Differences in the Climate-Growth Relationship of Scots Pine: A Case Study from Poland and Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Climate Data
2.2. Chronology Building
3. Results
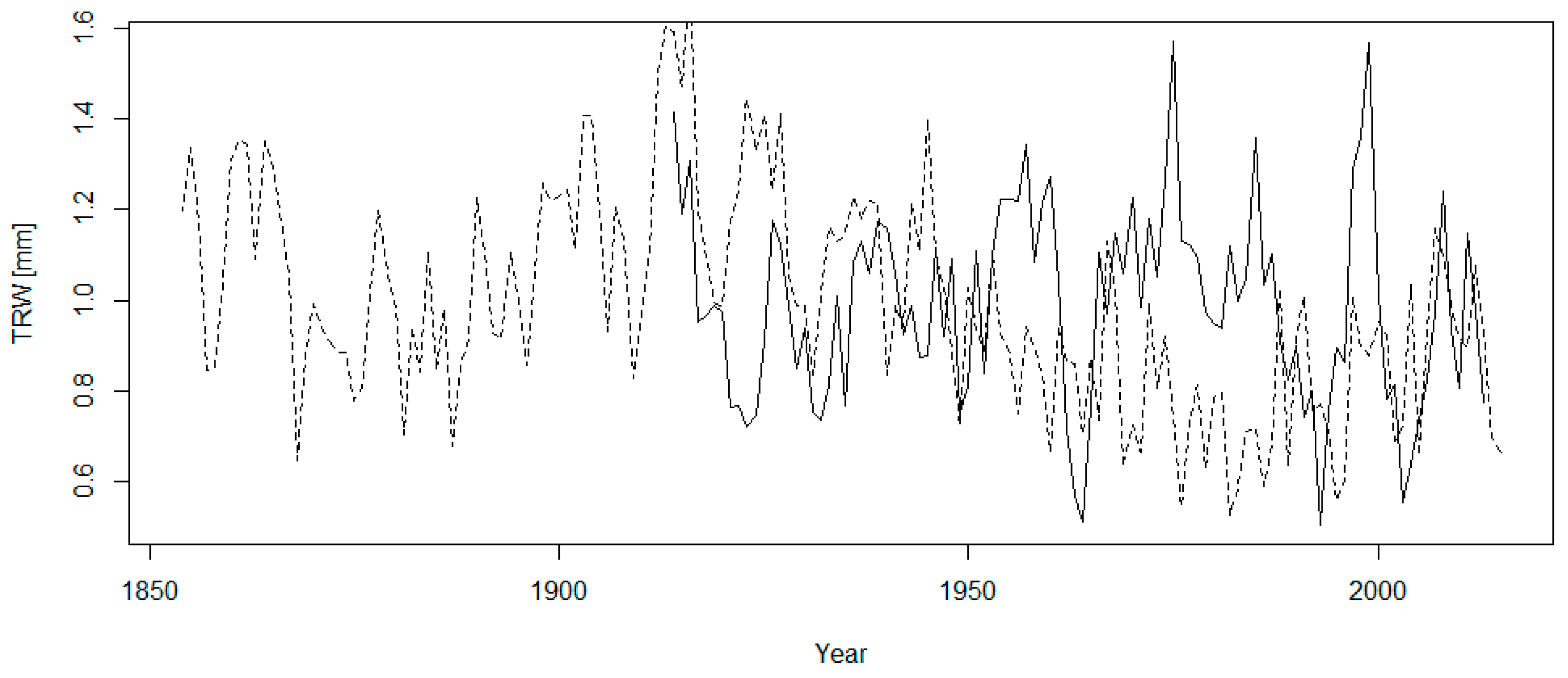
3.1. Tree-Ring Chronology
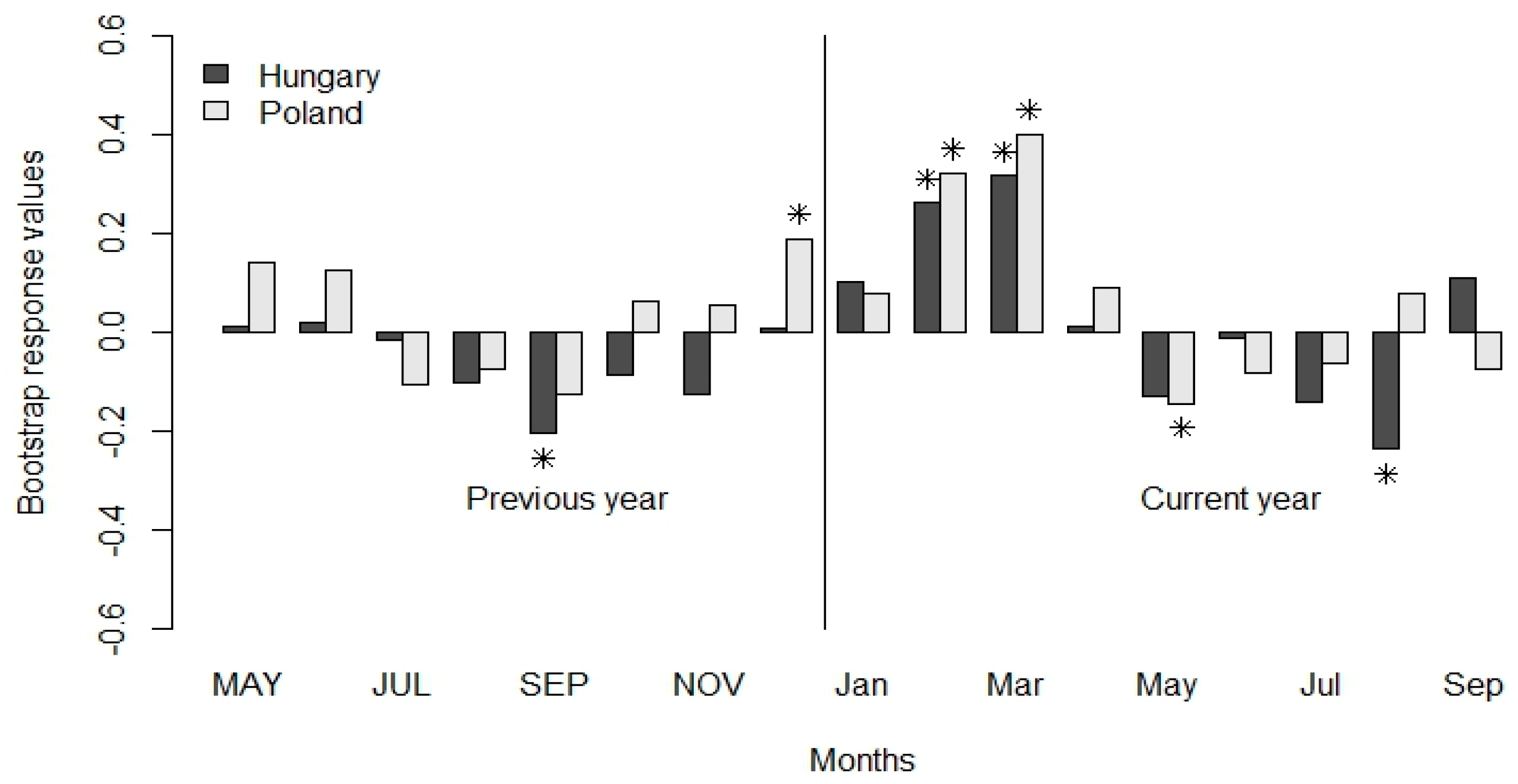
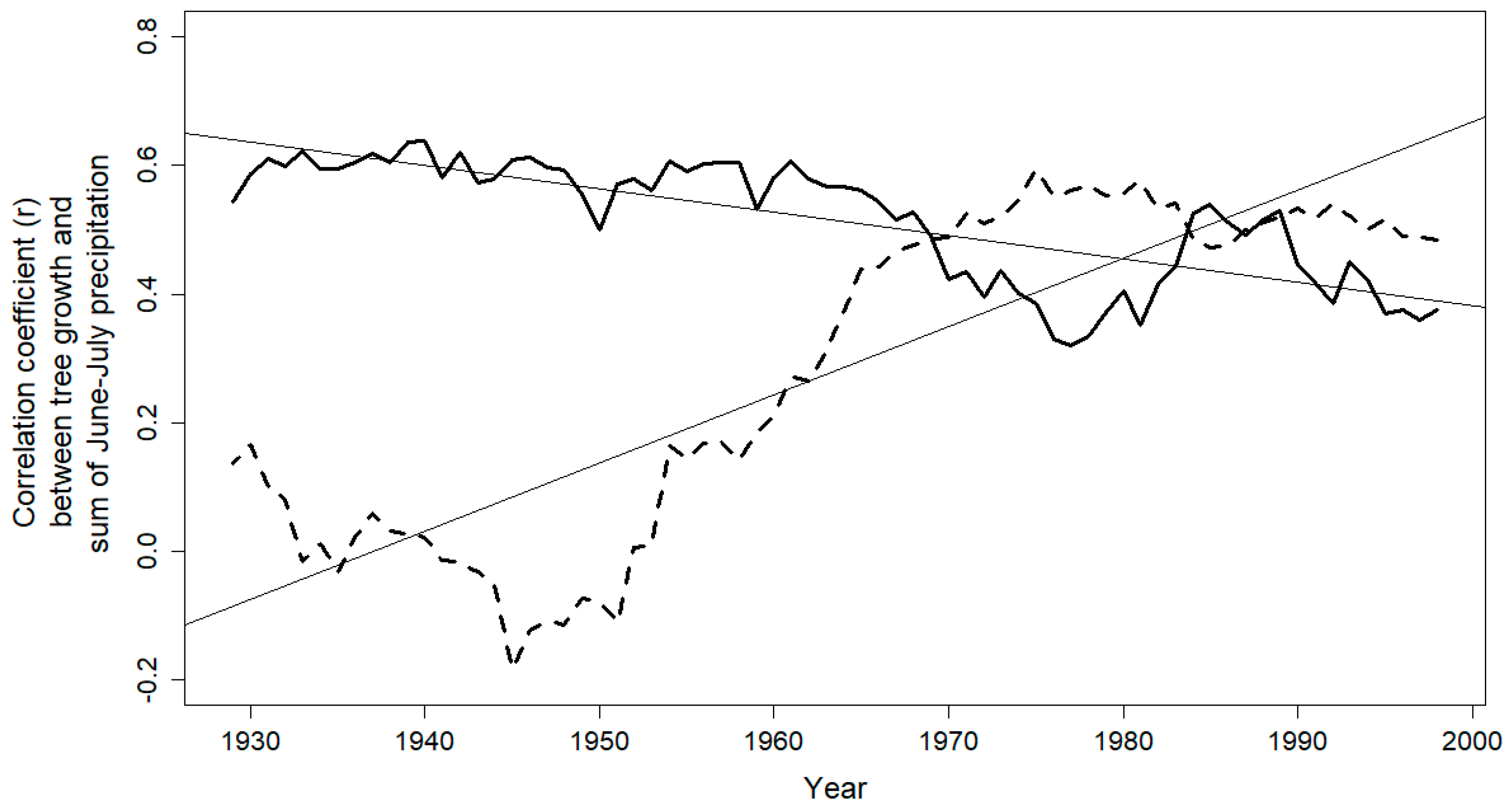
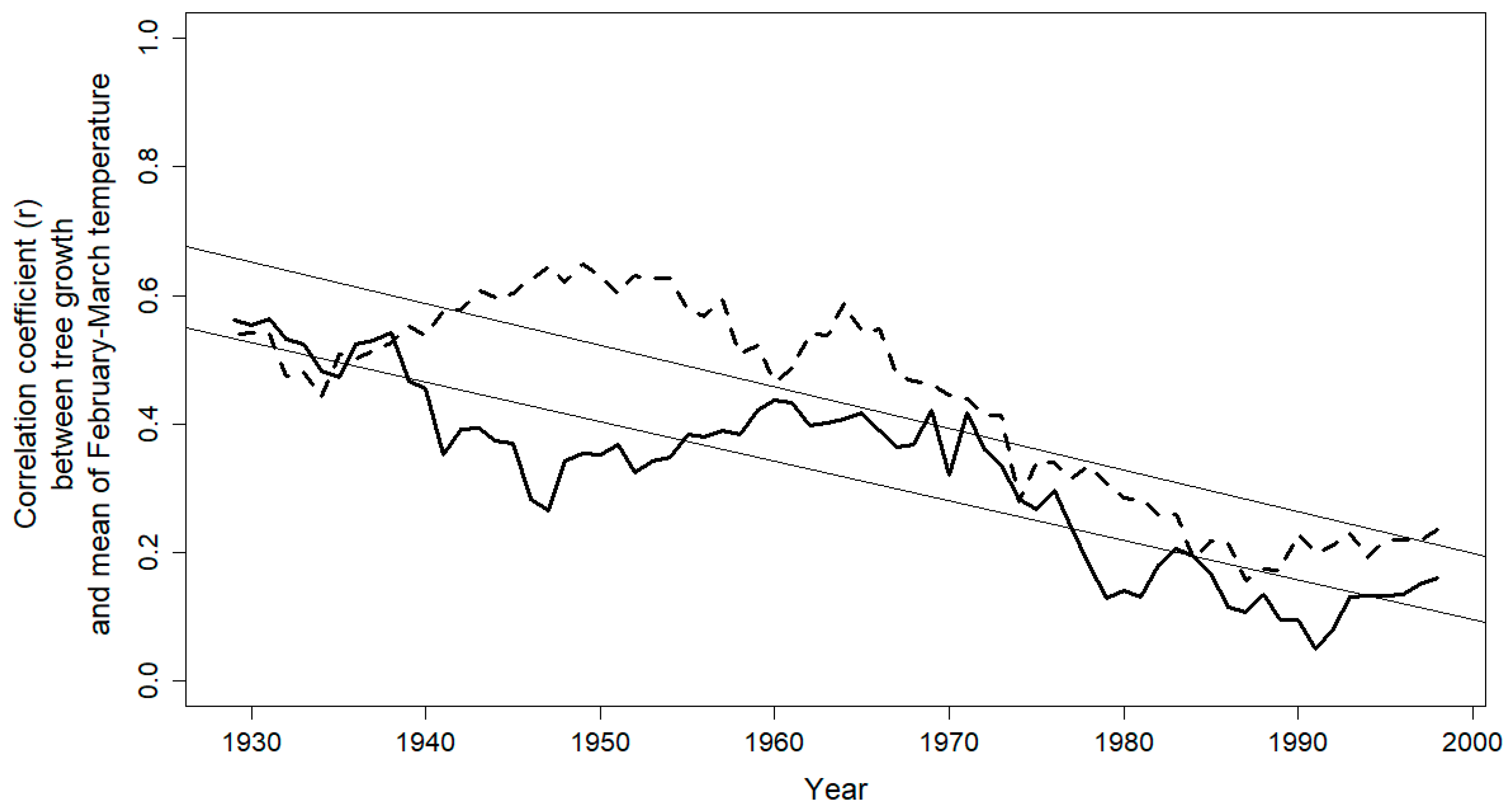
3.2. Climate-Growth Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Białobok, S.; Boratyński, A.; Bugała, W. Biologia Sosny Zwyczajnej; Sorus: Poznań, Kórnik, Poland, 1993; ISBN 8385599215. (In Polish with English Abstracts). [Google Scholar]
- Rivas-Martínez, S.; Rivas-Sáenz, S.; Penas-Merino, A. Worldwide bioclimatic classification system. Glob. Geobot. 2011, 1, 1–638. [Google Scholar]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2012, 3, 203–207. [Google Scholar] [CrossRef]
- Zielski, A.; Krąpiec, M.; Koprowski, M. Dendrochronological Data. In Polish Climate in European Context An Historical Overview; Przybylak, R., Majorowicz, J., Brázdil, R., Kejna, M., Eds.; Springer: Dodrecht, The Netherlands, 2010; pp. 87–100, ISBN 13: 9789048131662. [Google Scholar]
