The Impact of Different Management Scenarios on the Availability of Potential Forest Habitats for Wildlife on a Landscape Level: The Case of the Black Stork Ciconia nigra (Linnaeus, 1758)
Abstract
:1. Introduction
2. Materials and Methods
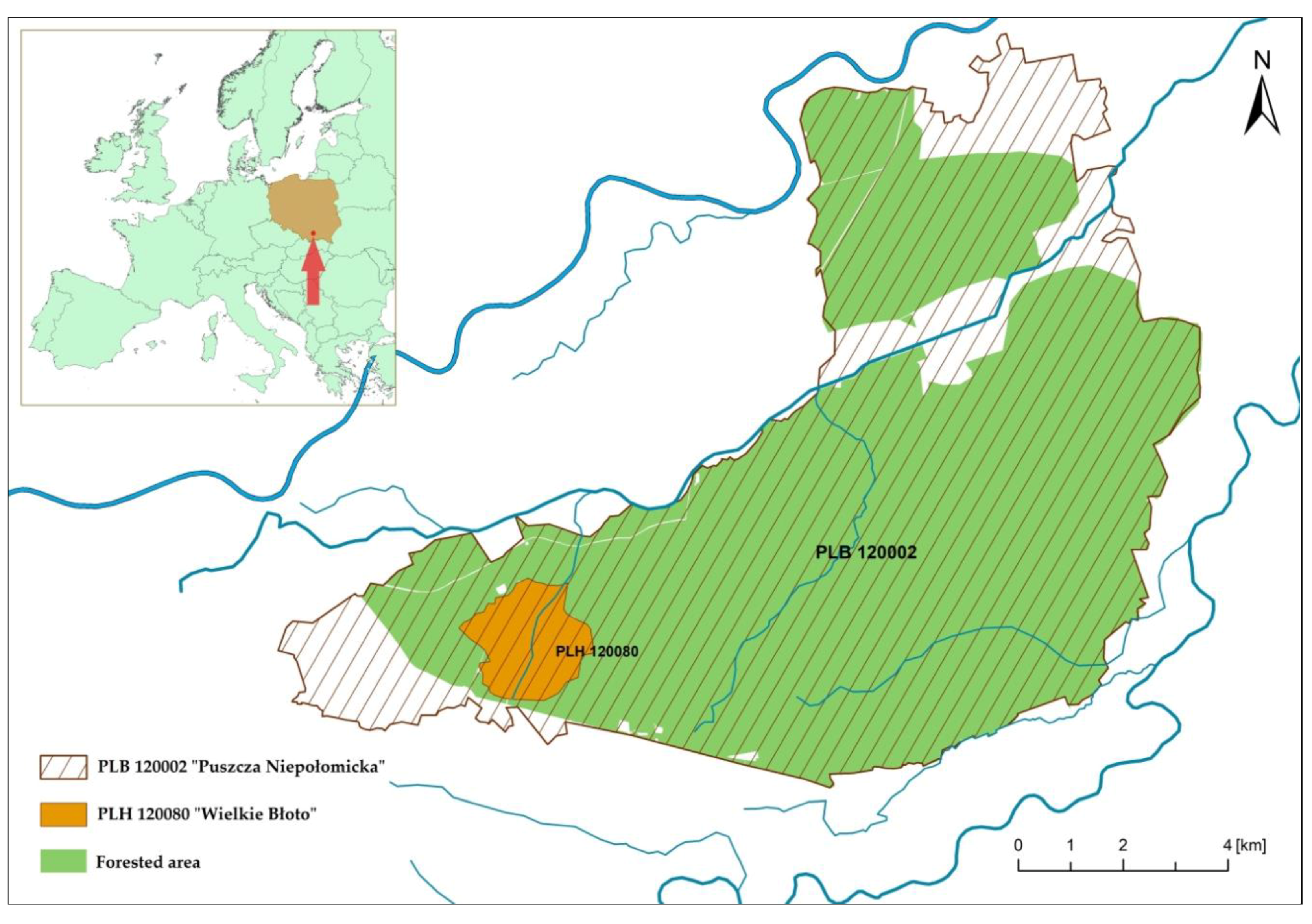
2.1. Study Area and Input Data
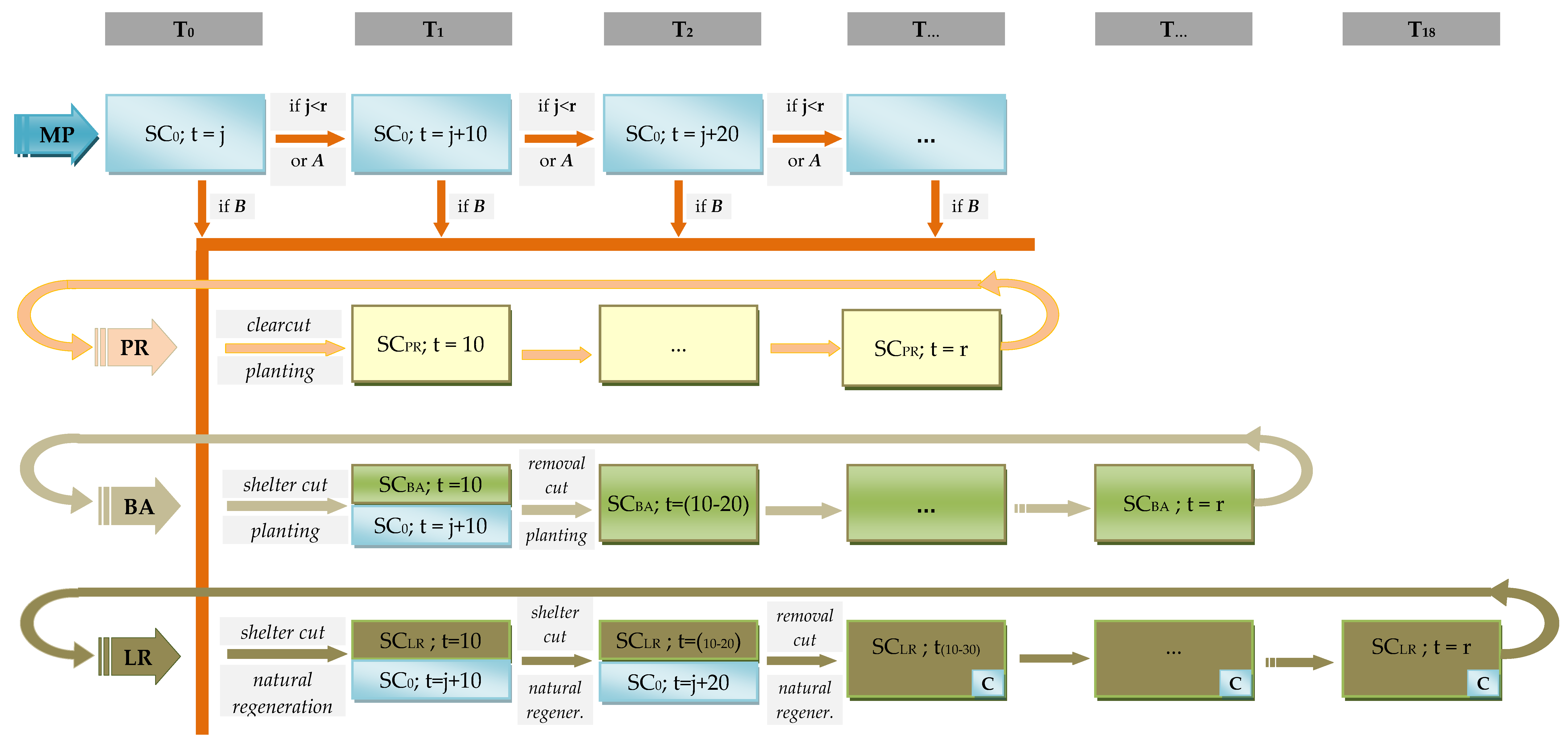
2.2. Management Scenarios
- The Productive (PR) scenario—the objective of this management scenario is to maximize timber production. Rotation ages correspond to the highest mean annual volume increment. This scenario predominantly involves monospecific stands with the dominant species being Scots pine (rotation age of 90 years) harvested by the clearcut method and regenerated artificially by planting. Two-species stands managed by the shelterwood system with a short (10 years) regeneration period are only found on eutrophic sites.
