Abstract
The aim of this study was to investigate the potential mixing effects on degradation of lignin and cellulose in mixed leaf litter from Pinus massoniana Lamb., Cupressus funebris Endl., and/or Quercus variabilis Bl., and elucidate the interactions with abiotic factors. The litter bag method was used in the field experiment, and the three predominant species in the Three Gorges Reservoir region were treated as single-, pair-, and tri-species combinations with equal proportions of litter mass. Lignin and cellulose losses in the litter treatments were measured, and the mixing effects were evaluated based on the sampling phase and decomposition period. At the end of the one-year decomposition period, mixing species increased lignin loss by 3.3% for the cypress + oak combination and cellulose loss by 3.9%, 1.8%, and 0.8% for the pine + oak, cypress + oak, and pine + cypress + oak combinations, respectively. The pine + oak and cypress + oak combinations exhibited greater lignin and cellulose loss than the tri-species mixture. Accelerated lignin degradation also apparently occurred in the pine + cypress combination as decomposition proceeded. Generalized linear models suggested that the investigated environmental factors (in terms of average temperature and cumulative precipitation) and changing litter quality (lignin, cellulose, and lignin/cellulose) had significant effects on nonadditive lignin loss, whereas only the changing litter quality factors significantly affected nonadditive cellulose loss. In summary, mixing two or three of the studied species alters cycling of recalcitrant substrates in plantations, and mixed planting with Quercus appears to strengthen both the lignin and cellulose degradation processes.
1. Introduction
As key components of forest ecosystems, tree leaf litter, deadwood, fine and coarse roots, as well as non-woody litter from understory vegetation such as herbs and shrubs, have a strong influence on the structures and material cycling of forest ecosystems [1]. However, leaf litter decomposition is a key ecological process in forest ecosystems [2], particularly in forest plantations, where nutrient cycling is one limitation to establishing sustainable plantation ecosystems [3] and leaf litter production accounted for a relatively high proportion of the total biomass of the ecosystem. As one of the most abundant carbon sources in plant litter, cellulose exerts considerable control on the decomposition process, and its degradation can dominate carbon fluxes as litter decomposition proceeds [4]. Moreover, lignin is a recalcitrant component of the litter substrate and thus can exert considerable control over the litter decomposition rate [5]. Therefore, thorough understanding of changes in lignin and cellulose in decomposing litter is indispensable for better understanding soil carbon and nutrient cycling.
According to studies of single-litter decomposition, the lignin and cellulose degradation processes in decomposing litter can be regulated by climate, litter quality, and decomposers in multiple forms and through many processes [6,7,8]. In general, a greater lignin degradation rate often occurs in the later stage of decomposition due to its recalcitrance as a substrate [9]. In addition, the cellulose degradation rate increases during the later phase of litter decomposition according to the hypotheses of Berg and McClaugherty [9] because the labile litter components in plant cell walls are surrounded by lignin. However, a debate has also arisen regarding lignin and cellulose loss. Recent studies declared that a greater loss rate can also be found during the early phase of decomposition due to microenvironmental factors, such as hydrology [10], snow cover [4,5], and solar radiation [11], which can influence the degradation process directly or indirectly. However, the effects of species mixing on lignin and cellulose degradation have seldom been reported to date.
Heterogeneity in litter composition on the forest floor can also interact with the microclimate to alter the decomposition environment, and in turn, influence lignin and cellulose degradation. Theoretically, mixed litters consisting of different individual species with different chemical and physical traits contribute to a heterogeneous microenvironment and food resources [12]. Accordingly, the physical abrasion or breakdown of litter is complex and involves diverse processes [13]. Scarce materials may be transferred from nutrient-rich litter to nutrient-poor litter by decomposers (i.e., via the fungal mycelial process) [14,15,16], thereby contributing to litter decomposition. It is therefore important to select species with readily decomposable litter to promote the decomposition of more recalcitrant species in litter mixtures [17,18]. In contrast, component species with specific substrates, such as tannins and polyphenols, may cause antagonism for decomposition [19,20,21]. However, little attention has been paid to lignin and cellulose degradation in leaf litter driven by species mixing under the scenario of mixed plantation development.
Masson pine (Pinus massoniana Lamb.), cypress (Cupressus funebris Endl.), and oak (Quercus variabilis Bl.) have been the main species of developed plantation forests in Three Gorges Reservoir area, China [22]. Forest managers practically implement mixed planting with two of the three species or with all three species to ensure sustainable development of plantations [23]. Relevant research in this area has documented the characteristics of the mass loss rate and nutrient release (i.e., carbon, nitrogen and phosphorus) from monospecific and mixed litters among these species [23,24,25]. These studies revealed slow decomposition of cypress and Masson pine litter because of their low litter quality and further declared that mixed afforestation of the three predominant tree species facilitated litter decomposition for each species. In particular, the ability of oak to improve the nutrient cycling associated with litter mixture decomposition makes it a preferential tree species for mixed planting with cypress and Masson pine. However, the simultaneous degradation process of recalcitrant materials has not been addressed to date, which may preclude generalizations. In this paper, we report the mixing effects on lignin and cellulose degradation based on a whole-year field litter decomposition experiment (published in Zeng et al. [25]) using the three species under seven treatments (with equal mass proportions of single-, pair-, and tri-species combinations of Masson pine, cypress, and oak litter). Given the superior characteristics of the litter quality of oak species, we hypothesized that oak species would facilitate lignin and cellulose degradation in the litter mixtures.