- Kern, Z.; Patkó, M.; Kázmér, M.; Fekete, J.; Kele, S.; Pályi, Z. Multiple tree-ring proxies (earlywood width, latewood width and d13C) from pedunculate oak (Quercus robur L.), Hungary. Quat. Int. 2013, 293, 257–267. [Google Scholar] [CrossRef]
- Garamszegi, B.; Kern, Z. Climate influence on radial growth of Fagus sylvatica growing near the edge of its distribution in Bükk Mts., Hungary. Dendrobiology 2014, 72, 93–102. [Google Scholar] [CrossRef]
- Misi, D.; Nafradi, K. Possibility of identification of negative extreme climatic events using Pinus sylvestris tree-rings in Transdanubia, Hungary. Dendrobiology 2016, 75, 45–54. [Google Scholar] [CrossRef]
- Misi, D.; Náfrádi, K. Growth response of Scots pine to changing climatic conditions over the last 100 years: A case study from Western Hungary. Trees Struct. Funct. 2017, 31, 919–928. [Google Scholar] [CrossRef]
- Bauwe, A.; Koch, M.; Kallweit, R.; Konopatzky, A.; Strohbach, B.; Lennartz, B. Tree-ring growth response of Scots pine (Pinus sylvestris L.) to climate and soil water availability in the lowlands of north-eastern Germany. Balt. For. 2013, 19, 212–225. [Google Scholar]
