Soil Organic Carbon Content and Microbial Functional Diversity Were Lower in Monospecific Chinese Hickory Stands than in Natural Chinese Hickory–Broad-Leaved Mixed Forests
Abstract
1. Introduction
2. Materials and Methods
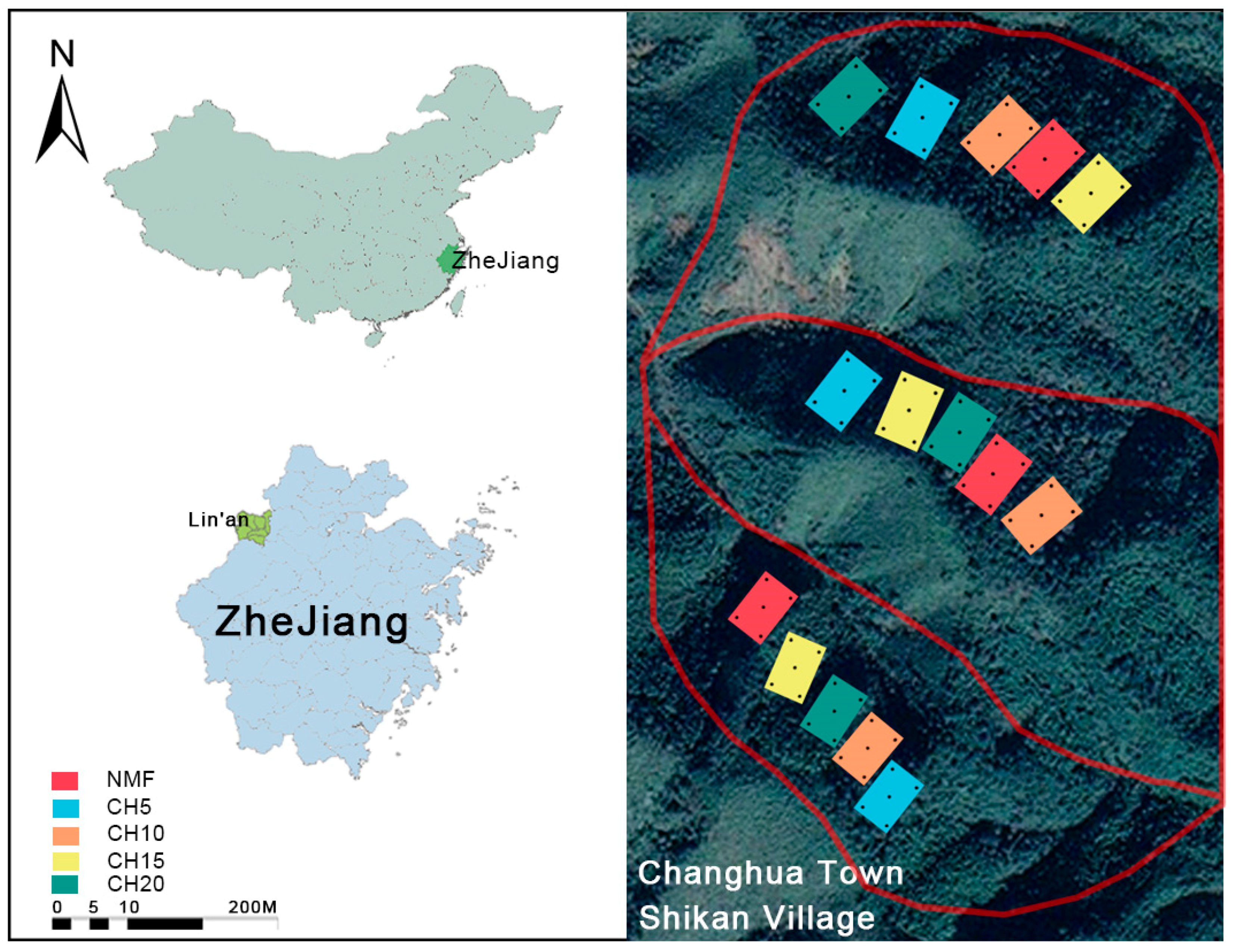
2.1. Research Area Overview
2.2. Experimental Design
2.3. Soil Analyses
2.4. Statistical Analyses
3. Results
3.1. Soil Organic Carbon Pool
3.2. Soil Organic Carbon Structure
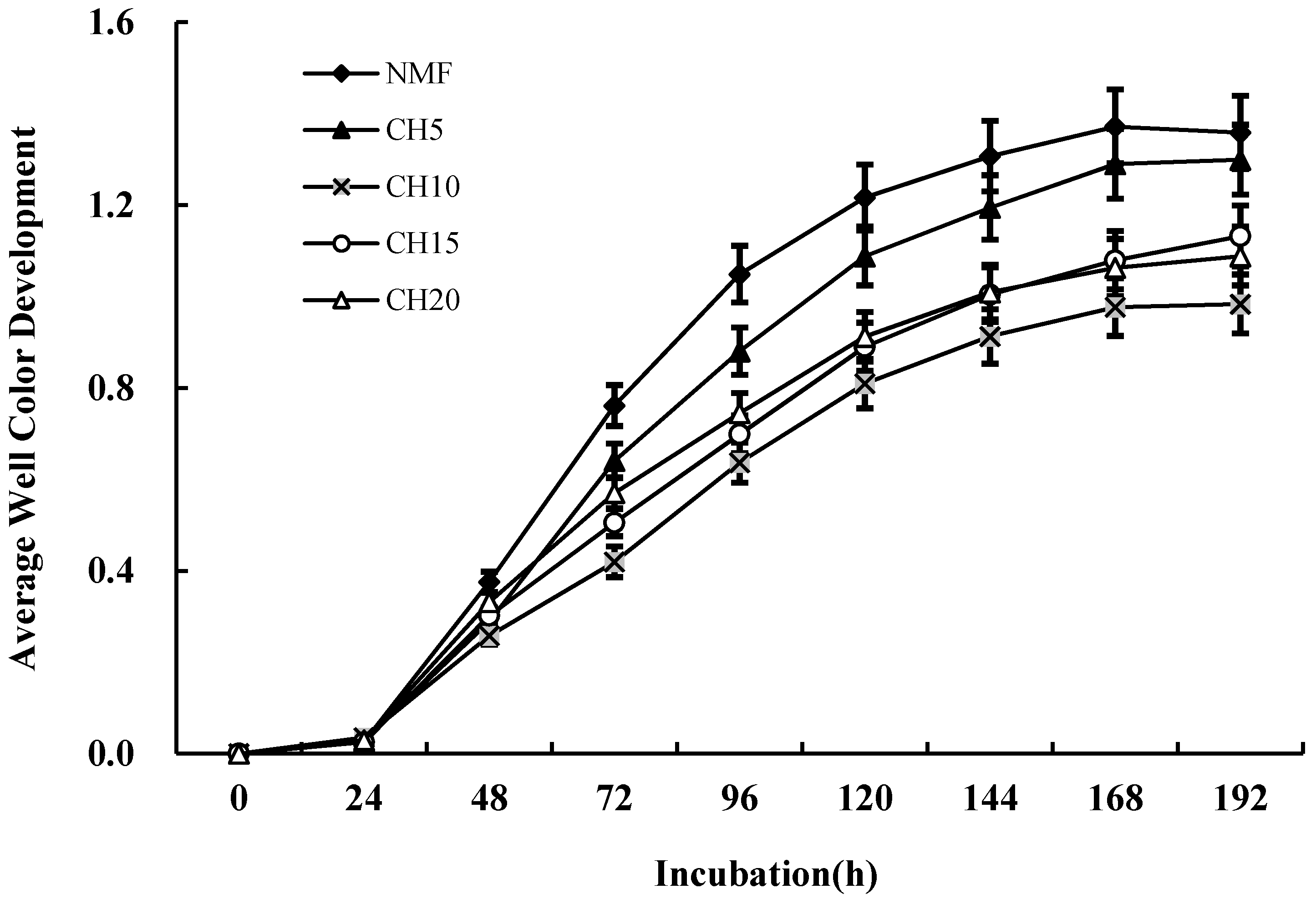
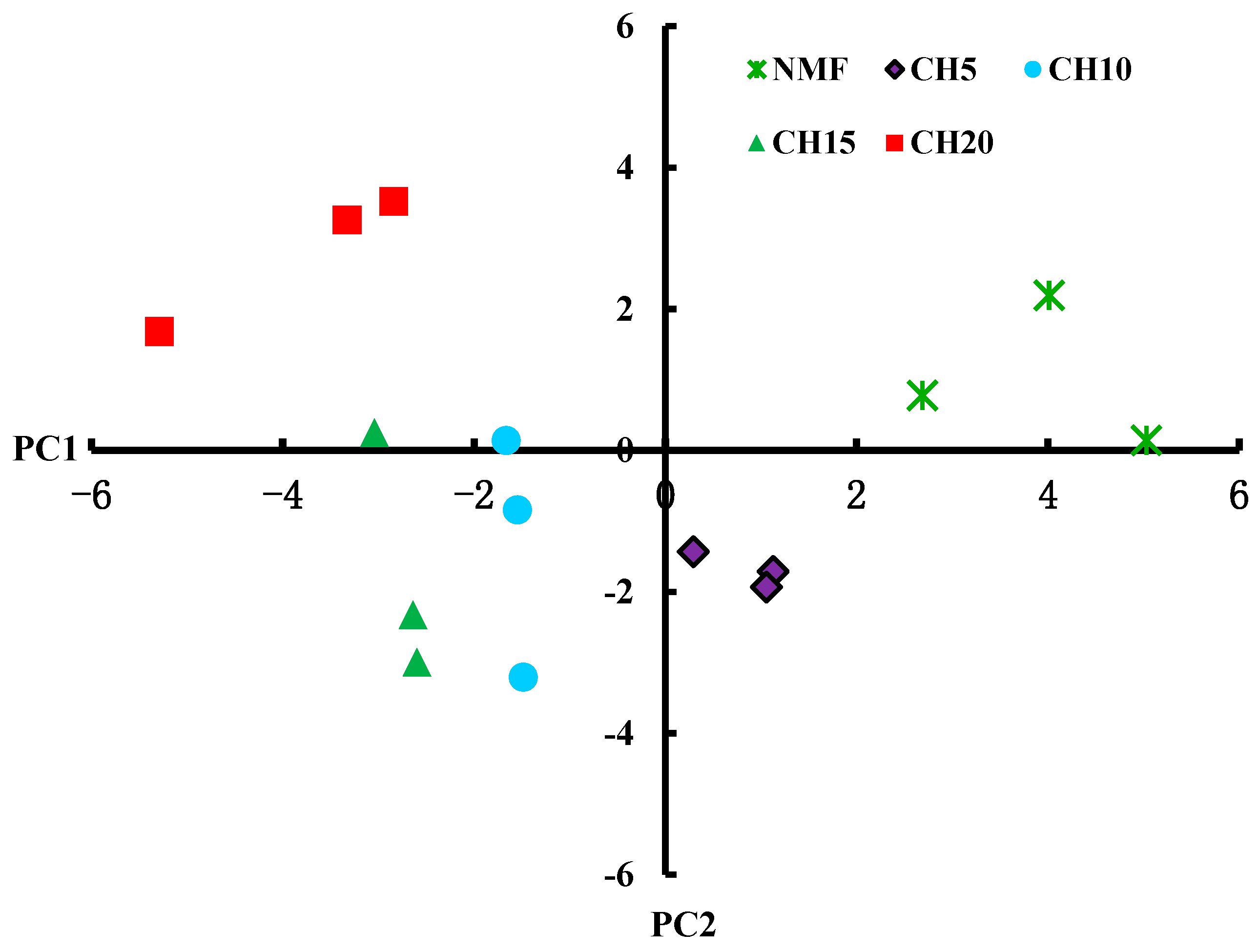
3.3. Differences in Microbial Carbon Source Usage
4. Discussion
4.1. Effects of Intensive Management on Soil Organic Carbon Pool in Chinese Hickory Stands
4.2. Effects of Intensive Management on Soil Microbial Functional Diversity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lal, R. Social values of soil carbon. J. Soil Water Conserv. 2014, 69, 186A–192A. [Google Scholar] [CrossRef]
- Dai, W.; Fu, W.; Jiang, P.; Zhao, K.; Li, Y.; Tao, J. Spatial pattern of carbon stocks in forest ecosystems of a typical subtropical region of southeastern China. For. Ecol. Manag. 2018, 409, 288–297. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Ed.; Soil Sci. Soc. Am Publication No. 35; SSSA: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar]
- Catoni, M.; D’Amico, M.E.; Zanini, E.; Bonifacio, E. Effect of pedogenic processes and formation factors on organic matter stabilization in alpine forest soils. Geoderma 2016, 263, 151–160. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, B.C.; Lin, J.Y. A basin-scale estimation of carbon stocks of a forest ecosystem characterized by spatial distribution and contributive features in the Liuxihe river basin of Pearl river delta. Forests 2016, 7, 299. [Google Scholar] [CrossRef]
- Guillaume, T.; Maranguit, D.; Murtilaksono, K.; Kuzyakov, Y. Sensitivity and resistance of soil fertility indicators to land-use changes: New concept and examples from conversion of Indonesian rainforest to plantations. Ecol. Indic. 2016, 67, 49–57. [Google Scholar] [CrossRef]
- Fu, W.J.; Zhao, K.L.; Zhang, C.S.; Wu, J.S.; Tunney, H. Outlier identification of soil phosphorus and its implication for spatial structure modeling. Precis. Agric. 2016, 17, 121–135. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Selvalakshmi, S.; Vasu, D.; Liu, Q.Q.; Cheng, H.; Guo, F.T.; Ma, X.Q. Identification of indicators for evaluating and monitoring the effects of Chinese fir monoculture plantations on soil quality. Ecol. Indic. 2018, 93, 547–554. [Google Scholar]