- The Basic (BA) scenario—the objectives include timber production as well as non-production forest functions. In contrast to the PR scenario, BA does not use clearcutting or monospecific stands; instead it relies on two- or three-species stands, preferably containing pine and oak (enabling a high yield of merchantable timber), with a medium rotation age of 100 and 140 years, respectively, managed mainly by the shelterwood system and regenerated by planting.
- The Long Rotation (LR) scenario—in comparison with BA, LR focuses even more on ecological functions by enhancing species and age diversity and increasing the proportion of old stands. This scenario involves multispecies stands with a dominance of deciduous trees and a long rotation age: 110 and 180 years for pine and oak, respectively. The shelterwood system is implemented with different durations of the regeneration period (10–30 years). Regeneration is essentially natural, but in the transition period the desired species absent from the stand are introduced by planting. About 5% of the mature stand area and volume is retained during harvest for natural death as a source of deadwood (retention trees). In addition, swamp sites are entirely excluded from wood production due to their great significance to black storks, their low share in the overall forest area, and difficulties associated with implementing silvicultural activities (implying low economic viability of wood production).
2.3. Simulation of Future Forest Development
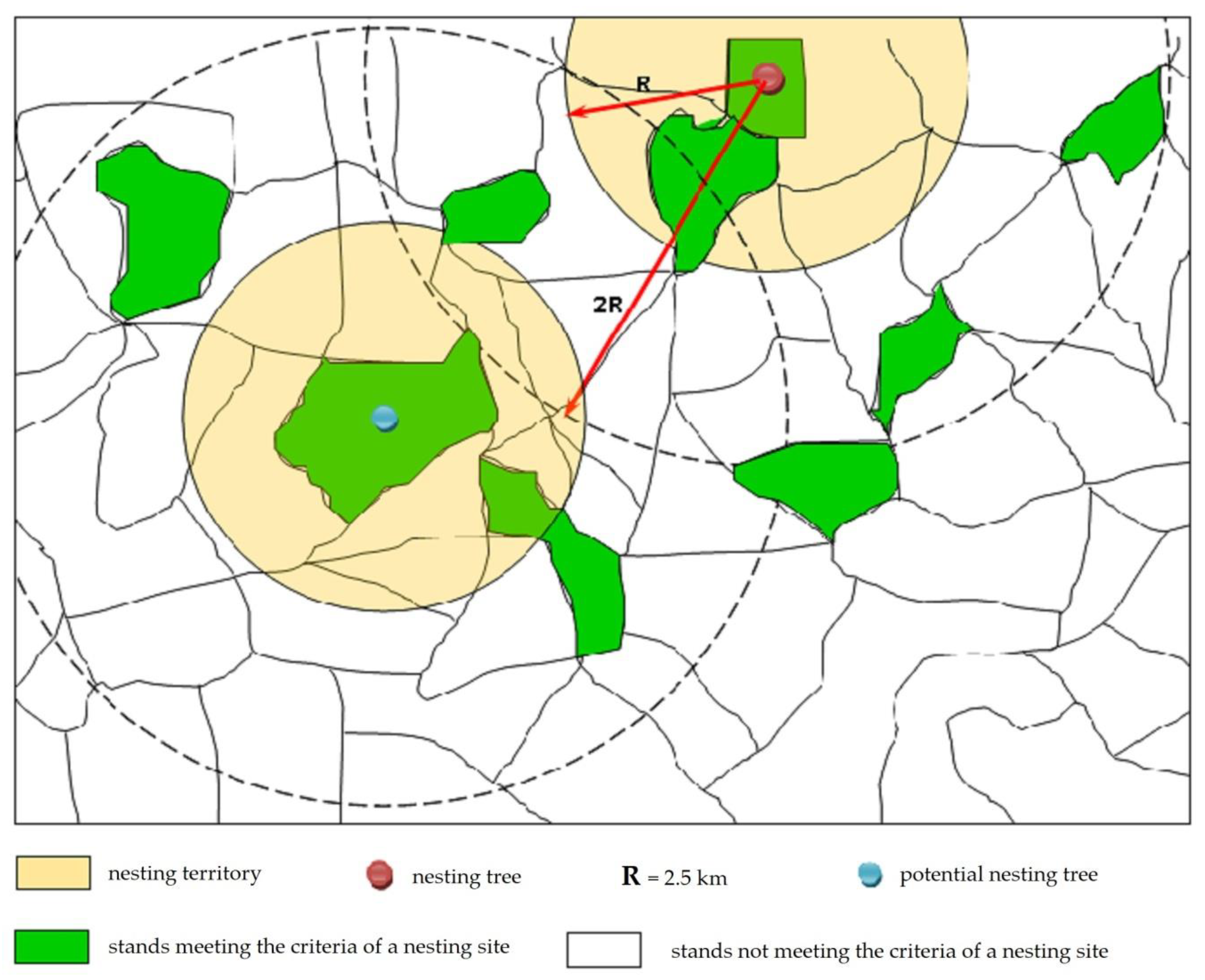
2.4. Identification of Habitats Suitable for Black Storks
2.5. Forest Indicators
2.5.1. Overall Area of Black Stork Nesting Sites
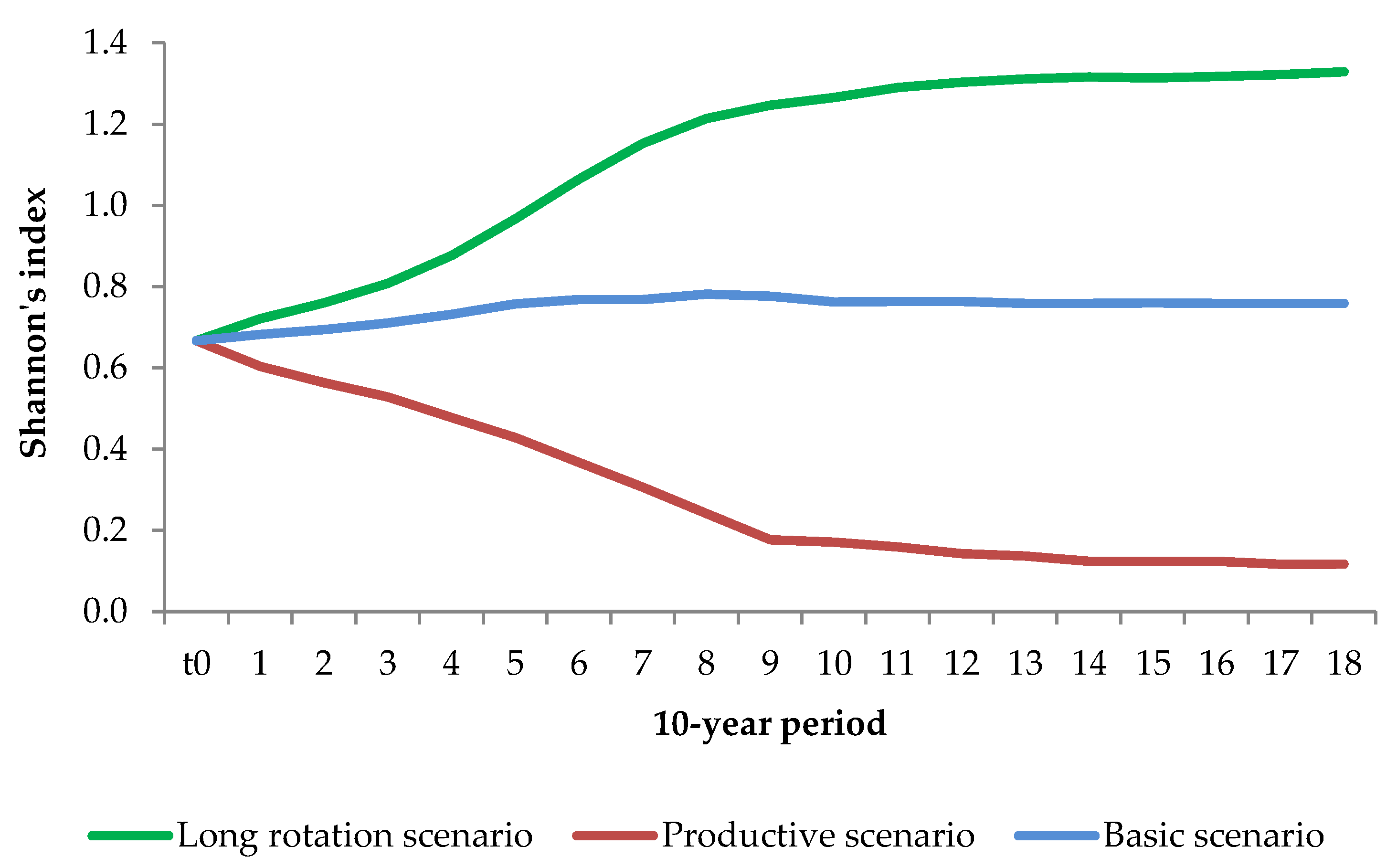
2.5.2. Tree Species Diversity
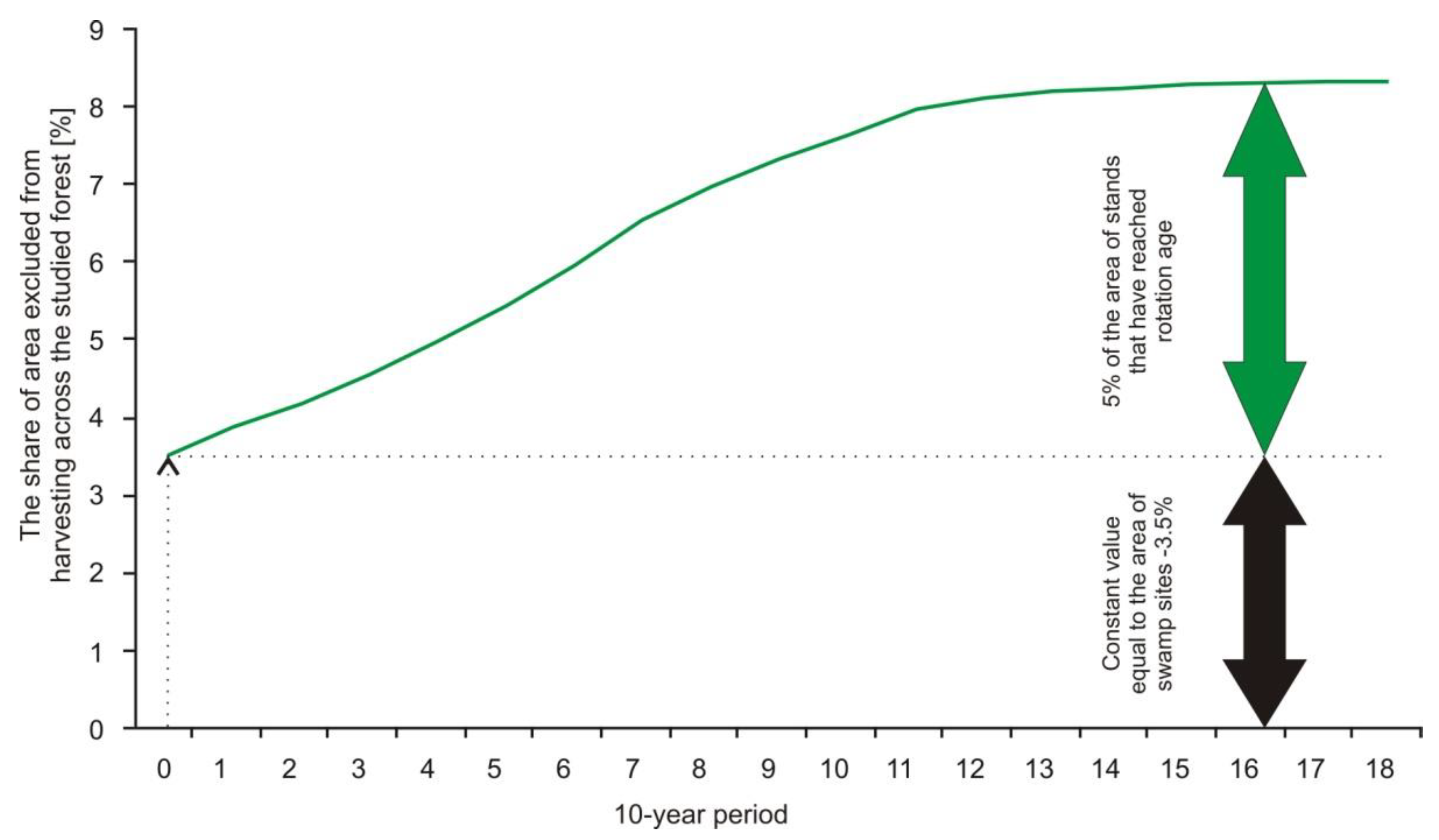
2.5.3. Area of Stands Excluded from Harvesting
2.5.4. Large Trees
3. Results
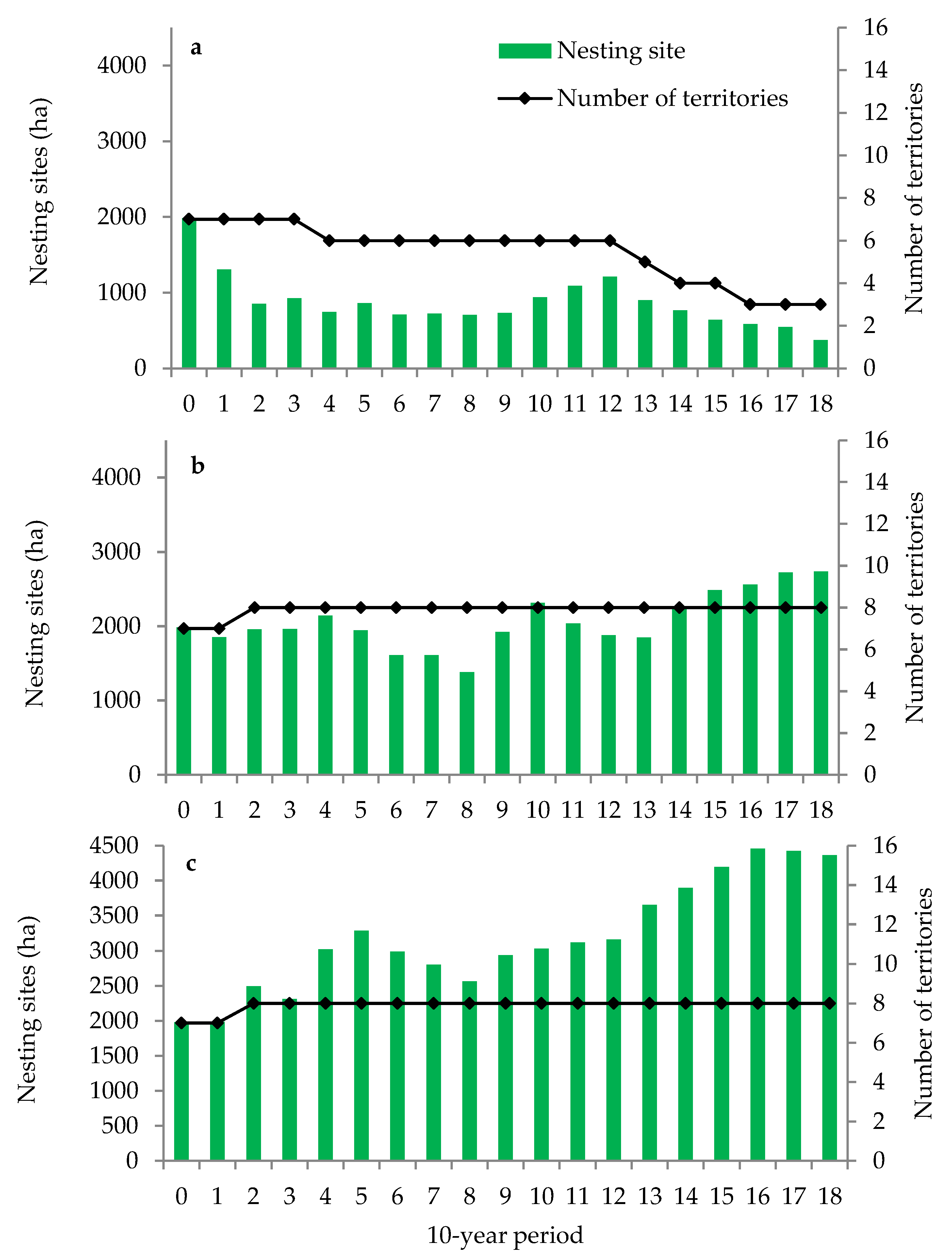
3.1. Habitats Suitable for Black Stork
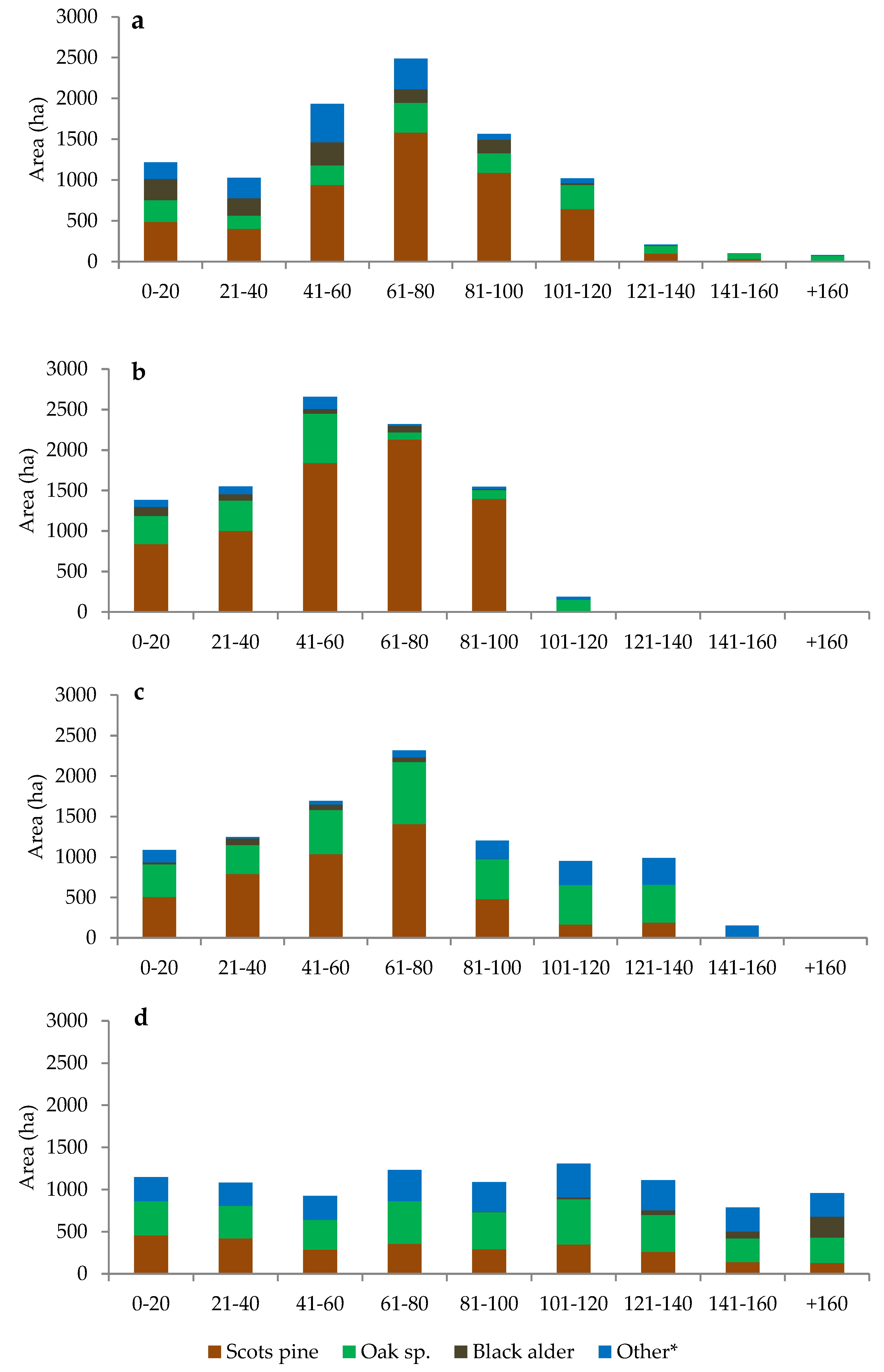
3.2. Age Class, Species Composition of Stands, and Harvested Volume
3.3. Large Trees and Area of Stands Excluded from Harvesting
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkar, A.U. Sustainability, sustainable development and forest resources. Int. J. Sustain. Dev. World Ecol. 1998, 5, 164–171. [Google Scholar] [CrossRef]
- Vierikko, K.; Vehkamäki, S.; Niemelä, J.; Pellikka, J.; Lindén, H. Meeting the ecological, social and economic needs of sustainable forest management at a regional scale. Scand. J. For. Res. 2008, 23, 431–444. [Google Scholar] [CrossRef]
- Heinonen, T.; Pukkala, T.; Mehtätalo, L.; Asikainen, A.; Kangas, J.; Peltola, H. Scenario analyses for the effects of harvesting intensity on development of forest resources, timber supply, carbon balance and biodiversity of Finnish forestry. For. Policy Econ. 2017, 80, 80–98. [Google Scholar] [CrossRef]
- Eggers, J.; Lindhagen, A.; Lind, T.; Lämås, T.; Öhman, K. Balancing landscape-level forest management between recreation and wood production. Urban For. Urban Green. 2018, 33, 1–11. [Google Scholar] [CrossRef]