- Mácová, M. Dendroclimatological comparison of native Pinus sylvestris and invasive Pinus strobus in different habitats in the Czech Republic. Preslia 2008, 80, 277–289. [Google Scholar]
- Vitas, A.; Erlickytė, R. Influence of droughts to the radial growth of Scots pine (Pinus sylvestris L.). Ekológia (Bratislava) 2008, 27, 367–378. [Google Scholar]
- Xenakis, G.; Ray, D.; Mencuccini, M. Effects of climate and site characteristics on Scots pine growth. Eur. J. For. Res. 2012, 131, 427–439. [Google Scholar] [CrossRef]
- Oleksyn, J.; Tjoelker, M.G.; Reich, P.B. Adaptation to changing environment in Scots pine populations across a latitudinal gradient. Silva Fenn. 1998, 32, 129–140. [Google Scholar] [CrossRef]
- Borhidi, A. Magyarország Növénytársulásai; Akadémiai Kiadó: Budapest, Hungary, 2003. (In Hungarian) [Google Scholar]
- Majer, A. Fenyves a Bakonyalján; Akadémiai Kiadó: Budapest, Hungary, 1988. (In Hungarian) [Google Scholar]
- Dziekoński, H. How were the Tabórz pine trees stands established? Sylwan 1994, 138, 47–55, (In Polish with English Abstract). [Google Scholar]