- Sofo, A.; Ricciuti, P.; Fausto, C.; Mininni, A.; Grecchio, C.; Scagliola, M.; Malerba, A.D.; Xiloyannis, C.; Dichio, B. The metabolic and genetic diversity of soil bacterial communities depends on the soil management system and C/N dynamics: The case of sustainable and conventional olive groves. Appl. Soil Ecol. 2019, 137, 21–28. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, M.; Urbanskia, L.; Hobleya, E.; Langc, B.; von Lützowa, M.; Marin-Spiottad, E.; van Wesemaele, B.; Rabotf, E.; Ließ, M.; Garcia-Franco, N.; Wollschläger, U.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Burton, J.; Chen, C.R.; Xu, Z.H.; Ghadiri, H. Soil microbial biomass, activity and community composition in adjacent native and plantation forests of subtropical Australia. J. Soils Sediments 2010, 10, 1267–1277. [Google Scholar] [CrossRef]
- Fan, S.H.; Guan, F.Y.; Xu, X.L.; Forrester, D.; Ma, W.; Tang, X.L. Ecosystem carbon stock loss after land use change in subtropical forests in China. Forests 2016, 7, 142. [Google Scholar] [CrossRef]
- Mellin, C.; MacNeil, M.; Cheal, A.; Emslie, M.; Caley, M. Marine Protected Areas Increase Resilience among Coral Reef Communities. Ecol. Lett. 2016, 19, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.L.; Fu, W.J.; Ye, Z.Q.; Zhang, C.S. Contamination and spatial variation of heavy metals in the soil-rice system in Nanxun County, southeastern China. Int. J. Environ. Res. Public Health 2015, 12, 1577–1594. [Google Scholar] [CrossRef]
- Burns, R.; De Forest, J.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Yang, K.; Shi, W.; Zhu, J.J. The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil 2013, 368, 535–546. [Google Scholar] [CrossRef]
- Zhao, K.L.; Fu, W.J.; Liu, X.M.; Zhang, C.S.; Ye, Z.Q.; Xu, J.M. Spatial variations of concentrations of Copper and its speciation in the soil-rice system in Wenling of southeastern China. Environ. Sci. Pollut. Res. 2014, 21, 7165–7176. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.H.; Liu, G.B.; Zhao, L.; Zhao, G.C. The response of carbon storage to the age of three forest plantations in the Loess Hilly Regions of China. Catena 2017, 159, 106–114. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Bhatti, J.S.; Chang, S.X.; Sidders, D. Ecosystem carbon stocks and distribution under different land-use in north central Alberta. For. Ecol. Manag. 2009, 257, 1776–1785. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Meng, M.; Guo, X.; Wu, Y.; Liu, X.; Zhao, K.; Ding, L.; Shao, Y.; Fu, W. Forest-type shift and subsequent intensive management affected soil organic carbon and microbial community in southeastern China. Eur. J. For. Res. 2017, 136, 689–697. [Google Scholar] [CrossRef]
- Fu, W.J.; Zhao, K.L.; Tunney, H.; Zhang, C.S. Using GIS and Geostatistics to Optimize Soil Phosphor and Magnesium Sampling in Temperate Grassland. Soil Sci. 2013, 178, 240–247. [Google Scholar] [CrossRef]
- Monkai, J.; Goldberg, S.; Hyde, K.D.; Harrison, R.D.; Mortimer, P.E.; Xu, J.C. Natural forests maintain a greater soil microbial diversity than that in rubber plantations in Southwest China. Agric. Ecosyst. Environ. 2018, 265, 190–197. [Google Scholar] [CrossRef]
- Dorsaf, K.; Tripathi, B.M.; Dong, K.; Go, R.; Adams, J.M. Rainforest Conversion to Rubber Plantation May Not Result in Lower Soil Diversity of Bacteria, Fungi, and Nematodes. Microb. Ecol. 2016, 72, 359–371. [Google Scholar]
- Alberti, G.; Fornasier, C.N.; Scherer-Lorenzen, M.; Nobili, M.; Peressotti, A.; Hoenig, L.; Bruelheide, H.; Bauhus, J. Tree functional diversity influences belowground ecosystem functioning. Appl. Soil Ecol. 2017, 120, 160–168. [Google Scholar] [CrossRef]
- Wu, J.S.; Lin, H.P.; Meng, C.F.; Jiang, P.K.; Fu, W.J. Effects of intercropping grasses on soil organic carbon and microbial community functional diversity under Chinese hickory (Carya cathayensis Sarg.) stands. Soil Res. 2014, 52, 575–583. [Google Scholar] [CrossRef]
- Fu, W.; Fu, Z.; Ge, H.; Ji, B.; Jiang, P.; Li, Y.; Wu, J.; Zhao, K. Spatial variation of biomass carbon density in a subtropical region of southeastern China. Forests 2015, 6, 1966–1981. [Google Scholar] [CrossRef]
- Wang, Z.J.; Huang, X.Z.; Tang, X.H.; Hang, J.Q.; Qian, L.F.; Li, Z.J. Analysis on economic and ecological benefits of no-tillage management of Carya cathayensis. Acta Ecol. Sin. 2011, 31, 2281–2289. (In Chinese) [Google Scholar]