- Angelstam, P.; Roberge, J.-M.; Lõhmus, A.; Bergmanis, M.; Brazaitis, G.; Dönz-Breuss, M.; Edenius, L.; Kosinski, Z.; Kurlavicius, P.; Lārmanis, V.; et al. Habitat modelling as a tool for landscape-scale conservation—A review of parameters for focal forest birds. Ecol. Bull. 2004, 51, 427–453. [Google Scholar] [CrossRef]
- Gao, T.; Nielsen, A.B.; Hedblom, M. Reviewing the strength of evidence of biodiversity indicators for forest ecosystems in Europe. Ecol. Indic. 2015, 57, 420–434. [Google Scholar] [CrossRef]
- Cramp, S. Handbook of the Birds of Europe, the Middle East, and North Africa. The Birds of the Western Palearctic: Ostrich to Ducks; Oxford University Press: Oxford, UK, 1980; Volume I. [Google Scholar]
- Roberge, J.M.; Angelstam, P.; Villard, M.A. Specialised woodpeckers and naturalnesss in hemiboreal forests—Deriving quantitative targets for conservation planning. Biol. Conserv. 2008, 141, 997–1012. [Google Scholar] [CrossRef]
- Pirovano, A.R.; Zecca, G. Black Woodpecker Dryocopus martius habitat selection in the Italian Alps: Implications for conservation in Natura 2000 network. Bird Conserv. Int. 2014, 24, 299–315. [Google Scholar] [CrossRef]
- Michalczuk, J.; Michalczuk, M. Nesting preferences of Syrian Woodpeckers Dendroccopos syriacus in the agricultural landscape of SE Poland. Acta Ornithol. 2016, 51, 71–81. [Google Scholar] [CrossRef]