- Stopa-Boryczka, M.; Boryczka, J.; Wawer, J.; Grabowska, K.; Dobrowolska, M.; Osowiec, M.; Błażek, E.; Skrzypczuk, J.; Rzęda, M. Climate of North-Eastern Poland Based on J. Kondracki and J. Ostrowski’s Physiographic Division in: Atlas of Interdependence of Meteorological and Geographical Parameters in Poland; University of Warsaw Faculty of Geography and Regional Studies: Warsaw, Poland, 2013; ISBN 9788363245528. [Google Scholar]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3. 10 Dataset. Int. J. Climatol. 2014, 642, 623–642. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Vessella, F.; Schirone, B. Predicting potential distribution of Quercus suber in Italy based on ecological niche models: Conservation insights and reforestation involvements. For. Ecol. Manag. 2013, 304, 150–161. [Google Scholar] [CrossRef]
- Kreyling, J.; Buhk, C.; Backhaus, S.; Hallinger, M.; Huber, G.; Huber, L.; Jentsch, A.; Konnert, M.; Thiel, D.; Wilmking, M.; et al. Local adaptations to frost in marginal and central populations of the dominant forest tree Fagus sylvatica L. as affected by temperature and extreme drought in common garden experiments. Ecol. Evol. 2014, 4, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, C.; Wohlgemuth, T.; Ghazoul, J.; Moser, B. Cold temperature extremes during spring do not limit the range shift of Mediterranean pines into regions with intermittent frost. Funct. Ecol. 2015, 30, 856–865. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Guarino, L.; Cruz, M.; Rojas, E. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genet. Resour. 2001, 127, 15–19. [Google Scholar]
- Hijmans, R.J.; Guarino, L.; Mathur, P. DIVA-GIS Version 7.5. Manual 2012. Available online: https://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf (accessed on 6 March 2018).
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers. An Analysis of their Distribution, Biogeography, Diversity and Conservation Status; Brill: Leiden, The Netherlands; Boston, MA, USA, 2013; ISBN 9789004211803. [Google Scholar]
- Rinn, F. TSAP-Win Time Series Analysis and Presentation for Dendrochronology and Related Applications. 2003. Available online: www.rinntech.de (accessed on 6 March 2018).