- Li, Y.F.; Hu, S.D.; Chen, J.H.; Müller, K.; Li, Y.C.; Fu, W.J.; Lin, Z.W.; Wang, H.L. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources (WRB). A Framework for International Classification, Correlation and Communication; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Wu, J.S. Study on the Characteristics of Soil Organic Carbon under Carya cathayensis Stands. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2014. (In Chinese). [Google Scholar]
- Dai, W.; Zhao, K.; Fu, W.; Jiang, P.; Li, Y.; Zhang, C.; Gielen, G.; Gong, X.; Li, Y.; Wang, H.; et al. Spatial variation of organic carbon density in topsoils of a typical subtropical forest, southeastern China. Catena 2018, 167, 181–189. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods for Soil and Agro-Chemistry; China Agr Sci. Technol. Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Dai, W.; Li, Y.; Fu, W.; Jiang, P.; Zhao, K.; Li, Y.; Penttinen, P. Spatial variability of soil nutrients in forest areas: A case study from subtropical China. J. Plant Nutr. Soil Sci. 2018, 181, 827–835. [Google Scholar] [CrossRef]
- Liang, B.C.; Mackenzie, A.F.; Schnitzer, M. Management-induced change in labile soil organic matter under continuous corn in eastern Canadian soils. Biol. Fertil. Soils 1998, 26, 88–94. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Mathers, N.J.; Xu, Z.H.; Berners-Price, S.J.; Perera, S.; Saffigna, P.G. Hydrofluoric acid pre-treatment for improving 13C CPMAS NMR spectral quality of forest soils in southeast Queensland, Australia. Aust. J. Soil Res. 2002, 40, 655–674. [Google Scholar]
- Gomez, E.; Ferreras, L.; Toresani, S. Soil bacterial functional diversity as influenced by organic amendment application. Bioresour. Technol. 2006, 97, 1484–1489. [Google Scholar] [CrossRef]
- Selmants, P.C.; Hart, S.C.; Boyle, S.I.; Stark, J.M. Red alder (Alnus rubra) alters community-level soil microbial function in conifer forests of the Pacific Northwest, USA. Soil Biol. Biochem. 2005, 37, 1860–1868. [Google Scholar] [CrossRef]
- Fu, W.; Fu, Z.; Zhao, K.; Tunney, H.; Zhang, C. Variation of soil P and other nutrients in a long-term grazed grassland P experiment field. Arch. Agron. Soil Sci. 2014, 60, 1459–1466. [Google Scholar] [CrossRef]
