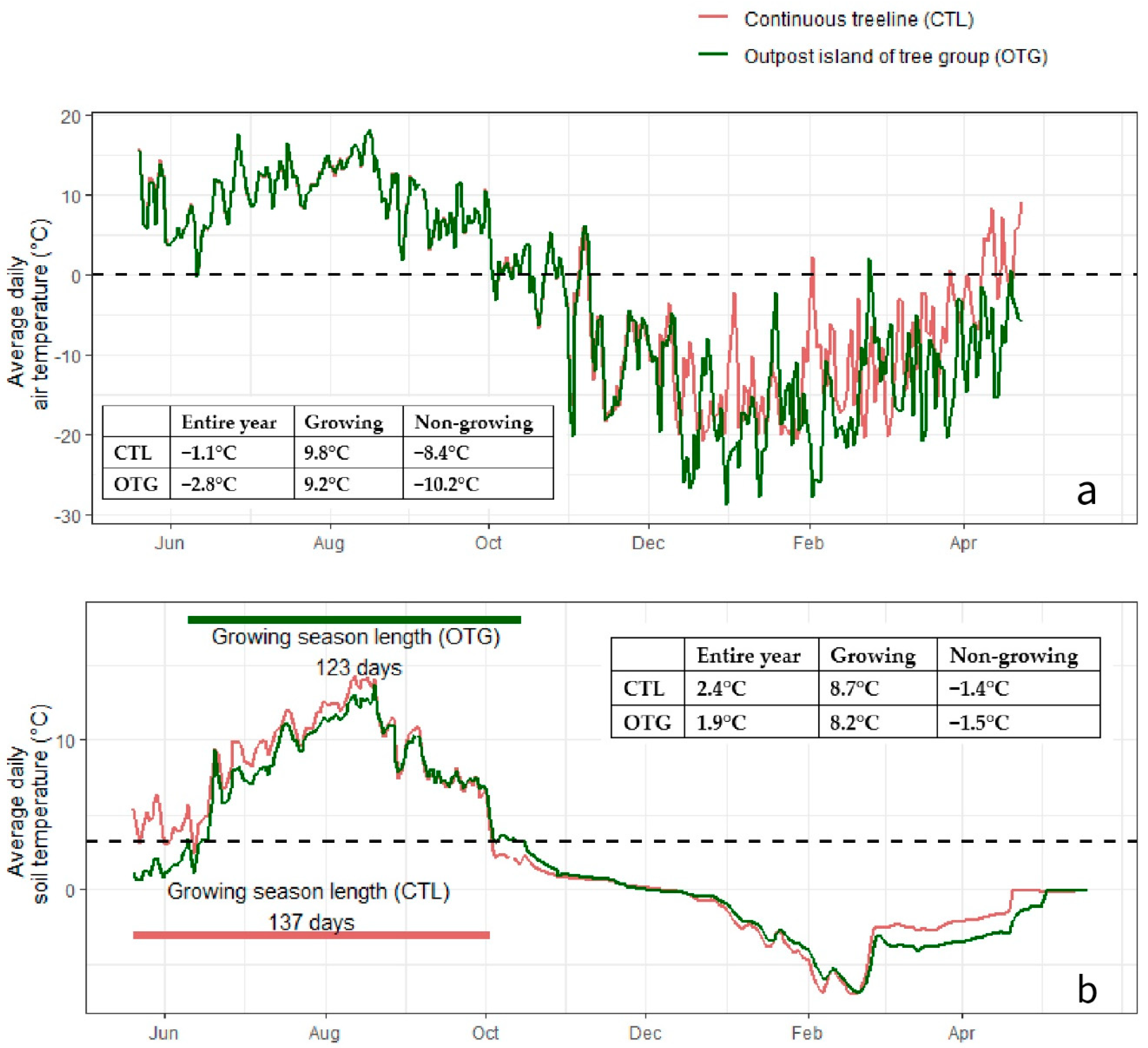
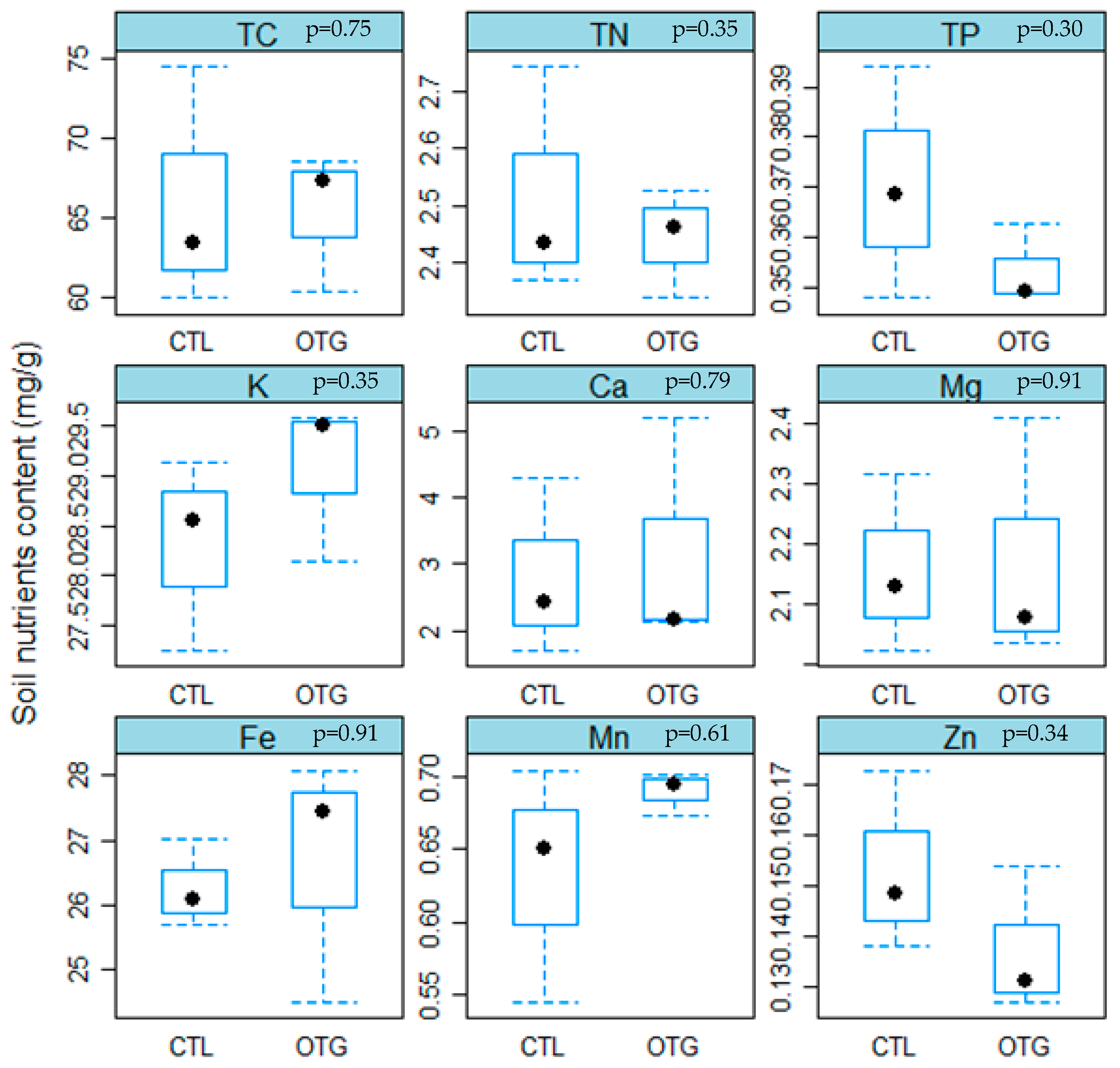
3.1. Climate Conditions and Soil Properties
3.2. Plant Nutrients
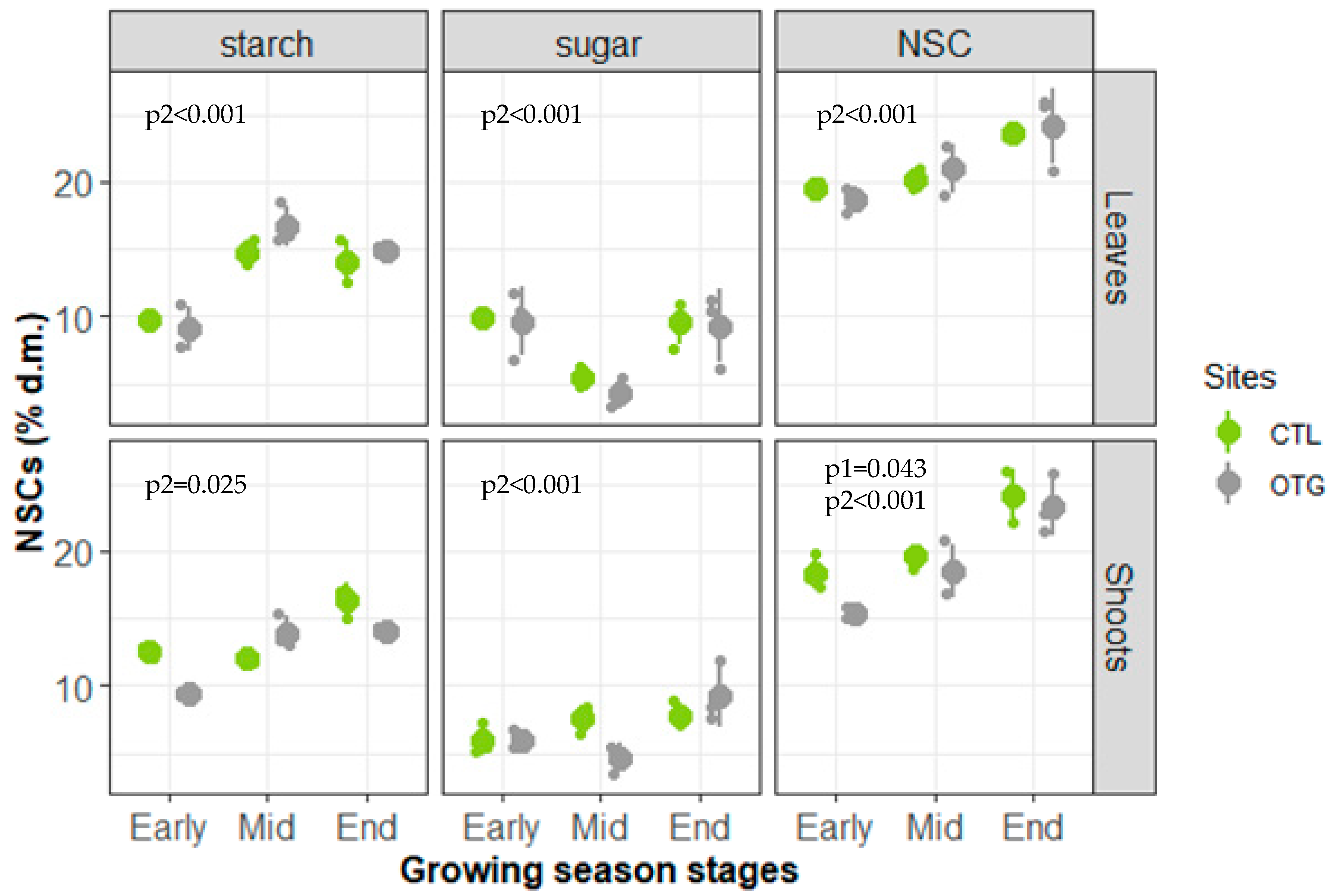
3.3. Non-Structural Carbohydrates (NSCs)
3.4. Relationships between Nutrients and NSC
4. Discussion
4.1. Microsite-Modulated Climate in Growing and Non-Growing Seasons
4.2. Similar Soil Nutrient Concentrations
4.3. Generally Similar Ecophysiological Performances
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wieser, G.; Holtmeier, F.-K.; Smith, W.K. Treelines in a changing global environment. In Trees in a Changing Environment; Springer: Dordrecht, The Netherlands, 2014; pp. 221–263. [Google Scholar]
- Wieser, G.; Tausz, M. Current concepts for treelife limitation at the upper timberline. In Trees at Their Upper Limit; Springer: Dordrecht, The Netherlands, 2007; pp. 1–18. [Google Scholar]
- Gehrig-Fasel, J.; Guisan, A.; Zimmermann, N.E. Evaluating thermal treeline indicators based on air and soil temperature using an air-to-soil temperature transfer model. Ecol. Model. 2008, 213, 345–355. [Google Scholar] [CrossRef]
- Li, M.-H.; NK, K. The state of knowledge on alpine treeline and suggestions for future research. J. Sichuan For. Sci. Technol. 2005, 26, 36–42. [Google Scholar]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gricar, J.; Rossi, S.; Cufar, K.; Ellison, A. Critical minimum temperature limits xylogenesis and maintains treelines on the Tibetan Plateau. Available online: https://www.biorxiv.org/content/early/2016/12/13/093781.full.pdf (accessed on 4 May 2019).