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Cook, E.R. A Time Series Analysis Approach to Tree-Ring Standardization. Ph.D. Thesis, The University of Arizona, Tucson, AZ, USA, 1985. [Google Scholar]
- Wigley, T.; Briffa, K.; Jones, P. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Cook, E.; Krusic, P. ARSTAN4.1b_XP 2006. Available online: http://Ideo.columbia.edu (accessed on 6 March 2018).
- Zang, C.; Biondi, F. treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Durrant Houston, T.; de Rigo, D.; Caudullo, G. Pinus sylvestris in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxemburg, 2016; pp. 132–133. [Google Scholar]
- Castro, J.; Zamora, R.; Hódar, J.A. Mechanisms blocking Pinus sylvestris colonization of Mediterranean mountain meadows. J. Veg. Sci. 2002, 13, 725–731. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hodar, J.A.; Gomez, J.M.; Hódar, J.A.; Gómez, J.M. Seedling establishment of a boreal tree species (Pinus sylvestris) at its southernmost distribution limit: Consequences of being in a marginal Mediterranean habitat. J. Ecol. 2004, 92, 266–277. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Leuschner, C. Seedling emergence and establishment of Pinus sylvestris in the Mongolian forest-steppe ecotone. Plant Ecol. 2013, 214, 139–152. [Google Scholar] [CrossRef]
- Michelot, A.; Bréda, N.; Damesin, C.; Dufrêne, E. Differing growth responses to climatic variations and soil water deficits of Fagus sylvatica, Quercus petraea and Pinus sylvestris in a temperate forest. For. Ecol. Manag. 2012, 265, 161–171. [Google Scholar] [CrossRef]
- Panayotov, M.P.; Zafirov, N.; Cherubini, P. Fingerprints of extreme climate events in Pinus sylvestris tree rings from Bulgaria. Trees Struct. Funct. 2013, 27, 211–227. [Google Scholar] [CrossRef]
- Hutchinson, G. The Multivariate Niche. Concluding Remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–421. [Google Scholar] [CrossRef]
- Vetaas, O.R. Realized and potential climate niches: A comparison of four Rhododendron tree species. J. Biogeogr. 2002, 29, 545–554. [Google Scholar] [CrossRef]
- Zielski, A.; Krąpiec, M.; Wilczyński, S.; Szychowska-Krąpiec, E. Chronologies of radial growth of Scots pine In Poland. Sylwan 2001, 145, 105–120, (In Polish with English Abstract). [Google Scholar]
- Koprowski, M. Spatial distribution of introduced Norway spruce growth in lowland Poland: The influence of changing climate and extreme weather events. Quat. Int. 2013, 283, 139–146. [Google Scholar] [CrossRef]