- Zhao, K.L.; Fu, W.J.; Qiu, Q.Z.; Ye, Z.Q.; Li, Y.F.; Tunney, H.; Dou, C.Y.; Zhou, K.N.; Qian, X.B. Spatial patterns of potentially hazardous metals in paddy soils in a typical electrical waste dismantling area and their pollution characteristics. Geoderma 2019, 337, 453–462. [Google Scholar] [CrossRef]
- Fu, W.J.; Tunney, H.; Zhang, C.S. Spatial variation of soil nutrients in a dairy farm and its implications for site-specific fertilizer application. Soil Tillage Res. 2010, 106, 185–193. [Google Scholar] [CrossRef]
- Fu, W.J.; Zhao, K.L.; Tunney, H.; Zhang, C.S. Using Moran’s I and geostatistics to identify spatial patterns of soil nutrients in two different long-term phosphorus application plots. J. Plant Nutr. Soil Sci. 2011, 174, 785–798. [Google Scholar] [CrossRef]
- Mujuru, L.; Gotora, T.; Velthorst, E.J.; Nyamangara, J.; Hoosbeek, M.R. Soil carbon and nitrogen sequestration over an age sequence of Pinus patula plantations in Zimbabwean Eastern Highlands. For. Ecol. Manag. 2014, 303, 254–265. [Google Scholar] [CrossRef]
- Battle-Bayer, L.; Batjes, N.H.; Bindraban, P.S. Changes in organic carbon stocks upon land use conversion in the Brazilian Cerrado. Agric. Ecosyst. Environ. 2010, 137, 47–58. [Google Scholar] [CrossRef]
- Fu, W.J.; Jiang, P.K.; Zhao, K.L.; Zhou, G.M.; Li, Y.F.; Wu, J.S.; Du, H.Q. The carbon storage in Moso Bamboo plantation and its spatial variation in Anji County of southeastern China. J. Soils Sediments 2014, 14, 320–329. [Google Scholar] [CrossRef]
- Wu, J.S.; Huang, J.Q.; Liu, D.; Li, J.W.; Zhang, J.C.; Wang, H.L. Effect of 26 Years of Intensively Managed Carya cathayensis Stands on Soil Organic Carbon and Fertility. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Chen, G.S.; Yang, Y.S.; Yang, Z.J.; Xie, J.S.; Guo, J.F.; Gao, R.; Yin, Y.F.; Robinson, D. Accelerated soil carbon turnover under tree plantations limits soil carbon storage. Sci. Rep. 2016, 6, 19693. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Jiang, P.K.; Chang, S.X.; Xu, Q.F.; Lin, Y. Dissolved soil organic carbon and nitrogen were affected by conversion of native forests to plantations in subtropical China. Can. J. Soil Sci. 2010, 90, 27–36. [Google Scholar] [CrossRef]
- Ussira, D.A.N.; Johnson, C.E. Characterization organic mater in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods. Geoderma 2003, 111, 123–149. [Google Scholar] [CrossRef]