- Friedrich-Karl, H.; Gabriele, B. Feedback effects of clonal groups and tree clusters on site conditions at the treeline: Implications for treeline dynamics. Clim. Res. 2017, 73, 85–96. [Google Scholar]
- Scherrer, D.; Körner, C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr. 2011, 38, 406–416. [Google Scholar] [CrossRef]
- Drik, W.; Roland, P.; Jörg, L. Alpine Soil Temperature Variability at Multiple Scales. Arct. Antarct. Alp. Res. 2010, 42, 117–128. [Google Scholar]
- Schirmer, M.; Lehning, M. Persistence in intra-annual snow depth distribution: 2. Fractal analysis of snow depth development. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef]
- Stoeckel, D.M.; Miller-Goodman, M.S. Seasonal nutrient dynamics of forested floodplain soil influenced by microtopography and depth. Soil Sci. Soc. Am. J. 2001, 65, 922–931. [Google Scholar] [CrossRef]
- Derbyshire, E.; Hails, J.R.; Gregory, K.J. Geomorphological Processes: Studies in Physical Geography; Elsevier: London, UK, 2013. [Google Scholar]
- Erickson, T.A.; Williams, M.W.; Winstral, A. Persistence of topographic controls on the spatial distribution of snow in rugged mountain terrain, Colorado, United States. Water Resour. Res. 2005, 41. [Google Scholar] [CrossRef]
- Litaor, M.I.; Williams, M.; Seastedt, T.R. Topographic controls on snow distribution, soil moisture, and species diversity of herbaceous alpine vegetation, Niwot Ridge, Colorado. J. Geophys. Res. Biogeosci. 2008, 113. [Google Scholar] [CrossRef]
- Hughes, N.M.; Johnson, D.M.; Akhalkatsi, M.; Abdaladze, O. Characterizing Betula Litwinowii Seedling Microsites at the Alpine-Treeline Ecotone, Central Greater Caucasus Mountains, Georgia. Arct. Antarct. Alp. Res. 2009, 41, 112–118. [Google Scholar] [CrossRef][Green Version]
- Johnson, A.C.; Yeakley, J.A. Seedling Regeneration in the Alpine Treeline Ecotone: Comparison of Wood Microsites and Adjacent Soil Substrates. Mt. Res. Dev. 2016, 36, 443–451. [Google Scholar] [CrossRef]
- Mayr, S.; Hacke, U.; Schmid, P.; Schwienbacher, F.; Gruber, A. Frost drought in conifers at the alpine timberline: Xylem dysfunction and adaptations. Ecology 2006, 87, 3175–3185. [Google Scholar] [CrossRef]
- Malanson, G.P.; Butler, D.R.; Fagre, D.B.; Walsh, S.J.; Tomback, D.F.; Daniels, L.D.; Resler, L.M.; Smith, W.K.; Weiss, D.J.; Peterson, D.L.; et al. Alpine treeline of Western North America: Linking organism-to-landscape dynamics. Phys. Geogr. 2007, 28, 378–396. [Google Scholar] [CrossRef]
- Renard, S.M.; McIntire, E.J.; Fajardo, A. Winter conditions—Not summer temperature -influence establishment of seedlings atwhite spruce alpine treeline in Eastern Quebec. J. Veg. Sci. 2016, 27, 29–39. [Google Scholar] [CrossRef]
- Mamet, S.D.; Kershaw, G.P. Age-dependency, climate, and environmental controls of recent tree growth trends at subarctic and alpine treelines. Dendrochronologia 2013, 31, 75–87. [Google Scholar] [CrossRef]
- Barbeito, I.; Brucker, R.L.; Rixen, C.; Bebi, P. Snow Fungi-Induced Mortality of Pinus cembra at the Alpine Treeline: Evidence from Plantations. Arct. Antarct. Alp. Res. 2013, 45, 455–470. [Google Scholar] [CrossRef]
- Hofgaard, A.; Dalen, L.; Hytteborn, H. Tree recruitment above the treeline and potential for climate-driven treeline change. J. Veg. Sci. 2009, 20, 1133–1144. [Google Scholar] [CrossRef]