- Bijak, S. Tree-ring chronology of Silver fir and its dependence on climate of the Kaszubskie Lakeland (northern Poland). Geochronometria 2010, 35, 91–94. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Luterbacher, J.; Dietrich, D.; Xoplaki, E.; Grosjean, M.; Wanner, H. European Seasonal and Annual Temperature Variability, Trends, and Extremes since 1500. Science 2004, 303, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Xoplaki, E.; Luterbacher, J.; Paeth, H.; Dietrich, D.; Steiner, N.; Grosjean, M.; Wanner, H. European spring and autumn temperature variability and change of extremes over the last half millennium. Geophys. Res. Lett. 2005, 32, L15713. [Google Scholar] [CrossRef]
- Parry, M.; Parry, M.L.; Canziani, O.; Palutikof, J.; Van der Linden, P.; Hanson, C. Climate Change 2007—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Fourth Assessment Report of the IPCC (Vol. 4); Cambridge University Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Gulyás, K.; Bidló, A.; Horváth, A. Causes of the Forest Die-off in a Pinus Forest (Pinus sylvestris) in Fenyőfő. In Proceedings of the Local and Regional Challenges of Climate Change Adaptation and Green Technologies, H-Sopron, Hungary, 19 September 2014; Polgár, A., Bazsó, T., Nagy, G., Gálos, B., Eds.; The University of West Hungary: Sopron, Hungary, 2014. [Google Scholar]
- Misi, D.; Náfrádi, K. Late winter—Early spring thermal conditions and their long-term effect on tree-ring growth in Hungary. Balt. For. 2016, 22, 203–211. [Google Scholar]
- Koprowski, M.; Przybylak, R.; Zielski, A.; Pospieszyńska, A. Tree rings of Scots pine (Pinus sylvestris L.) as a source of information about past climate in northern Poland. Int. J. Biometeorol. 2012, 56, 1–10. [Google Scholar] [CrossRef]
- Briffa, K.R.; Schweingruber, F.H.; Jones, P.D.; Osborn, T.J. Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 1998, 391, 678–682. [Google Scholar] [CrossRef]
- Jacoby, G.C.; D’Arrigo, R.D. Tree ring width and density evidence of climatic and potential forest change in Alaska. Glob. Biogeochem. Cycles 1995, 9, 227–234. [Google Scholar] [CrossRef]
- Wilson, R.J.; Luckman, B.H. Dendroclimatic reconstruction of maximum summer temperatures from upper treeline sites in Interior British Columbia, Canada. Holocene 2003, 13, 851–861. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Wilson, R.; Liepert, B.; Cherubini, P. On the “Divergence Problem” in Northern Forests: A review of the tree-ring evidence and possible causes. Glob. Planet. Chang. 2008, 60, 289–305. [Google Scholar] [CrossRef]