To test this hypothesis, the degradation rates of lignin and cellulose in litter treatments from the three predominant afforestation trees with wide variation in litter quality were measured every 60 days for one year. We focused on determining (1) whether effects of tree mixing on the loss of lignin and cellulose existed during decomposition and, if they existed, (2) how the degree of changing litter quality and environmental hydrothermal dynamics influenced the mixing effect on lignin and cellulose loss. The results will be valuable and will provide theoretical suggestions for plantation management of multiple tree species with regard to degradation of recalcitrant materials in the Three Gorges Reservoir area of China.
2. Materials and Methods
2.1. Study Site and Experimental Design
The field litter bag experiment was carried out in a representative Masson pine (Pinus massoniana) forest in the Three Gorges Reservoir area of China (in Zigui County, 110°00′–111°18′ E, 30°38′–31°11′ N, 362 m a.s.l. (above sea level)). The annual average temperature is 18 °C, with maximum and minimum temperatures of 44 °C and −2.5 °C, respectively. The annual sunshine time is 1620 h; the relative air humidity is 77%; and the number of frost-free days ranges from 300 to 340. Approximately 75% (1000 to 1025 mm) of annual precipitation occurs from April to September. Local trees are dominated by Pinus massoniana Lamb., Cupressus funebris Endl. and Quercus variabilis Bl.. Cunninghamia lanceolata (Lamb.) Hook. and Pinus tabuliformis Carr. are also common. The understory shrubs are dominated by Camellia oleifera Abel., Loropetalum chinense var. rubrum Yieh, Viburnum erosum Thunb., Echinochloa crusgalli (L.) Beauv., Dicranopteris linearis (Burm.) Underw., and other species. Soils are dominated by yellow and/or brown soil. The aim of this study was to assess the potential nonadditive effects of tree species mixing on litter lignin and cellulose degradation during decomposition. The full descriptive details of the study site were published in Zeng et al. [25], and the experiments were performed in three replicate plots (5 × 5 m) that were located at least 200 m from one another within the Masson pine forest (Figure 1).

Figure 1.
Study region in the Three Gorges Reservoir area of China and the experimental layout showing the litter bag distribution in the selected Masson pine (Pinus massoniana Lamb.) forest.
2.2. Litter Processing
Newly shed leaves from Masson pine (Pinus massoniana), cypress (Cupressus funebris), and oak (Quercus variabilis) were collected from the floor of the natural forest in November 2014 and air-dried for two weeks at room temperature. Then, 15.0 g of air-dried litter samples for each treatment were placed in prepared nylon litterbags (15 cm × 15 cm, 1 mm mesh size) and sealed. The treatment combinations were as follows: Individual litter bags for Masson pine, cypress, and oak; pairwise combinations of the three species (i.e., pine + cypress, pine + oak, and cypress + oak, 7.5 g for each component species); and a combination of all three species (pine + cypress + oak, 5 g for each component species). In addition, we weighed three replicates of 10-g air-dried samples for each species, oven-dried the samples at 65 °C for 48 h to calculate the moisture correction factor and then analyzed their initial elemental concentrations (Table S1).
To avoid the “home-field” advantage [26,27] and differences in the initial microbial compositions, we collected topsoil from the 0–10 cm layer of the three natural forests from which the litters were collected. After the coarse materials were removed, these soils were mixed, sieved (2-mm sieve), and used to fill plastic pots (with holes at bottom) that were 15 cm (diameter) × 20 cm (height). The basic soil properties were as follows: pH, 4.65 ± 0.02; organic C (carbon), 22.42 ± 0.20 g kg−1; total N (nitrogen), 0.75 ± 0.01 g kg−1; total P (phosphorus), 0.25 ± 0.01 g kg−1; and available P, 5.00 ± 0.20 mg kg−1. On 6 December 2014, a total of 378 plastic pots with litterbags (7 litter treatments × 3 plots × 3 replicates × 6 sampling events) were randomly placed in the established experimental plots (prepared litter bags containing air-dried litter were randomly placed on the topsoil in plastic pots at one bag per pot). To exclude litterfall from the standing forest, we placed nylon gauze over the experimental plots (a detailed report can be found in the Zeng et al. [25]).
2.3. Sampling and Microenvironmental Measurements
To quantify the temporal mixture effects and the relationships between the changing litter substrates and microenvironmental factors, we sampled the litterbags six times at 60-day intervals during the year-long incubation. Temperature and precipitation data were collected from local weather station (Figure 2).
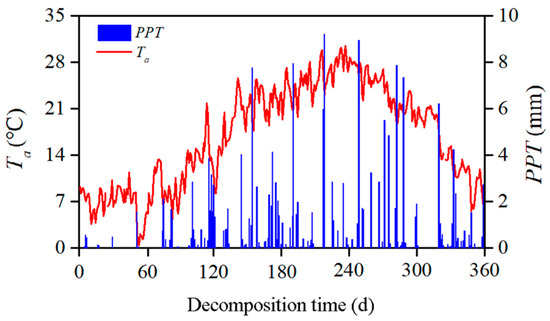
Figure 2.