- Spaccini, R.; Mbagwu, J.S.C.; Conte, P.; Piccolo, A. Changes of humic substances characteristics from forested to cultivated soils in Ethiopia. Geoderma 2006, 132, 9–19. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A.; Haberhauer, G.; Gerzabek, M.H. Transformation of organic matter from maize residues into labile and humic fractions of three European soils as revealed by 13C distribution and CPMAS-NMR spectra. Eur. J. Soil Sci. 2000, 51, 583–594. [Google Scholar] [CrossRef]
- Garland, J.L. Analysis and interpretation of community-level physiological profiles in microbial ecology. Microb. Ecol. 1997, 24, 289–300. [Google Scholar] [CrossRef]
- Bending, G.D.; Putland, C.; Rayns, F. Changes in microbial community metabolism and labile organic matter fractions as early indicators of the impact of management on soil biological quality. Biol. Fertil. Soils 2000, 31, 78–84. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Buma, D.S.; De Boer, W.; Kinkhamer, P.G.L.; Veen, J.A.V. Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Anton. Leeuw. 2002, 81, 509–520. [Google Scholar] [CrossRef]
- He, M.J.; Shen, H.R.; Li, Z.T.; Wang, L.; Wang, F.; Zhao, K.L.; Liu, X.M.; Wendroth, O.; Xu, J.M. Ten-year regional monitoring of soil-rice grain contamination by heavy metals with implications for target remediation and food safety. Environ. Pollut. 2019, 244, 431–439. [Google Scholar] [CrossRef]
- Yu, S.Y.; Chen, Z.L.; Zhao, K.L.; Ye, Z.Q.; Zhang, L.Y.; Dong, J.Q.; Shao, Y.F.; Zhang, C.S.; Fu, W.J. Spatial Patterns of Potentially Hazardous Metals in Soils of Lin’an City, Southeastern China. Int. J. Environ. Res. Public Health 2019, 16, 246. [Google Scholar] [CrossRef]
- Johal, G.S.; Huber, D.M. Glyphosate effects on diseases of plants. Eur. J. Agron. 2009, 31, 144–152. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Yang, F.; E, Y.Y.; Raza, W.; Huang, Q.W.; Shen, Q.R. Application of bioorganic fertilizer significantly increased apple yields and shaped bacterial community structure in orchard soil. Microb. Ecol. 2017, 73, 404–416. [Google Scholar] [CrossRef]
| Stand Types | Stand Density (tree ha−1) | DBH (cm) | Height (m) | Canopy Density (%) | Canopy Structure | Main Tree Species |