- Mikusiński, G.; Gromadzki, M.; Chylarecki, P. Woodpeckers as indicators of forest bird diversity. Conserv. Biol. 2001, 15, 208–217. [Google Scholar] [CrossRef]
- Sergio, F.; Newton, I.; Marchesi, L. Top predators and biodiversity. Nature 2005, 436, 192. [Google Scholar] [CrossRef] [PubMed]
- Sergio, F.; Newton, I.; Marchesi, L. Top predators and biodiversity: much debate, few data. J. Appl. Ecol. 2008, 45, 992–999. [Google Scholar] [CrossRef]
- Roberge, J.M.; Angelstam, P.E.R. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Branton, M.; Richardson, J.S. Assessing the value of the umbrella-species concept for conservation planning with meta-analysis. Conserv. Biol. 2011, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Maslo, B.; Leu, K.; Faillace, C.; Weston, M.A.; Pover, T.; Schlacher, T.A. Selecting umbrella species for conservation: A test of habitat models and niche overlap for beach-nesting birds. Biol. Conserv. 2016, 203, 233–242. [Google Scholar] [CrossRef]
- Moreno-Opo, R.; Fernández-Olalla, M.; Guil, F.; Arredondo, Á.; Higuero, R.; Martín, M.; Soria, C.; Guzmán, J. The role of ponds as feeding habitat for an umbrella species: best management practices for the black stork Ciconia nigra in Spain. Oryx 2011, 45, 448–455. [Google Scholar] [CrossRef]
- Council of the European Communities. Council Directive 79/409/EEC on the Conservation of Wild Birds. 1979. Available online: http://europa.eu/legislation_sum maries/environment/nature_and_biodiversity/ (accessed on 25 March 2019).