Dynamics of the daily average atmospheric temperature (Ta, red line) and precipitation (PPT, blue column) from 6 December 2014, to 30 November 2015 (totaling 360 days of exposure, the data are from the local weather station located in Zigui County). The x-axis shows the decomposition time in days, with the time of litterbag placement as day 0.
2.4. Analyses and Calculations
After removal of foreign materials from the litterbags, the retrieved litter was oven dried at 65 °C to a constant mass and weighed. The oven-dried samples were ground in a mill with a 0.3-mm mesh screen to measure the lignin and cellulose concentrations. The acid detergent lignin and cellulose concentrations in the powdered leaf litter from both the initial and retrieved samples were determined according to the acid detergent lignin method [28], with some modifications (the detailed modification steps can be found in our previous study by He et al. [5]).
To characterize the dynamics of environmental factors, we calculated the average temperature (AT) and cumulative precipitation from the daily mean temperatures and precipitation (Table S2) for each phase (defined as the duration between the current and previous sampling dates) [4]. The loss of lignin and cellulose (L) in each phase during decomposition was calculated as
where C(t−1) and Ct represent the lignin or cellulose content on the current and previous sampling dates, respectively, and Ct0 is the initial lignin or cellulose content. The cumulative lignin or cellulose loss with the decomposition time was the sum of the corresponding values in the different phases [25].
To assess nonadditive effects, the expected loss (EL, calculated from loss characteristic of component for single-species) of the litter mixtures was calculated as the following [29]:
where N is the number of species in the mixture and Lnt is the measured lignin or cellulose loss in the bag containing only the nth species.
2.5. Statistical Analyses
An independent t-test with an alpha level of 0.05 was used to determine the difference between the observed and expected values for loss of lignin and cellulose. Mixing effects were classified as nonadditive effects when significant difference occurred between the observed and expected values, otherwise the additive effect was determined (when there is no significant difference between the two kind of values) [30]. In the current study, an observed value greater than the expected value represented a positive nonadditive effect (synergistic effect), whereas an observed value less than the expected value represented a negative nonadditive effect (antagonistic effect).
Detailed post hoc mean comparisons of significant differences in the litter variables among treatments were performed using Tukey’s HSD after the general ANOVA hypothesis was verified. The sampling time and a nested factor of litter mixture treatments served as fixed factors and were input into the univariate ANOVA, and a nested model of general linear models was used to examine the effects of the two factors on the loss of lignin and cellulose over time. In addition, a univariate regression analysis was performed with dynamics of chemical traits or abiotic factors as predictor variables and lignin or cellulose loss in different phases as a response variable. A stepwise linear regression analysis was conducted to examine the main factors (changing chemical traits or abiotic factors) that influenced the nonadditive loss of lignin and cellulose, and the best general linear model was selected according to the lowest Akaike information criterion (AIC) value [31]. The statistical Product and Service Solutions program (SPSS 21.0 for Windows; SPSS Inc., Chicago, IL, USA) and R (R Development Core Team, 2015, http://www.R-project.org/) were used to perform all analyses.
3. Results
3.1. Effects on the Lignin and Cellulose Concentrations
The lignin concentrations tended to increase during the year-long incubation regardless of the litter treatment (Figure 3a,c). Conversely, the cellulose concentrations decreased during the first 60 days in all litter treatments and then exhibited a minor increase as decomposition proceeded (Figure 3b,d). However, for all mixtures, the lignin and cellulose concentrations were not lower than the lowest values or higher than the highest values for the treatments of single-species litter.
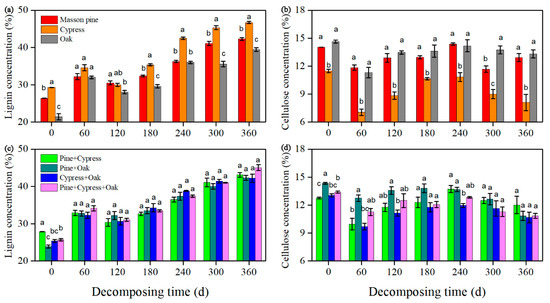
Figure 3.
Exponential regression of the lignin concentration (a,c) and cellulose concentration (b,d) for the treatments (Pine + Cypress, Pinus massoniana + Cupressus funebris; Pine + Oak, Pinus massoniana + Quercus variabilis; and so on) against the decomposition time (mean ± SE, n = 3).
3.2. Effects on Lignin and Cellulose Loss
At the end of the 360 day decomposition period, the loss of lignin and cellulose from all treatments ranged from 16.0% to 34.5% and from 51.8% to 70.1%, respectively (Figure 4a–d). Compared with the highest loss values from the single treatments (oak litter), the mixing effect increased lignin loss by 3.3% for the cypress + oak combination and cellulose loss by 3.9%, 1.8%, and 0.8% for the pine + oak, cypress + oak, and pine + cypress + oak combinations, respectively.
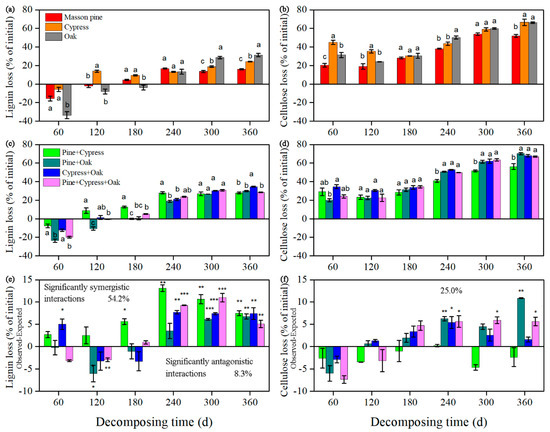
Figure 4.