- Bauwe, A.; Jurasinski, G.; Scharnweber, T.; Schröder, C.; Lennartz, B. Impact of climate change on tree-ring growth of scots pine, common beech and pedunculate Oak in northeastern Germany. IForest 2016, 9, 1–11. [Google Scholar] [CrossRef]
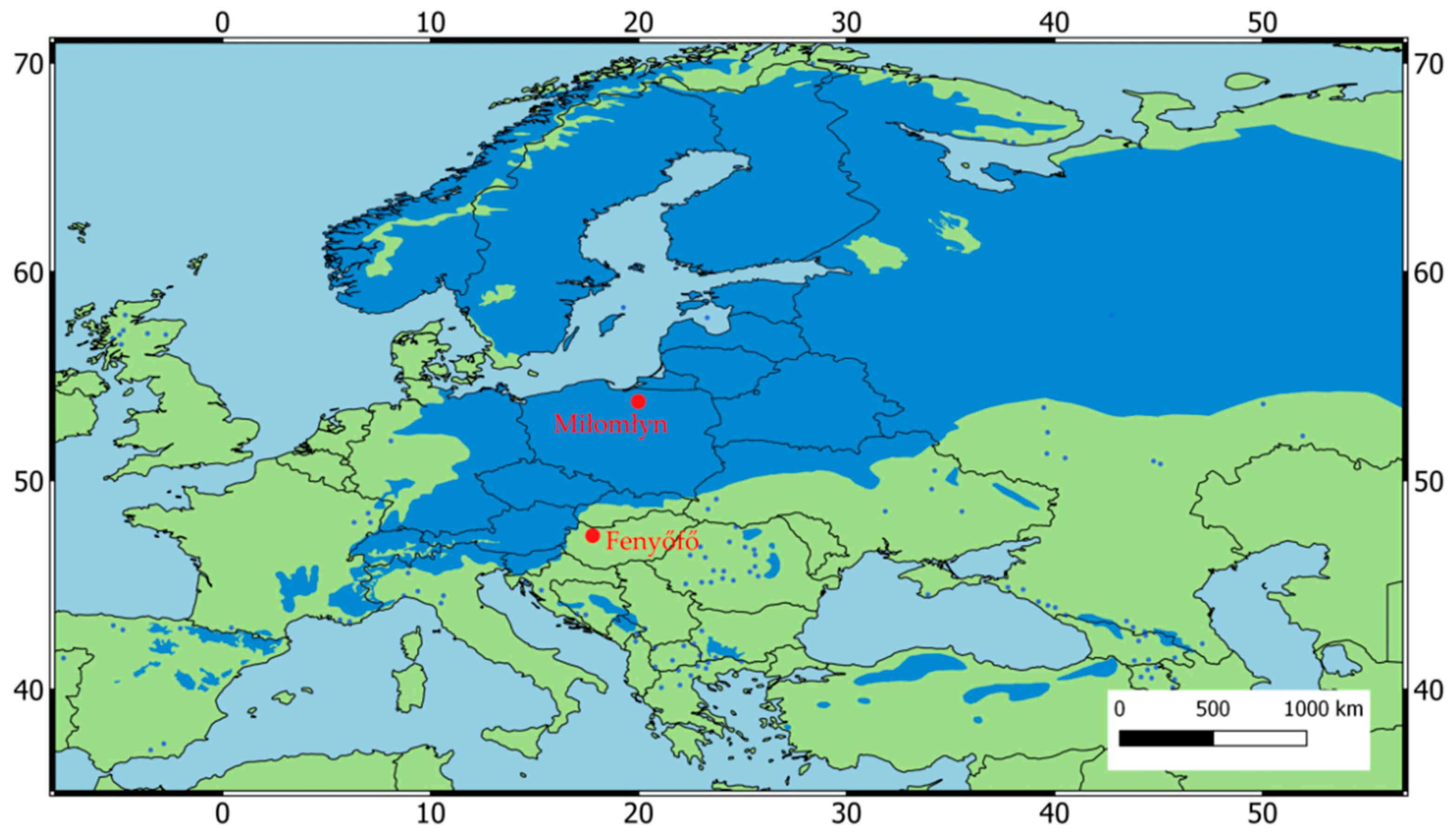

| Bioclimatic Variable | Poland | Hungary |
|---|---|---|
| Isothermality (2/7) × 100 | 24 | 30 |
| Temperature Seasonality (Standard deviation × 100) | 816.9 | 759.6 |
| Max Temperature of Warmest Month | 23 °C | 25.8 °C |
| Min Temperature of Coldest Month | −7.6 °C | −4.8 °C |
| Temperature Annual Range (5–6) | 30.6 °C | 30.6 °C |
| Mean Temperature of Wettest Quarter | 17.1 °C | 19 °C |
| Mean Temperature of Driest Quarter | 1.8 °C | 0.9 °C |
| Mean Temperature of Warmest Quarter | 17.1 °C | 19 °C |
| Mean Temperature of Coldest Quarter | −3.9 °C | −0.6 °C |
| Precipitation of Wettest Month | 81 mm | 71 mm |
| Precipitation of Driest Month | 31 mm | 32 mm |
| Precipitation Seasonality (Coefficient of Variation) | 30 | 27 |
| Precipitation of Wettest Quarter | 235 mm | 189 mm |
| Precipitation of Driest Quarter | 103 mm | 103 mm |
| Precipitation of Warmest Quarter | 235 mm | 189 mm |
| Precipitation of Coldest Quarter | 120 mm | 117 mm |
| Hungarian Site | Polish Site | |
|---|---|---|
| Time-span | 1914–2013 | 1855–2015 |
| Correlation with Master | 0.651 | 0.571 |
| Mean ring width | 2.36 | 1.44 |
| Standard deviation | 1.168 | 0.734 |
| Autocorrelation | 0.785 | 0.797 |
| Mean sensitivity | 0.234 | 0.244 |
| Expressed Population Signal (EPS) | 0.97 | 0.87 |
| Rbar | 0.44 | 0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misi, D.; Puchałka, R.; Pearson, C.; Robertson, I.; Koprowski, M. Differences in the Climate-Growth Relationship of Scots Pine: A Case Study from Poland and Hungary. Forests 2019, 10, 243. https://doi.org/10.3390/f10030243
Misi D, Puchałka R, Pearson C, Robertson I, Koprowski M. Differences in the Climate-Growth Relationship of Scots Pine: A Case Study from Poland and Hungary. Forests. 2019; 10(3):243. https://doi.org/10.3390/f10030243
Chicago/Turabian StyleMisi, Dávid, Radosław Puchałka, Charlotte Pearson, Iain Robertson, and Marcin Koprowski. 2019. "Differences in the Climate-Growth Relationship of Scots Pine: A Case Study from Poland and Hungary" Forests 10, no. 3: 243. https://doi.org/10.3390/f10030243
APA StyleMisi, D., Puchałka, R., Pearson, C., Robertson, I., & Koprowski, M. (2019). Differences in the Climate-Growth Relationship of Scots Pine: A Case Study from Poland and Hungary. Forests, 10(3), 243. https://doi.org/10.3390/f10030243