- Strazds, M. Conservation Ecology of the Black Stork in Latvia. Ph.D. Thesis, University of Latvia, Riga, Latvia, 2011. [Google Scholar]
- Zawadzka, D.; Olech, B.; Zawadzki, J. Population density, reproduction and food of the Black stork in the Kampinoski National Park in years 1979–1987. Notatki Ornitologiczne 1990, 31, 5–20. [Google Scholar]
- Lõhmus, A.; Sellis, U. Foraging habitats of the Black Stork in Estonia. Hirundo 2001, 14, 109–112. [Google Scholar]
- Tucker, G.M.; Heath, M.F. Birds in Europe: Their Conservation Status; BirdLife International: Cambridge, UK, 1994; Conservation, 3. [Google Scholar]
- Treinys, R.; Lõhmus, A.; Stončius, D.; Skuja, S.; Drobelis, E.; Šablevičius, B.; Rumbutis, S.; Dementavičius, D.; Naruŝevičius, V.; Petraŝka, A.; et al. At the border of ecological change: Status and nest sites of the Lithuanian Black Stork Ciconia nigra population 2000–2006 versus 1976–1992. J. Ornithol. 2008, 149, 75–81. [Google Scholar] [CrossRef]
- Rosenvald, R.; Lõhmus, A. Nesting of the black stork (Ciconia nigra) and white-tailed eagle (Haliaeetus albicilla) in relation to forest management. For. Ecol. Manag. 2003, 185, 217–223. [Google Scholar] [CrossRef]
- Lõhmus, A.; Sellis, U.; Rosenvald, R. Have recent changes in forest structure reduced the Estonian black stork Ciconia nigra population? Biodivers. Conserv. 2005, 14, 1421–1432. [Google Scholar] [CrossRef]
- Strazds, M. Conservation status of the Black stork in Europe and in the World. Aves 2003, 40, 12–13. [Google Scholar]
- Chevallier, D.; Le Maho, Y.; Brossault, P.; Baillon, F.; Massemin, S. The use of stopover sites by Black Storks (Ciconia nigra) migrating between West Europe and West Africa as revealed by satellite telemetry. J. Ornithol. 2011, 152, 1–13. [Google Scholar] [CrossRef]
- Pugacewicz, E. Stan populacji bociana czarnego (Ciconia nigra) na Nizinie Północnopodlaskiej w latach 1985–1994. Notatki Ornitologiczne 1994, 35, 297–308. [Google Scholar]
- Sikora, A.; Rohde, Z.; Gromadzki, M.; Neubauer, G.; Chylarecki, P. Bocian czarny Ciconia nigra. In Atlas Rozmieszczenia Ptaków Lęgowych Polski 1985–2004; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2007. [Google Scholar]
- Zieliński, P.; Stopczyński, M.; Janic, B.; Gapys, A.; Bańbura, J. Czy ochrona strefowa miejsc gniazdowych bociana czarnego Ciconia nigra jest wystarczająca? Studia i Materiały CEPL w Rogowie 2011, 27, 49–56. [Google Scholar]
- Newton, I. Population Limitation in Birds; Academic Press Inc.: Londyn, UK, 1998. [Google Scholar]
- Bettinger, P.; Boston, K.; Kim, Y.H.; Zhu, J. Landscape-level optimization using tabu search and stand density-related forest management prescriptions. Eur. J. Oper. Res. 2007, 176, 1265–1282. [Google Scholar] [CrossRef]
- McComb, W.C.; McGrath, M.T.; Spies, T.A.; Vesely, D. Models for mapping potential habitat at landscape scales: An example using northern spotted owls. For. Sci. 2002, 48, 203–216. [Google Scholar] [CrossRef]
- Aberg, J.; Swenson, J.; Per, A. The habitat requirements of hazel grouse (Bonasa bonasia) in managed boreal forest and applicability of forest stand descriptions as a tool to identify suitable patches. For. Ecol. Manag. 2003, 175, 437–444. [Google Scholar] [CrossRef]
- Suorsa, P.; Huhta, E.; Jäntti, A.; Nikula, A.; Helle, H.i.; Kuitunen, M.; Koivunen, V.; Hakkarainen, H. Thresholds in selection of breeding habitat by the Eurasian treecreeper (Certhia familiaris). Biol. Conserv. 2005, 121, 443–452. [Google Scholar] [CrossRef]
- Loehle, C.; Wigley, T.B.; Rutzmoser, S.; Gerwin, J.A.; Keyser, P.D.; Lancia, R.A.; Reynolds, C.J.; Thill, R.E.; Weih, R.; White, D.; et al. Managed forest landscape structure and avian species richness in the southeastern US. For. Ecol. Manag. 2005, 214, 279–293. [Google Scholar] [CrossRef]
- Loehle, C.; van Deusen, P.; Wigley, T.B.; Mitchell, M.S.; Rutzmoser, S.H.; Aggett, J.; Beebe, J.A.; Smith, M.L. A method for landscape analysis of forestry guidelines using bird habitat models and the Habplan harvest scheduler. For. Ecol. Manag. 2006, 232, 56–67. [Google Scholar] [CrossRef]
- Mitchell, M.S.; Reynolds-Hogland, M.J.; Smith, M.L.; Wood, P.B.; Beebe, J.A.; Keyser, P.D.; Loehle, C.; Reynolds, C.J.; van Deusen, P.; White, D., Jr. Projected long-term response of Southeastern birds to forest management. For. Ecol. Manag. 2008, 256, 1884–1896. [Google Scholar] [CrossRef]
- Marušák, R.; Kašpar, J. Spatially-constrained harvest scheduling with respect to environmental requirements and silvicultural system. For. J. 2015, 61, 71–77. [Google Scholar] [CrossRef]
- Vopěnka, P.; Kašpar, J.; Marušák, R. GIS tool for optimization of forest harvest-scheduling. Comput. Electron. Agric. 2015, 113, 254–259. [Google Scholar] [CrossRef]
- Kašpar, J.; Hlavatỳ, R.; Kuželka, K.; Marušák, R. The impact of assumed uncertainty on long-term decisions in forest spatial harvest scheduling as a part of sustainable development. Forests 2017, 8, 335. [Google Scholar] [CrossRef]
- Central Statistical Office (CSO). Forestry; Central Statistical Office: Warsaw, Poland, 2014.
- Wielkoobszarowa inwentaryzacja stanu lasu. Wyniki za okres 2009–2013; Biuro Urządzania Lasu i Geodezji Leśnej: Sękocin Stary, Poland, 2014. [Google Scholar]
- Krzyżanowski, A.; Zajączkowski, S.; Zielony, R. Struktura siedlisk leśnych w Polsce oraz kierunki zmian. Inżynieria Ekologiczna 2002, 6, 38–46. [Google Scholar]
- Zarządzenie nr 36 Dyrektora Generalnego Lasów Państwowych z dnia 19 maja 2004 r. w Sprawie zmian w Instrukcji Urządzania lasu; Załącznik nr 1: Warsaw, Poland, 2004.