Lignin loss (a,c) and cellulose loss (b,d) for monospecific and mixed leaf litter of Masson pine (Pinus massoniana), cypress (Cupressus funebris), and oak (Quercus variabilis) during decomposition (mean ± SE, n = 3). Treatments: Pine + Cypress, Pinus massoniana + Cupressus funebris; Pine + Oak, Pinus massoniana + Quercus variabilis; and so on. Two-way ANOVA (the litter mixture treatment was treated as a nested factor) indicates significant effects of the litter mixture treatment (F(6,84) = 29.060 to 44.549, p < 0.001) and decomposition time (F(35,84) = 75.017 to 134.210, p < 0.001) on the lignin and cellulose loss rates. Differences between the observed and expected values for given variables are shown in (e) and (f), where a positive value indicates an observed value > the expected value and vice versa. Statistical significance level for comparisons between the observed and expected values: ***, p < 0.001, **, p < 0.01 and *, p < 0.05. Inset values indicate the proportion of the corresponding significant interactions (synergistic and antagonistic interactions lie above and below the zero line, respectively) for a total of 24 cases.
Over the course of decomposition, negative values for lignin loss (accumulation) were observed in all treatments in the early phase of this study, although a loss pattern was shown for the entire year. In contrast, cellulose showed a relatively consistent loss pattern. Lignin was significantly lost from oak litter for the entire year and after 300 days of incubation (F = 45.0 to 48.0, p < 0.001), but showed a greater loss from Masson pine or cypress litter before 300 days of incubation. A similar tendency was detected for cellulose loss, whereas the largest values in oak litter were found after 180 days (F = 5.4 to 16.2, p < 0.05). Compared with the other mixtures, the pine + cypress combination had considerable lignin loss that continued to 240 days of incubation (F = 13.6 to 32.5, p < 0.01) and then displayed the lowest value among the mixtures (F = 4.2 to 19.8, p < 0.05). Moreover, the lignin loss in the pine + oak or cypress + oak combination was greater than that in the pine + cypress + oak combination at the end of the study. Similarly, the cellulose loss was mostly lowest in the pine + cypress combination (F = 5.7 to 41.3, p < 0.05), whereas the losses in the pine + oak and cypress + oak combinations were greater than those in the pine + cypress + oak combination after incubation for 120 days. In addition, lignin loss in the pine + cypress combination was higher than that in the litter of either of the two single species as decomposition proceeded.
A comparison of decomposition among the different phases showed that the greatest lignin accumulation and cellulose loss occurred during the first 60 days of incubation regardless of the treatment. Apparent losses of both lignin and cellulose also occurred from 181–240 days. Across the entire experimental period, lignin and cellulose losses from the litter of both the single species and the mixtures were significantly correlated with changes in the litter quality based on lignin, cellulose, lignin/cellulose, and environmental factors, such as the average temperature and cumulative precipitation (except for the effect of average temperature on cellulose loss) (Table 1).

Table 1.
Regression analyses of changing chemical traits and environmental factors and loss of lignin and cellulose in litter (%, per phase) of the single-species treatments (A) and mixtures (B).
3.3. Synergism and Antagonism in the Litter Mixtures
For all tested mixtures (over the incubation time), an immediate positive nonadditive effect (significant synergistic interaction) on lignin loss was found for the cypress+ oak combination during the first 60 days of incubation (Figure 4e). In addition, positive nonadditive effects on lignin loss regardless of the species treatments were found over the later decomposition phases and accounted for 54.2% (13 of 24 cases) of the cases. Only two cases of antagonistic interactions were detected during the first 120 days. A quarter of all cases representing positive nonadditive effects on cellulose loss were found after 240 days of decomposition (Figure 4f).
In all tested mixtures (with six sampled phases), a decrease in the incidence of nonadditive effects was detected for both lignin and cellulose loss as compared to incubation time (Figure 5). Significant synergistic and antagonistic interactions accounted for 29.2% and 12.5% of all cases of lignin loss, whereas the corresponding values for cellulose loss were 8.3% and 4.2%, respectively (seven and two of 24 cases and two and one of 24 cases, respectively). Path analysis revealed that the mixing effects on lignin loss were strongly correlated with environmental factors (average temperature and cumulative precipitation) and changing litter quality in terms of lignin, cellulose, and lignin/cellulose, whereas the effects on cellulose loss were only strongly correlated with the changing litter quality (Table 2; Figure 6).
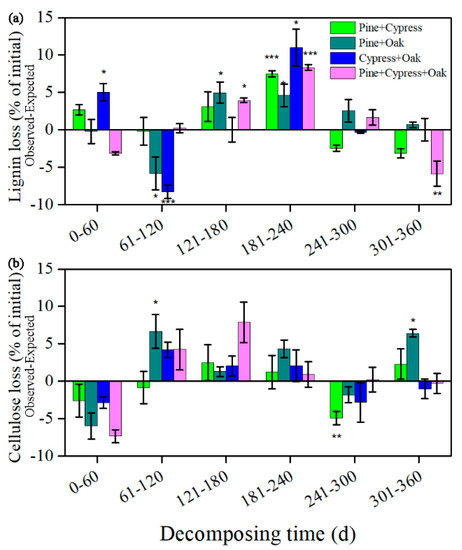
Figure 5.
