Robinia pseudoacacia L. in Short Rotation Coppice: Seed and Stump Shoot Reproduction as well as UAS-based Spreading Analysis
Abstract
1. Introduction
- (1)
- What is the average germination of black locust seeds after six different seed treatments?
- (2)
- How many stump shoots survive in short rotation coppices depending on shoot age?
- (3)
- What is the average sprouting distance of black locust, depending on neighboring forest, meadow, farmland, and along a dirt road analyzed via OBIA in UAS images?
- (4)
- What is the accuracy and loss of black locust classification in single UAS images under varying conditions by using a CNN?
2. Materials and Methods
2.1. Site Description
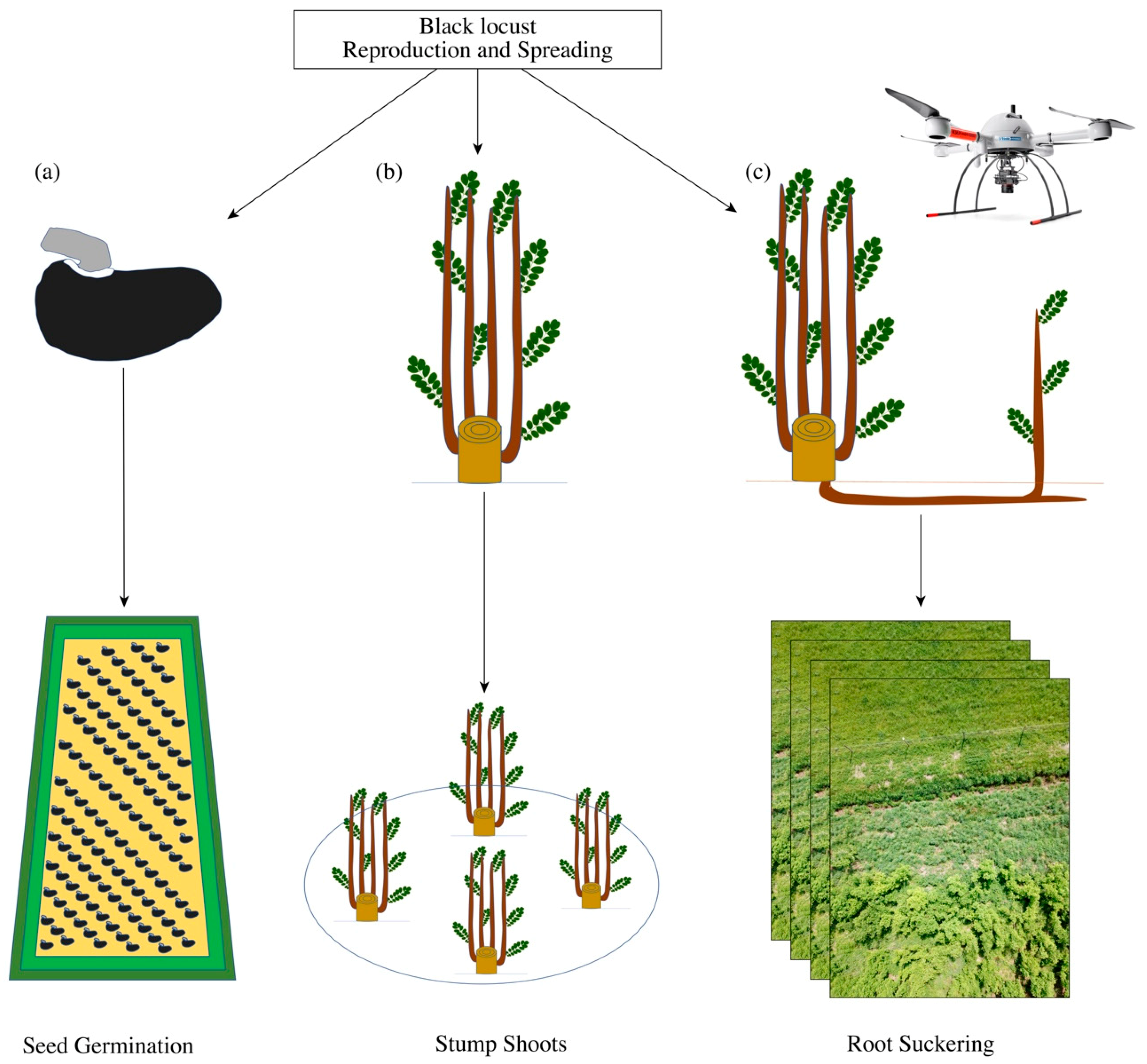
2.2. Reproduction Analysis
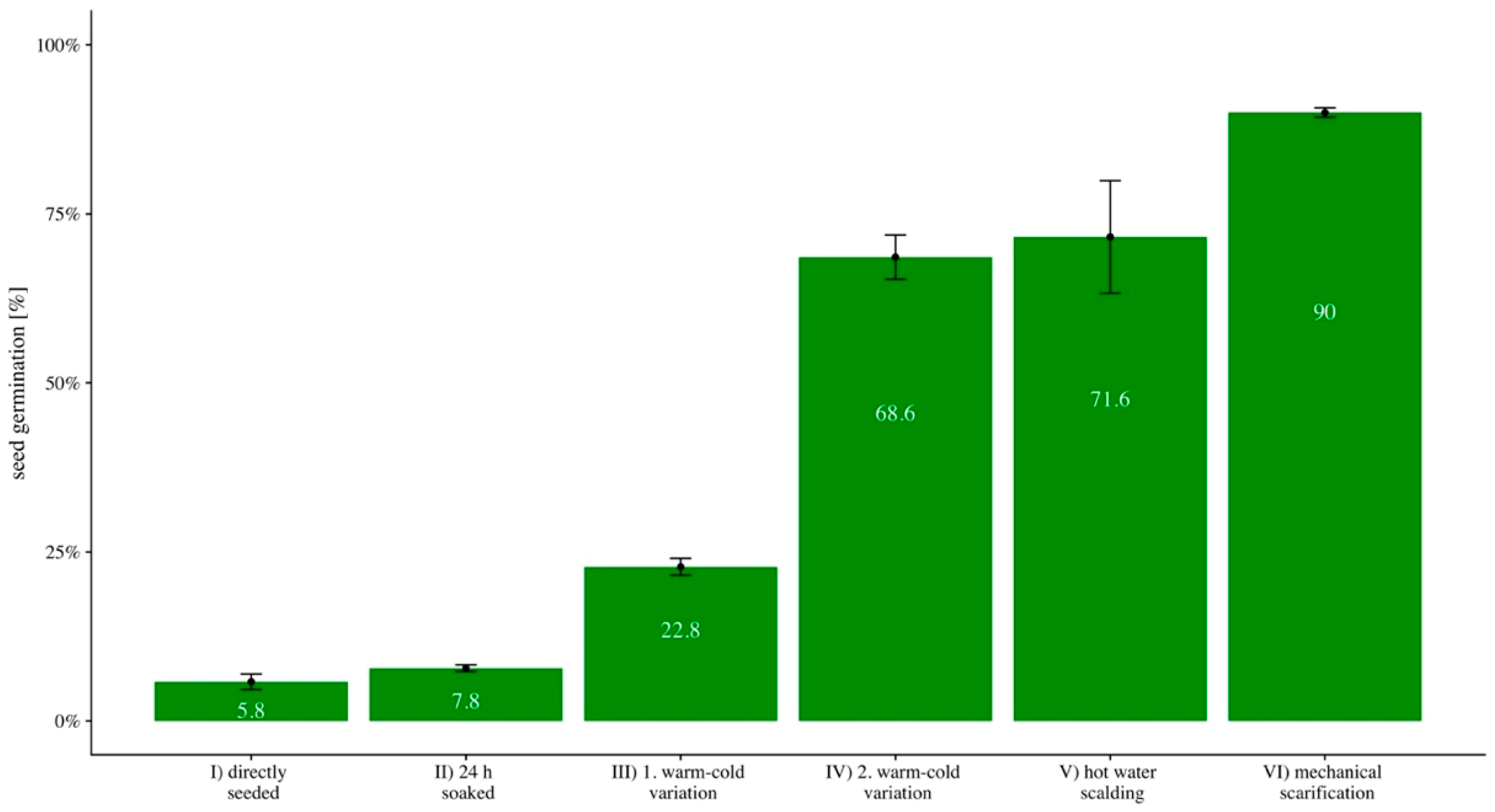
2.3. Seed Germination
- (I)
- seeds were seeded and watered,
- (II)