- Wilk, T. Niepolomice Forest. In Important Bird Areas of International Importance in Poland; OTOP: Marki, Poland, 2010; pp. 409–411. [Google Scholar]
- Górniak, J. Prognoza Oddziaływania na Środowisko Projektu Planu Urządzenia lasu Nadleśnictwa Niepołomice na Okres Gospodarczy od 1 Stycznia 2012 r. do 31 Grudnia 2021 r.; Biuro Urządzania Lasu i Geodezji Leśnej Oddział w Krakowie: Kraków, Poland, 2011. [Google Scholar]
- Plan Urządzania Lasu (PUL). Nadleśnictwa Niepołomice na lata 2012–2021; Biuro Urządzania Lasu i Geodezji Leśnej: Kraków, Poland, 2012. [Google Scholar]
- Państwowe Gospodarstwo Leśne Lasy Państwowe. Zasady Hodowli Lasu (ZHL); ZILP: Warsaw, Poland, 2012. [Google Scholar]
- Państwowe Gospodarstwo Leśne Lasy Państwowe. Manual of Forest Management (IUL); ZILP: Warsaw, Poland, 2012. [Google Scholar]
- Jones, J. Habitat Selection Studies in Avian Ecology: A Critical Review. auk 2001, 118, 557–562. [Google Scholar] [CrossRef]
- Janssen, G.; Hormann, M.; Rohde, C. Der Schwarzstorch; Westarp Wissenschaften: Hohenwarsleben, Germany, 2004. [Google Scholar]
- Konovalov, A.; Kaldma, K.; Bokotey, A.; Brossault, P.; Chapalain, F.; Dmitrenok, M.; Dzyubenko, N.; Sellis, U.; Strazds, M.; Strenna, L.; et al. Spatio-temporal variation in nestling sex ratio among the Black Stork Ciconia nigra populations across Europe. J. Ornithol. 2015, 156, 381–387. [Google Scholar] [CrossRef]
- Murray, A.T.; Weintraub, A. Scale and unit specification influences in harvest scheduling with maximum area restrictions. For. Sci. 2002, 48, 779–789. [Google Scholar] [CrossRef]
- Yoshimoto, A.; Brodie, J.D. Comparative analysis of algorithms to generate adjacency constraints. Can. J. For. Res. 1994, 24, 1277–1288. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Malden/Oxford, UK, 2004. [Google Scholar]
- Lõhmus, A. Nest-tree and nest-stand characteristics of forest-dwelling raptors in east-central Estonia: implications for forest management and conservation. Proc. Estonian Acad. Biol. Ecol. 2006, 56, 31–50. [Google Scholar]
- Banaś, J.; Bujoczek, L.; Zięba, S.; Drozd, M. The effects of different types of management, functions, and characteristics of stands in Polish forests on the amount of coarse woody debris. European journal of forest research. Euro. J. For. Res. 2014, 133, 1095–1107. [Google Scholar] [CrossRef]
- Hagemeijer, W.J.M.; Blair, M.J. The EBCC Atlas of European Breeding Birds: Their Distribution and Abundance; Poyser: London, UK, 1997. [Google Scholar]
- Bobek, M.; Hampl, R.; Peške, L.; Pojer, F.; Šimek, J.; Bureš, S. African Odyssey project–satellite tracking of black storks Ciconia nigra breeding at a migratory divide. J. Avian Biol. 2008, 39, 500–506. [Google Scholar] [CrossRef]
- European Environment Agency. European Forest Ecosystems. State and Trends; EEA Report no 5; Publications Office of the European Union: Copenhagen, Denmark, 2016. [Google Scholar]
- Buczek, T. Ciconia nigra (L., 1758)–Bocian Czarny. In Ptaki (część 1). Poradniki Ochrony Siedlisk i Gatunków–Podręcznik Metodyczny; Gromadzki, M., Ed.; Ministerstwo Środowiska: Warszawa, Poland, 2004; pp. 81–85. [Google Scholar]
- Treinys, R.; Mozgeris, G.; Skuja, S. Can intensified forestry be responsible for changes in habitat usage by the forest-dwelling Black Stork? Eur. J. For. Res. 2016, 135, 1175–1186. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lacht, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Paillet, Y.; Archaux, F.; Du Puy, S.; Bouget, C.; Boulanger, V.; Debaive, N.; Gilg, O.; Gosselin, F.; Guilbert, E. The indicator side of tree microhabitats: A multi-taxon approach based on bats, birds and saproxylic beetles. J. Appl. Ecol. 2018, 55, 2147–2159. [Google Scholar] [CrossRef]
- Noss, R.F. Local priorities can be too parochial for biodiversity. Nature 2010, 463, 424. [Google Scholar] [CrossRef] [PubMed]
- Kangas, J.; Kangas, A. Multiple criteria decision support in forest management—The approach, methods applied, and experiences gained. For. Ecol. Manag. 2005, 207, 133–143. [Google Scholar] [CrossRef]
- Roberge, J.M.; Öhman, K.; Lämås, T.; Felton, A.; Ranius, T.; Lundmark, T.; Nordin, A. Modified forest rotation lengths: Long-term effects on landscape-scale habitat availability for specialized species. J. Environ. Manag. 2018, 210, 1–9. [Google Scholar] [CrossRef]
- Strazds, M. Micro-Reserves for the Black Stork in Latvia—An Important Measure for Habitat Conservation. In Schutzstrategien für Schwarzstorch und Rauchfußhühner; Naturschutzzentrum Vasserschloß Mitwitz: Mitwitz, Germany, 1993; pp. 39–45. [Google Scholar]
- Rozporządzenie Ministra Środowiska w Sprawie Ochrony Gatunkowej Zwierząt, Dz.U. 2016 poz. 2183, 16 Grudnia 2016; DZIENNIK USTAW: Warsaw, Poland, 2016.