|---|---|---|---|---|---|---|
| CH5 | 450 ± 50 b | 6.0 ± 1.0 c | 5.0 ± 0.6 b | 30 ± 5 d | Trees + herbs | C. cathayensis |
| CH10 | 450 ± 45 b | 8.0 ± 1.2 b | 6.0 ± 0.7 b | 50 ± 8 c | Trees + herbs | C. cathayensis |
| CH15 | 450 ± 40 b | 10.0 ± 1.3 ab | 7.0 ± 0.6 ab | 70 ± 9 b | Trees + herbs | C. cathayensis |
| CH20 | 435 ± 38 b | 12.0 ± 1.2 a | 8.0 ± 0.8 a | 80 ± 10 a | Trees + herbs | C. cathayensis |
| NMF | 1350 ± 120 a | 10.0 ± 2.5 ab | 8.0 ± 1.3 a | 80 ± 12 a | Trees + shrubs + herbs | C. cathayensis, Schima superb, Cyclobalanopsis glauca, Castanopsis sclerophylla |
| Stand Types | pH | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | Sand (%) | Silt (%) | Clay (%) | Soil Texture Class | BD (g cm−3) |
|---|---|---|---|---|---|---|---|---|---|
| CH5 | 5.7 ± 0.1 a | 179.6 ± 13.4 a | 3.7 ± 0.4 c | 120.8 ± 18.3 ab | 26.5 ± 2.8 a | 52.3 ± 3.9 a | 21.2 ± 1.5 a | silt loam | 1.05 ± 0.08 a |
| CH10 | 5.8 ± 0.3 a | 175.7 ± 15.3 a | 4.7 ± 0.8 b | 125.0 ± 20.6 ab | 27.4 ± 3.6 a | 51.6 ± 2.8 a | 21.0 ± 3.2 a | silt loam | 1.07 ± 0.10 a |
| CH15 | 5.8 ± 0.2 a | 161.8 ± 10.6 ab | 4.9 ± 1.0 b | 131.7 ± 17.6 a | 29.6 ± 2.8 a | 50.0 ± 1.6 a | 20.4 ± 1.8 a | silt loam | 1.10 ± 0.11 a |
| CH20 | 5.6 ± 0.3 a | 145.1 ± 12.1 b | 6.7 ± 1.5 a | 119.2 ± 10.3 b | 30.5 ± 4.5 a | 49.3 ± 1.5 a | 20.2 ± 2.0 a | loam | 1.15 ± 0.12 a |
| NMF | 5.6 ± 0.2 a | 150.6 ± 12.8 b | 3.5 ± 0.3 c | 124.2 ± 18.9 ab | 26.2 ± 2.4 a | 52.1 ± 3.4 a | 21.7 ± 2.3 a | silt loam | 1.03 ± 0.06 a |
| Stand Types | TOC (g kg−1) | MBC (mg kg−1) | WSOC (mg kg−1) | MBC/TOC (%) | WSOC/TOC (%) |
|---|---|---|---|---|---|
| CH5 | 20.17 ± 2.01 b | 148.83 ± 13.23 b | 117.62 ± 12.31 b | 0.7 ± 0.1 ab | 0.6 ± 0.1 b |
| CH10 | 19.39 ± 3.01 b | 134.75 ± 14.42 b | 107.77 ± 12.42 b | 0.6 ± 0.0 b | 0.6 ± 0.1 b |
| CH15 | 18.15 ± 0.91 b | 123.73 ± 13.25 b | 96.52 ± 10.35 b | 0.6 ± 0.0 b | 0.5 ± 0.1 b |
| CH20 | 17.28 ± 2.34 b | 115.39 ± 12.37 b | 90.39 ± 11.87 b | 0.6 ± 0.0 b | 0.5 ± 0.0 b |
| NMF | 28.16 ± 1.91 a | 225.84 ± 23.75 a | 251.80 ± 24.25 a | 0.8 ± 0.1 a | 0.9 ± 0.1 a |
| Stand Types | Alkyl C (%) | N-Alkyl C (%) | O-Alkyl C (%) | Acetal C (%) | Aromatic C (%) | Phenolic C (%) | Carbonyl C (%) | Alkyl C/O-Alkyl C | Hydrophilic C/Hydrophobic C | Aliphatic C/Aromatic C | Aromaticity (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH5 | 21.2 a | 14.5 b | 24.7 a | 12.8 a | 13.3 b | 5.9 b | 7.5 b | 0.54 b | 0.68 b | 2.74 a | 20.8 b |