- Resler, L.M.; Butler, D.R.; Malanson, G.P. Topographic shelter and conifer establishment and mortality in an alpine environment, Glacier National Park, Montana. Phys. Geogr. 2005, 26, 112–125. [Google Scholar] [CrossRef]
- Li, M.H.; Yang, J. Effects of microsite on growth of Pinus cembra in the subalpine zone of the Austrian Alps. Ann. For. Sci. 2004, 61, 319–325. [Google Scholar] [CrossRef]
- Li, M.H.; Yang, J.; Kräuchi, N. Growth responses of Picea abies and Larix decidua to elevation in subalpine areas of Tyrol, Austria. Can. J. For. Res. 2003, 33, 653–662. [Google Scholar]
- Greenwood, S.; Chen, J.C.; Chen, C.T.; Jump, A.S. Temperature and sheltering determine patterns of seedling establishment in an advancing subtropical treeline. J. Veg. Sci. 2015, 26, 711–721. [Google Scholar] [CrossRef]
- Scherrer, D.; Koerner, C. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Glob. Chang. Biol. 2010, 16, 2602–2613. [Google Scholar] [CrossRef]
- Boateng, J.O.; Heineman, J.L.; McClarnon, J.; Bedford, L. Twenty year responses of white spruce to mechanical site preparation and early chemical release in the boreal region of northeastern British Columbia. Can. J. For. Res. 2006, 36, 2386–2399. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. The carbon charging of pines at the climatic treeline: A global comparison. Oecologia 2003, 135, 10–21. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Growth, demography and carbon relations of Polylepis trees at the world’s highest treeline. Funct. Ecol. 2005, 19, 941–951. [Google Scholar] [CrossRef]
- Peili, S.; Christian, K.; Hoch, G. A test of the growth-limitation theory for alpine tree line formation in evergreen and deciduous taxa of the eastern Himalayas. Funct. Ecol. 2008, 22, 213–220. [Google Scholar]
- Günter, H.; Christian, K. Global patterns of mobile carbon stores in trees at the high-elevation tree line. Glob. Ecol. Biogeogr. 2011, 21, 861–871. [Google Scholar]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.; Schönbeck, L.; et al. Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiol. 2018, 9, 1345–1355. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef]
- Yu, D.P.; Wang, Q.W.; Liu, J.Q.; Zhou, W.M.; Qi, L.; Wang, X.Y.; Zhou, L.; Dai, L.M. Formation mechanisms of the alpine Erman’s birch (Betula ermanii) treeline on Changbai Mountain in Northeast China. Trees 2014, 28, 935–947. [Google Scholar] [CrossRef]
- Chen, L.Z.; Bao, X.C.; Li, C.G. Structural characteristics of certain dominant species on the northern slope of Changbai mountain in Jilin province. J. Plant Ecol. 1964, 2, 207–225. [Google Scholar]
- Yu, D.P.; Wang, G.G.; Dai, L.M.; Wang, Q.L. Dendroclimatic analysis of Betula ermanii forests at their upper limit of distribution in Changbai Mountain, Northeast China. For. Ecol. Manag. 2007, 240, 105–113. [Google Scholar] [CrossRef]
- Du, H.; Liu, J.; Li, M.H.; Büntgen, U.; Yang, Y.; Wang, L.; Wu, Z.; He, H.S. Warming-induced upward migration of the alpine treeline in the Changbai Mountains, northeast China. Glob. Chang. Biol. 2018, 24, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Tamir, K.; Yann, V.; Günter, H. Coordination between growth, phenology and carbon storage in three coexisting deciduous tree species in a temperate forest. Tree Physiol. 2016, 36, 847–855. [Google Scholar]
- Mitsuru, O.; Takuro, S.; Toshiaki, T. Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil Sci. Plant Nutr. 1991, 37, 117–128. [Google Scholar]