- Zieliński, P.; Janic, B.; Kamiński, M.; Stopczyński, M.; Marszał, L.; Szpetmańska, H.; Bańbura, J. Wzrost liczebności i zagęszczenie bociana czarnego Ciconia nigra w Polsce środkowej. Chrońmy Przyrodę Ojczystą 2017, 72, 101–109. [Google Scholar]
- Olszewski, A.; Różycki, A.; Matusiak, J. Wybiórczość środowiskowa miejsc lęgowych oraz typy umiejscowienia gniazd bociana czarnego Ciconia nigra w Kampinoskim Parku Narodowym. Studia i Materiały CEPL w Rogowie 2017, 51, 169–185. [Google Scholar]
- Jactel, H.; Nicoll, B.C.; Branco, M.; Gonzalez-Olabarria, J.R.; Grodzki, W.; Långström, B.; Santos, H. The influences of forest stand management on biotic and abiotic risks of damage. Ann. For. Sci. 2009, 66, 701. [Google Scholar] [CrossRef]
- Mitchell, S.J. Wind as a natural disturbance agent in forests: a synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef]
- Forest Stewardship Council (FSC). Zasady, Kryteria i Wskaźniki Dobrej Gospodarki Leśnej w Polsce; Dokument Standardów w Obowiązujących w Certyfikacji Obszarów Leśnych w Systemie Forest Stewardship Council w Polsce; Forest Stewardship Council (FSC): Warsaw, Poland, 2012. [Google Scholar]
- Bujoczek, L.; Zięba, S.; Banaś, J. Ocena zasobów martwego drewna w lasach gospodarczych z uwzględnieniem typów siedliskowych lasu oraz bonitacji gatunku panującego. Sylwan 2016, 160, 320–327. [Google Scholar]
- Gutowski, J.M.; Bobiec, A.; Pawlaczyk, P.; Zub, K. Drugie Życie Drzewa; WWF: Warszawa-Hajnówka, Poland, 2004; ISBN 83-916021-6-8. [Google Scholar]
- Stokland, J.N.; Siitonen, J.; Jonsson, B.G. Biodiversity in Dead Wood; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Andersson, J.; Domingo Gomez, E.; Michon, S.; Roberge, J.M. Tree cavity densities and characteristics in managed and unmanaged Swedish boreal forest. Scand. J. For. Res. 2018, 33, 233–244. [Google Scholar] [CrossRef]
- Radu, S. The Ecological Role of Deadwood in Natural Forests. Nat. Conserv. 2006, 137–141. [Google Scholar] [CrossRef]
- Gábor, A.; Horváth, G.; Ortmann-né Ajkai, A.; Csicsek, G. Quantitative classification of macrohabitats for small mammals’ habitat segregation surveys in a forest reserve. Natura Somogyiensis 2015, 15, 123–134. [Google Scholar]
- Jancewicz, E.; Kielan, E. Importance of coarse woody debris in the functioning of small mammals populations. Sylwan 2017, 161, 519–528. [Google Scholar]
| Site Class (Gradient of Fertility) | Area (ha) | Species Composition 1 by Percent Area | |
|---|---|---|---|
| (Major Species) Share ≥ 5% | (Minor Species) Share < 5% | ||
| Oligotrophic | 4184.50 | SP 78, OK 6, | BI, EL, BE, AR, NS |
| Mesotrophic | 2934.10 | SP 56, OK 14, AR 13, BI 7, BE 6 | EL, HBM |
| Eutrophic | 2183.39 | OK 55, HBM 12, AR 11, AH 8, LI 6 | SP, BI, EL, BE, EM |
| Swamp | 338.69 | AR 79, AH 8, OK 6 | BI, SP, BE, NS |
| Total | 9640.68 | SP 51, OK 19, AR 11 | BI, BE, EL, HBM, AH, LI, NS, EM |
| Management Scenario | Site Class | Species Composition 1 (%) | Rotation Age (years) | Felling System/Regeneration Period (years) |
|---|---|---|---|---|
| Productive (PR) | Oligotrophic | SP 100 | 90 | Clearcut |
| Mesotrophic | SP 100 | 90 | Clearcut | |
| Eutrophic | OK 80, EM 20 | 120 | Shelterwood/10 | |
| Swamp | AR 100 | 70 | Clearcut | |
| Basis (BA) | Oligotrophic | SP 70, OK 30 | 100 | Shelterwood/10 |
| Mesotrophic | OK 40, SP 30, BE 30 | 140 | Shelterwood/20 | |
| Eutrophic | OK 60, BE 30, EM 10 | 140 | Shelterwood/20 | |
| Swamp | AR 100 | 80 | Clearcut | |
| Long rotation (LR) | Oligotrophic | SP 40, OK 30, EL 10, BI 10 | 110 | Shelterwood/10 |
| Mesotrophic | OK 40, SP 30, BE 10, LI 10, HBM 10 | 180 | Shelterwood/20 | |
| Eutrophic | OK 50, EM 20, BE 10, LI 10, HBM 10 | 180 | Shelterwood/30 | |
| Swamp | No interventions | |||
| Tree Age (years) | Species | DBH (cm) | Height (m) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | 95th Percentile | Mean | Standard Deviation | 95th Percentile | ||
| 81–90 | SP | 36.6 | 7.4 | 49.1 | 25.3 | 3.1 | 30.5 |
| OK | 37.2 | 11.2 | 53.3 | 24.0 | 4.1 | 29.0 | |
| 91–100 | SP | 39.3 | 7.3 | 52.1 | 25.7 | 2.9 | 31.0 |
| OK | 46.4 | 10.5 | 65.3 | 25.7 | 4.9 | 32.6 | |
| 101–110 | SP | 40.0 | 7.7 | 53.1 | 26.1 | 3.4 | 31.0 |
| OK | 50.8 | 12.1 | 71.1 | 27.4 | 3.7 | 33.0 | |
| 111–120 | SP | 42.1 | 8.0 | 55.3 | 25.7 | 3.0 | 31.0 |
| OK | 49.9 | 16.0 | 74.4 | 26.8 | 4.6 | 33.4 | |
| 121–140 * | SP | 44.3 | 8.5 | 57.6 | 26.8 | 4.0 | 33.6 |
| OK | 60.4 | 13.8 | 83.9 | 28.6 | 4.1 | 33.5 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaś, J.; Zięba, S.; Bujoczek, M.; Bujoczek, L. The Impact of Different Management Scenarios on the Availability of Potential Forest Habitats for Wildlife on a Landscape Level: The Case of the Black Stork Ciconia nigra (Linnaeus, 1758). Forests 2019, 10, 362. https://doi.org/10.3390/f10050362
Banaś J, Zięba S, Bujoczek M, Bujoczek L. The Impact of Different Management Scenarios on the Availability of Potential Forest Habitats for Wildlife on a Landscape Level: The Case of the Black Stork Ciconia nigra (Linnaeus, 1758). Forests. 2019; 10(5):362. https://doi.org/10.3390/f10050362
Chicago/Turabian StyleBanaś, Jan, Stanisław Zięba, Małgorzata Bujoczek, and Leszek Bujoczek. 2019. "The Impact of Different Management Scenarios on the Availability of Potential Forest Habitats for Wildlife on a Landscape Level: The Case of the Black Stork Ciconia nigra (Linnaeus, 1758)" Forests 10, no. 5: 362. https://doi.org/10.3390/f10050362
APA StyleBanaś, J., Zięba, S., Bujoczek, M., & Bujoczek, L. (2019). The Impact of Different Management Scenarios on the Availability of Potential Forest Habitats for Wildlife on a Landscape Level: The Case of the Black Stork Ciconia nigra (Linnaeus, 1758). Forests, 10(5), 362. https://doi.org/10.3390/f10050362