| CH10 | 23.1 a | 13.9 b | 24.1 a | 11.6 a | 12.5 bc | 6.2 ab | 8.7 a | 0.61 a | 0.72 ab | 2.65 ab | 20.5 b |
| CH15 | 23.3 a | 13.9 b | 24.4 a | 10.6 a | 14.7 ab | 6.0 ab | 7.1 b | 0.61 a | 0.79 a | 2.59 ab | 22.3 ab |
| CH20 | 22.9 a | 12.0 b | 23.8 a | 11.4 a | 15.4 a | 6.8 a | 7.7 b | 0.64 a | 0.82 a | 2.35 b | 24.1 a |
| NMF | 22.8 a | 16.0 a | 25.7 a | 11.1 a | 13.1 b | 5.4 b | 6.9 b | 0.55 b | 0.69 b | 2.99 a | 19.6 b |
| Stand Types | Shannon Index | Evenness Index |
|---|---|---|
| CH5 | 3.59 ± 0.09 a | 0.97 ± 0.01 a |
| CH10 | 3.34 ± 0.09 ab | 0.97 ± 0.01 ab |
| CH15 | 3.03 ± 0.02 bc | 0.94 ± 0.00 bc |
| CH20 | 3.02 ± 0.07 bc | 0.94 ± 0.00 bc |
| NMF | 3.61 ± 0.06 a | 0.97 ± 0.00 a |
| Evenness Index | Shannon Index | |
|---|---|---|
| TOC | 0.39 * | 0.42 * |
| MBC | 0.36 * | 0.34 * |
| WSOC | 0.42 * | 0.59 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Lin, H.; Fu, W.; Penttinen, P.; Li, Y.; Jin, J.; Zhao, K.; Wu, J. Soil Organic Carbon Content and Microbial Functional Diversity Were Lower in Monospecific Chinese Hickory Stands than in Natural Chinese Hickory–Broad-Leaved Mixed Forests. Forests 2019, 10, 357. https://doi.org/10.3390/f10040357
Wu W, Lin H, Fu W, Penttinen P, Li Y, Jin J, Zhao K, Wu J. Soil Organic Carbon Content and Microbial Functional Diversity Were Lower in Monospecific Chinese Hickory Stands than in Natural Chinese Hickory–Broad-Leaved Mixed Forests. Forests. 2019; 10(4):357. https://doi.org/10.3390/f10040357
Chicago/Turabian StyleWu, Weifeng, Haiping Lin, Weijun Fu, Petri Penttinen, Yongfu Li, Jin Jin, Keli Zhao, and Jiasen Wu. 2019. "Soil Organic Carbon Content and Microbial Functional Diversity Were Lower in Monospecific Chinese Hickory Stands than in Natural Chinese Hickory–Broad-Leaved Mixed Forests" Forests 10, no. 4: 357. https://doi.org/10.3390/f10040357
APA StyleWu, W., Lin, H., Fu, W., Penttinen, P., Li, Y., Jin, J., Zhao, K., & Wu, J. (2019). Soil Organic Carbon Content and Microbial Functional Diversity Were Lower in Monospecific Chinese Hickory Stands than in Natural Chinese Hickory–Broad-Leaved Mixed Forests. Forests, 10(4), 357. https://doi.org/10.3390/f10040357