Mixing effects of treatments on lignin (a) and cellulose (b) losses during the different decomposition phases. Values are the mean ± SE (n = 3). Treatments: Pine + Cypress, Pinus massoniana + Cupressus funebris; Pine + Oak, Pinus massoniana + Quercus variabilis; and so on. A positive value indicates an observed value > the expected value, and vice versa. Statistical significance level: ***, p < 0.001; **, p < 0.01; *, p < 0.05. Inset values indicate the proportion of the corresponding significant interactions (synergistic and antagonistic interactions lie above and below the zero line, respectively) for a total of 24 cases. Treatments: P. + C., P. massoniana + C. funebris; P. + Q., P. massoniana + Q. variabilis; and so on.

Table 2.
Summary of the generalized linear models for the effects of changing litter quality and environmental factors on the nonadditive loss of lignin and cellulose.
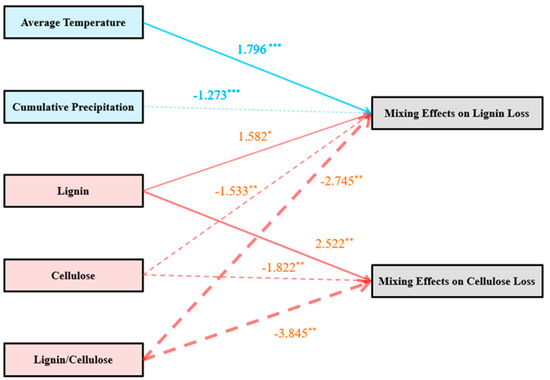
Figure 6.
Path diagram showing the relationships among changing chemical traits and environmental factors and the mixing effects on lignin and cellulose loss. Line widths indicate the relative strengths of the paths. Solid and broken lines represent positive and negative effects, respectively. Significance: * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
Assessing decomposition process of tree leaf litter is crucial for understanding the ecological process of nutrient cycling in forest ecosystems where leaf litter production accounts for a large proportion of the biomass relative to other litter pools, such as deadwood, roots, and non-wood litter of the ecosystem. Litter decomposition is a complex and successive process, and changes in the component chemical substrates are highly diverse and can significantly influence the subsequent decomposition rate [9]. In our study, the lignin concentration showed a consistent increase during the one-year experiment (Figure 3a,c), which was consistent with the classical theory that lignin was a recalcitrant material in litter compared to other substrates [32]. The obvious increasing lignin concentration in the litter during decomposition is due to other labile materials, which have relatively rapid loss rates. More importantly, regardless of the use of monospecific or mixed litters, we found that lignin accumulation occurred during the course of litter decomposition when the lignin concentration was lower than 30% (Figure 3a,c and Figure 4a,c). This finding coincided with the mechanisms proposed by McClaugherty and Berg [33], who declared that a decrease in the absolute lignin content occurred for litter species with a lignin concentration greater than 30%. The results further suggested that the potential nonadditive direction (accumulation or loss) for lignin dynamics during degradation was also regulated by the litter quality in the mixture. Moreover, more cellulose than lignin was lost in the litter during decomposition, although the fluctuation in the cellulose concentration was minor compared to that in the lignin concentration (Figure 3b,d). Reports indicated that a rich microbial community may be supported by the availability of soluble materials in leaf litter during the early phase of litter decomposition [34], which further facilitates the degradation process. As a result, the greatest lignin accumulation and cellulose loss were both detected during the first 60 days of decomposition.
In our study, we observed a greater loss of both lignin and cellulose in oak litter as decomposition proceeded (Figure 4a,b). Litter quality has long been considered one of the most important factors influencing the decomposition rate in a given ecosystem [35,36,37,38]. This finding is consistent with the current consensus that oak, with its superior litter quality characteristics, can display a rapid decomposition rate [25]. However, the hypothesis that oak species can accelerate lignin and cellulose degradation in litter mixtures due to its superior decay characteristics was only partly supported by our results. We only observed increased lignin loss in the cypress + oak combination and increased cellulose loss in the pine + oak, cypress + oak, and pine + cypress + oak combinations at the end of the study (Figure 4c,d). The underlying mechanism of the nonadditive effect in litter mixtures contributes to the changes in nutrient availability and the total litter surface physical characteristics [39]. The superior chemical and physical diversities disappear as decomposition proceeds due to loss of labile components and destruction of the litter shape during early rapid decomposition [9]. From this point, nonadditive interactions may occur for the pine + cypress combination for both lignin and cellulose loss and the pine + oak and pine + cypress + oak combinations for lignin degradation during the subsequent stage of decomposition [15,29]. In addition, although the strategy of placing litter bags in the plastic pots could control the influence of species interactions on mixing effects well, limitation on the exchange of soil moisture may occur, which may influence the decomposition process [9]. However, our results clearly suggest that mixed planting with oak in the Three Gorges Reservoir area can stimulate lignin and cellulose degradation in mixed plantations during the first-year incubation.