- Wang, Q.-W.; Qi, L.; Zhou, W.; Liu, C.-G.; Yu, D.; Dai, L. Carbon dynamics in the deciduous broadleaf tree Erman’s birch (Betula ermanii) at the subalpine treeline on Changbai Mountain, Northeast China. Am. J. Bot. 2018, 105, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J. Analyse Factorielle de Donnees Mixtes. Rev. Stat. Appl. 2004, 4, 93–111. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Körner, C. Alpine Treelines; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Li, M.H.; Xiao, W.F.; Shi, P.; Wang, S.G.; Zhong, Y.D.; Liu, X.L.; Wang, X.D.; Cai, X.H.; Shi, Z.M. Nitrogen and carbon source–sink relationships in trees at the Himalayan treelines compared with lower elevations. Plant Cell Environ. 2008, 31, 1377–1387. [Google Scholar] [CrossRef]
- Gaku, K. Intraspecific variation of leaf traits in several deciduous species in relation to length of growing season. Ecoscience 1996, 3, 483–489. [Google Scholar]
- Karlsson, P.S.; Nordell, K.O. Intraspecific variation in nitrogen status and photosynthetic capacity within mountain birch populations. Ecography 1988, 11, 293–297. [Google Scholar] [CrossRef]
- Manning, D.A. Mineral sources of potassium for plant nutrition. A review. Agron. Sustain. Dev. 2010, 30, 281–294. [Google Scholar] [CrossRef]
- Limin, R.; Peng, L. Review of manganese toxicity and the mechanisms of plant tolerance. Acta Ecol. Sin. 2007, 27, 357–367. [Google Scholar]
- Xu, W.B.; Shao, X.Q.; Wang, Y.T.; Wang, K. Advances in research on physiological effects of manganese on plants and manganese poisoning. Grassl. Lawns 2011, 3, 5–14. [Google Scholar]
- Epron, D.; Cabral, O.M.; Laclau, J.P.; Dannoura, M.; Packer, A.P.; Plain, C.; Battie-Laclau, P.; Moreira, M.Z.; Trivelin, P.C.; Bouillet, J.P.; et al. In situ 13CO2 pulse labelling of field-grown eucalypt trees revealed the effects of potassium nutrition and throughfall exclusion on phloem transport of photosynthetic carbon. Tree Physiol. 2016, 36, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Bader, M.K.F.; Leuzinger, S.; Mildner, M.; Schleppi, P.; Siegwolf, R.T.W.; Korner, C. Growth and carbon relations of mature Picea abies trees under 5years of free-air CO2 enrichment. J. Ecol. 2016, 104, 1720–1733. [Google Scholar] [CrossRef]
- Friedrich-Karl, H.; Gabriele, B. Landform Influences on Treeline Patchiness and Dynamics in a Changing Climate. Phys. Geogr. 2012, 33, 403–437. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Wang, Q.; Wang, X.; Dai, L.; Li, M. Microsite Effects on Physiological Performance of Betula ermanii at and Beyond an Alpine Treeline Site on Changbai Mountain in Northeast China. Forests 2019, 10, 400. https://doi.org/10.3390/f10050400
Yu D, Wang Q, Wang X, Dai L, Li M. Microsite Effects on Physiological Performance of Betula ermanii at and Beyond an Alpine Treeline Site on Changbai Mountain in Northeast China. Forests. 2019; 10(5):400. https://doi.org/10.3390/f10050400
Chicago/Turabian StyleYu, Dapao, Qingwei Wang, Xiaoyu Wang, Limin Dai, and Maihe Li. 2019. "Microsite Effects on Physiological Performance of Betula ermanii at and Beyond an Alpine Treeline Site on Changbai Mountain in Northeast China" Forests 10, no. 5: 400. https://doi.org/10.3390/f10050400
APA StyleYu, D., Wang, Q., Wang, X., Dai, L., & Li, M. (2019). Microsite Effects on Physiological Performance of Betula ermanii at and Beyond an Alpine Treeline Site on Changbai Mountain in Northeast China. Forests, 10(5), 400. https://doi.org/10.3390/f10050400