Interestingly, we detected more lignin and cellulose loss in the pine + oak and cypress + oak combinations than in the pine + cypress + oak combination (Figure 4c,d). A number of studies indicated that the species composition regulates the mixing effects in mixed litter [12,40], whereas others hold that species richness exerts a significant influence on the nonadditive effects [41,42]. Based on the extent of admixture of oak litter, our results further proved that the species composition had a greater effect on the loss of litter lignin and cellulose in mixed plantations of the three predominant species in the Three Gorges Reservoir area of China. In addition, during the incubation period, nonadditive effect can occur in one of the three two-species litter mixtures as long as it occurred in the three-species combination at the same incubation time, while the opposite may not occur (Figure 4b,d). As a result, magnitude of nonadditive loss in mixed litter decomposition appears to rely more on the species composition than on the species richness. The superior characteristic of abundant soluble materials in mixed litter associated with reasonable biotic activities can explain the underlying mechanism [16,43]. Furthermore, although Norby and Kozlowski [44] and Kil and Yim [45] argued that coniferous litters released more allelopathic compounds, we observed accelerated lignin loss in the mixture of two coniferous species. A previous theory declared that mixing litter could reduce the relative content of allelopathic compounds per unit mass of mixed litter [46], which might result in an accelerated decomposition process. Our findings supported the finding of a “dilution effect” for lignin degradation in the Three Gorges Reservoir area of China from the two coniferous afforestation species.
Previous studies also suggested that the decomposition time or duration strongly influenced the effects of biodiversity on biogeochemical cycling [12,15,47]. In our study, the incidence of nonadditive effects on both lignin and cellulose loss decreased when we compared the decomposition phase to decomposition over time (Figure 4e,f and Figure 5). The decreased incidence of nonadditive effects on lignin and cellulose loss during the decomposition phases indicated that the relative difficulty in degrading these materials induced less frequent facilitative and inhibitory mechanisms over the short course of decomposition. In line with previous hypotheses that a group of complex and recalcitrant materials could serve as a structural barrier in plant cell walls and impede the release of labile components from litter [11], the path analysis in the present experiment revealed that the dynamics of the litter lignin concentration could significantly affect nonadditive cellulose loss (Table 2; Figure 6). This result also supports the consensus that the incubation time is an important factor for litter lignin and cellulose degradation, which can further influence the nonadditive degradation process. In addition to the changing litter quality, microenvironmental factors have long been recognized as a major driver of litter decomposition because they can vary significantly over time [35,48]. However, the current study showed that the investigated environmental factors (average temperature and cumulative precipitation) only obviously affected the mixing effect on lignin degradation (Table 2; Figure 6). A previous study revealed that the photodegradation process of lignin was a pivotal pathway for lignin degradation in litter [11]. The current study further suggests that the abiotic factor of temperature, which is an index accompanied by the solar radiation intensity, quite likely plays a major role in photodegradative losses of lignin, and thus, profoundly influences nonadditive lignin loss during mixture decomposition. In contrast, because temperature and moisture are essential to the leaf litter decomposition process [48], the finding of an opposite trend in the interactions affecting cellulose loss (no significant influence of environmental factors on nonadditive cellulose degradation, Table 2; Figure 6) might be due to interactions among other litter traits or species-species interactions of the component single species that masked the synergistic and antagonistic processes and showed net additive or nonadditive cellulose losses [19]. Thus, no apparent relationship was exhibited between the investigated environmental factors and nonadditive cellulose loss in the current study.
5. Conclusions
Assessing lignin and cellulose degradation dynamics during litter mixture decomposition associated with predominant afforestation trees furthers our understanding and provides important information for scientific management of recalcitrant substrates in these plantation ecosystems. This information is also helpful for the selection of tree species with regards to recalcitrant materials cycling to establish sustainable plantation ecosystems. The results obtained in this in situ experiment in the Three Gorges Reservoir area clearly showed that mixing litter from Masson pine, cypress and oak trees could alter the dynamics of lignin and cellulose loss and induce nonadditive effects as decomposition proceeded. Our results confirmed the nonadditive lignin loss in the cypress + oak combination and nonadditive cellulose loss in the pine + oak, cypress + oak, and pine + cypress + oak combinations. These results indicated that litter quality was the key factor influencing nonadditive lignin and cellulose loss. Moreover, accelerated lignin degradation was found in the pine + cypress combination, and the absolute lignin and cellulose losses in the pine + oak and cypress + oak combinations were greater than those in the pine + cypress + oak combination. These findings further suggest that the magnitude of nonadditive loss in mixed litter decomposition relies more on the species composition than on the species richness. Accordingly, broad-leaf species (such as Q. variabilis) apparently are preferential tree species that are suitable for mixed planting with coniferous species (such as P. massoniana and C. funebris in the study area) because of the superior characteristics of the litter quality, which likely contributes to lignin and cellulose degradation, thereby beneficial for the release of elements bound in litter. In addition, we confirmed the degree of effects of the investigated environmental factors and changing litter quality in terms of the average temperature, cumulative precipitation, lignin concentration, cellulose concentration and lignin/cellulose on nonadditive lignin and cellulose degradation. Fallaciously, the current study exhibited no relationship between the investigated environmental factors and nonadditive cellulose loss. Thus, species-species interactions or interactions among other litter traits may have masked the synergistic and antagonistic processes that induced the net abiotic affects. Further complementary work should consider decomposition dynamics directly on the mixed forest floor, the realistic mass ratio of natural litter components and the species-species interactions among the components in combination.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/10/4/360/s1, Table S1: Initial litter chemistry (mean ± SE, n = 3) in litter treatments, Table S2: Average Temperature (°C) and Cumulative Precipitation (mm) in each phase of decomposition.
Author Contributions
L.Z., P.W. and W.X. designed the study. W.H., Z.M., J.P., M.T., Z.Y., Z.H., Z.Z. and X.L. performed the research. L.Z. proposed the structure of the paper and W.H. wrote the paper.
Funding
This work was supported by Chinese Ministry of Science and Technology (2017YFD0600304), the Fundamental Research Funds for the Central Non-profit Research Institution of CAF (No. CAFYBB2014QB033), the National Natural Science Foundation of China (No. 31800518, 31870611 and 31400531), the Fundamental Research Funds for the Central Universities (No. 2662016QD023 and 2662018PY084), Hubei province key technology innovation project (2016ABA111) and the Hubei Provincial Natural Science Foundation of China (2018CFB374).
Acknowledgments
We are grateful to Danju Zhang for constructive comments for improving the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Zhu, J.X.; Hu, H.F.; Tao, S.L.; Chi, X.L.; Li, P.; Jiang, L.L.; Ji, C.J.; Zhu, J.L.; Tang, Z.Y.; Pan, Y.D.; et al. Carbon stocks and changes of dead organic matter in China’s forests. Nat. Commun. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Lemma, B.; Nilsson, I.; Kleja, D.B.; Olsson, M.; Knicker, H. Decomposition and substrate quality of leaf litters and fine roots from three exotic plantations and a native forest in the southwestern highlands of Ethiopia. Soil Biol. Biochem. 2007, 39, 2317–2328. [Google Scholar] [CrossRef]
- He, W.; Wu, F.Z.; Zhang, D.J.; Yang, W.Q.; Bo, T.; Zhao, Y.Y.; Wu, Q.Q. The effects of forest gaps on cellulose degradation in the foliar litter of two shrub species in an alpine fir forest. Plant Soil 2015, 393, 109–122. [Google Scholar] [CrossRef]
- He, W.; Wu, F.Z.; Yang, W.Q.; Tan, B.; Zhao, Y.Y.; Wu, Q.Q.; He, M. Lignin degradation in foliar litter of two shrub species from the gap center to the closed canopy in an alpine fir forest. Ecosystems 2016, 19, 115–128. [Google Scholar]
- Cox, P.; Wilkinson, S.P.; Anderson, J.M. Effects of fungal inocula on the decomposition of lignin and structural polysaccharides in Pinus sylvestris litter. Biol. Fert. Soils. 2001, 33, 246–251. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA, 1979. [Google Scholar]
- Wang, L.F.; Zhang, J.; He, R.L. Impacts of soil fauna on lignin and cellulose degradation in litter decomposition across an alpine forest-tundra ecotone. Eur. J. Soil. Biol. 2018, 87, 53–60. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant litter. Decomposition, Humus Formation, Carbon Sequestration, 3rd ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Yue, K.; Wu, F.Z.; Yang, W.Q.; Zhang, C.; Peng, Y.; Tan, B.; Xu, Z.F.; Huang, C.P. Cellulose dynamics during foliar litter decomposition in an alpine forest meta-ecosystem. Forests 2016, 7, 176. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballare´, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef]
- Wu, D.D.; Li, T.T.; Wan, S.Q. Time and litter species composition affect litter-mixing effects on decomposition rates. Plant Soil 2013, 371, 355–366. [Google Scholar] [CrossRef]
- Swan, C.M.; Healey, B.; Richardson, D.C. The role of native riparian tree species in decomposition of invasive tree of heaven (Ailanthus altissima) leaf litter in an urban stream. Ecoscience 2008, 15, 27–35. [Google Scholar] [CrossRef]
- Finziab, A.C.; Canhamb, C.D. Non-additive effects of litter mixtures on net N mineralization in a southern New England forest. For. Ecol. Manag. 1998, 105, 129–136. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, Y.G.; Guo, K.; Zhao, H.W.; Qiao, X.G.; Wang, S.J.; Zhang, L.; Cai, X.L. Mixing litter from deciduous and evergreen trees enhances decomposition in a subtropical karst forest in southwestern China. Soil Biol. Biochem. 2016, 101, 44–54. [Google Scholar] [CrossRef]
- Meier, C.L.; Bowman, W.D. Links between plant litter chemistry, species diversity, and below-ground ecosystem function. Proc. Natl. Acad. Sci. USA 2008, 105, 19780–19785. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of eucalyptus with nitrogen fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Forrester, D.I.; Pares, A.; O’Hara, C.; Khanna, P.K.; Bauhus, J. Soil organic carbon is increased in mixed-species plantations of eucalyptus and nitrogen-fixing acacia. Ecosystems 2013, 16, 123–132. [Google Scholar] [CrossRef]
- De Marco, A.; Meola, A.; Maisto, G.; Giordano, M.; De Santo, A.V. Non-additive effects of litter mixtures on decomposition of leaf litters in a Mediterranean maquis. Plant Soil 2011, 344, 305–317. [Google Scholar] [CrossRef]
- Harrison, A.F. The inhibitory effect of oak leaf litter tannins on the growth of fungi, in relation to litter decomposition. Soil Biol. Biochem. 1971, 3, 167–172. [Google Scholar] [CrossRef]
- Vivanco, L.; Austin, A.T. Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J. Ecol. 2008, 96, 727–736. [Google Scholar] [CrossRef]
- Department of Forest Resources Management, State Forestry Administration. The 7th national forest inventory and status of forest resources. For. Resour. Manag. 2010, 1, 1–8. (In Chinese) [Google Scholar]
- Ge, X.G.; Zeng, L.X.; Xiao, W.F.; Huang, Z.L.; Zhou, B.Z. Dynamic of leaf litter stoichiometric traits dynamic and its relations with decomposition rates under three forest types in Three Gorges Reservoir Area. Acta Ecol. Sinica 2015, 35, 779–787, (In Chinese, English abstract). [Google Scholar]
- Sun, X.; Kang, H.Z.; Du, H.M.; Hu, H.B.; Zhou, J.B.; Hou, J.l.; Zhou, X.; Liu, C.J. Stoichiometric traits of oriental oak (Quercus variabilis) acorns and their variations in relation to environmental variables across temperate to subtropical China. Ecol. Res. 2012, 27, 765–773. [Google Scholar] [CrossRef]
- Zeng, L.X.; He, W.; Teng, M.J.; Luo, X.; Yan, Z.G.; Huang, Z.L.; Zhou, Z.X.; Wang, P.C.; Xiao, W.F. Effects of mixed leaf litter from predominant afforestation tree species on decomposition rates in the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 639, 679–686. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Desrochers, A.; Baldy, V. Home field advantage of litter decomposition in pure and mixed plantations under boreal climate. Ecosystems 2015, 18, 1014–1028. [Google Scholar] [CrossRef]
- Vanderbilt, K.L.; White, C.S.; Hopkins, O.; Craig, J.A. Aboveground decomposition in arid environments: Results of a longterm study in central New Mexico. J. Arid. Environ. 2008, 72, 696–709. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Mao, B.; Zeng, D.H. Non-additive effects vary with the number of component residues and their mixing proportions during residue mixture decomposition: A microcosm study. Geoderma 2012, 170, 112–117. [Google Scholar] [CrossRef]
- Mu, J.P.; Yang, Y.L.; Luo, Y.L. Pollinator preference and pollen viability mediated by flower color synergistically determine seed set in an Alpine annual herb. Ecol. Evol. 2017, 7, 2947–2955. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parkinson, D.; Parsons, W.F. Nitrogen and lignin content as predictors of litter decay rates: A microcosm test. Ecology 1989, 70, 97–104. [Google Scholar] [CrossRef]
- McClaugherty, C.; Berg, B. Cellulose, lignin and nitrogen concentrations as rate regulating factors in late stages of forest litter decomposition. Pedobiologia 1987, 30, 101–112. [Google Scholar]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Aerts, R.; De Caluwe, H. Nutritional and plant-mediated controls on leaf litter decomposition of Carex species. Ecology 1997, 78, 244–260. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Wang, Y.J.; Müller-Schärer, H.; van Kleunen, M.; Cai, A.M.; Zhang, P.; Yan, R.; Dong, B.C.; Yu, F.H. Invasive alien plants benefit more from clonal integration in heterogeneous environments than natives. New phytol. 2017, 216, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Z.; Peng, C.H.; Yang, W.Q.; Zhang, J.; Han, Y.; Mao, T. Admixture of alder (Alnus formosana) litter can improve the decomposition of eucalyptus (Eucalyptus grandis) litter. Soil Biol. Biochem. 2014, 73, 115–121. [Google Scholar] [CrossRef]
- Lecerf, A.; Risnoveanu, G.; Popescu, C.; Gessner, M.O.; Chauvet, E. Decomposition of diverse litter mixtures in streams. Ecology 2007, 88, 219–227. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Keith, A.M.; Van Der Wal, R.; Brooker, R.W.; Osler, G.H.R.; Chapman, S.J.; Burslem, D.F.R.P.; Elston, D.A. Increasing litter species richness reduces variability in a terrestrial decomposer system. Ecology 2008, 89, 2657–2664. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Norby, R.J.; Kozlowski, T.T. Allelopathic potential of ground cover species on Pinus resinosa seedlings. Plant Soil 1980, 57, 363–374. [Google Scholar] [CrossRef]
- Kil, B.S.; Yim, Y.J. Allelopathic effects of Pinus densiflora on undergrowth of red pine forest. Plant Soil 1983, 9, 1135–1151. [Google Scholar]
- Barantal, S.; Roy, J.; Fromin, N.; Schimann, H.; Hättenschwiler, S. Long-termpresence of tree species but not chemical diversity affect litter mixture effects on decomposition in a neotropical rainforest. Oecologia 2011, 167, 241–252. [Google Scholar] [CrossRef]
- Lecerf, A.; Marie, G.; Kominoski, J.S.; LeRoy, C.J.; Bernadet, C.; Swan, C.M. Incubation time, functional litter diversity, and habitat characteristics predict litter-mixing effects on decomposition. Ecology 2011, 92, 160–169. [Google Scholar] [CrossRef]
- Coûteaux, M.M.; Bottner, P.; Berg, B. Litter decomposition, climate and litter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).