- seeds were soaked for 24 h in water at a water temperature of 18 °C (64.4 °F) and then seeded and watered,
- (III)
- seeds were stored at an air temperature of 45 °C (113 °F) for two hours, and thereafter stored at an air temperature of −20 °C (−4 °F) for a further two hours and then seeded and watered,
- (IV)
- seeds were stored at an air temperature of 60 °C (140 °F) for two hours and thereafter stored for two hours at an air temperature of −20 °C (−4 °F) and then seeded and watered,
- (V)
- seeds were scalded with hot water, seeded and watered, and
- (VI)
- seeds were mechanically scarified, seeded and watered.
2.4. Stump Shoot Analysis
2.5. Root Suckering (Spreading)
2.5.1. Object-Based Analysis (OBIA)
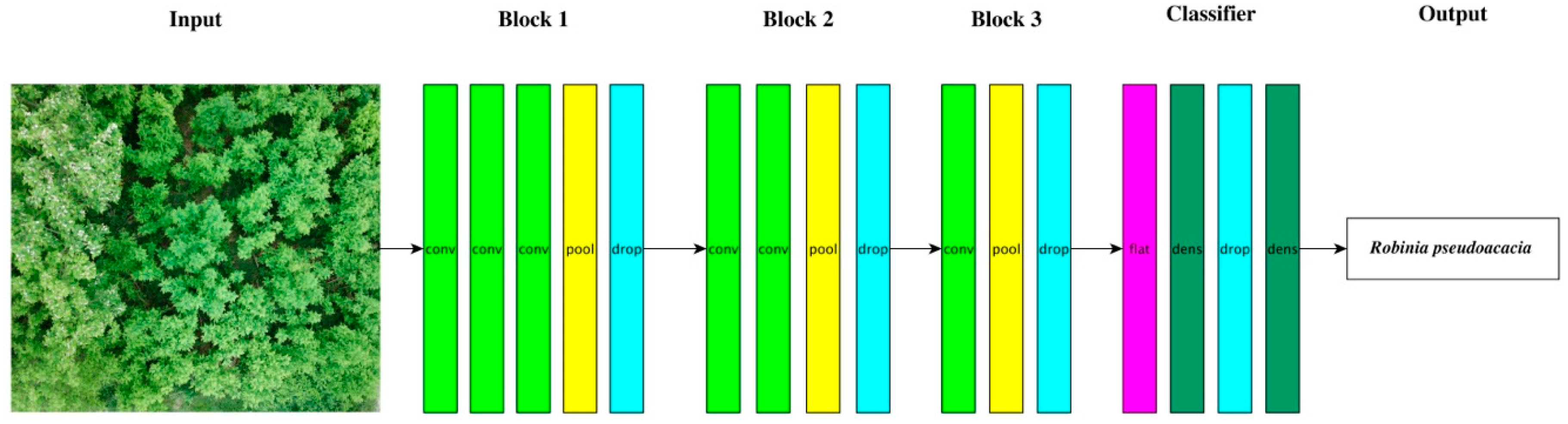
2.5.2. Classification via Deep Learning–CNN
3. Results
3.1. Seed Germination
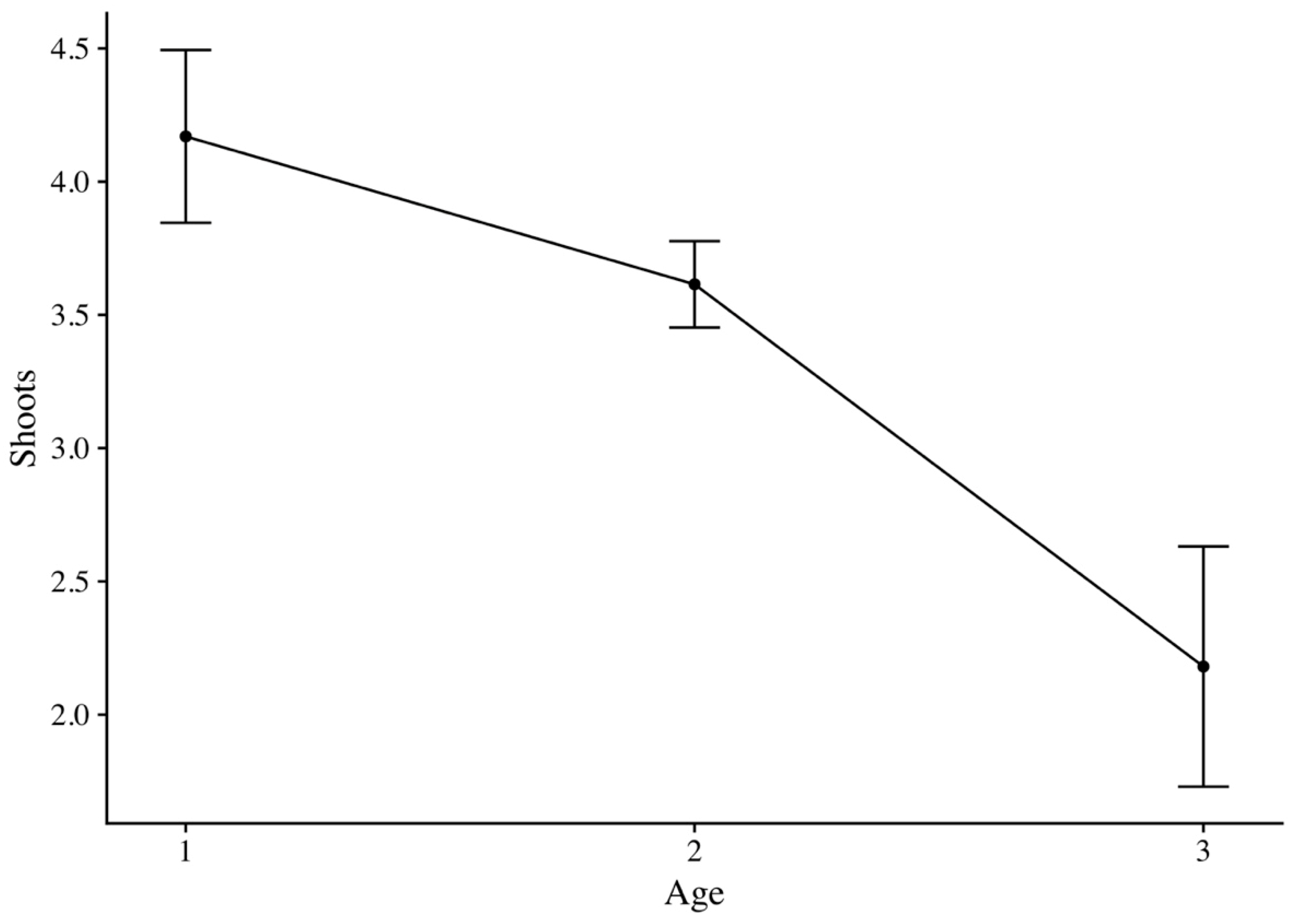
3.2. Stump Shoot
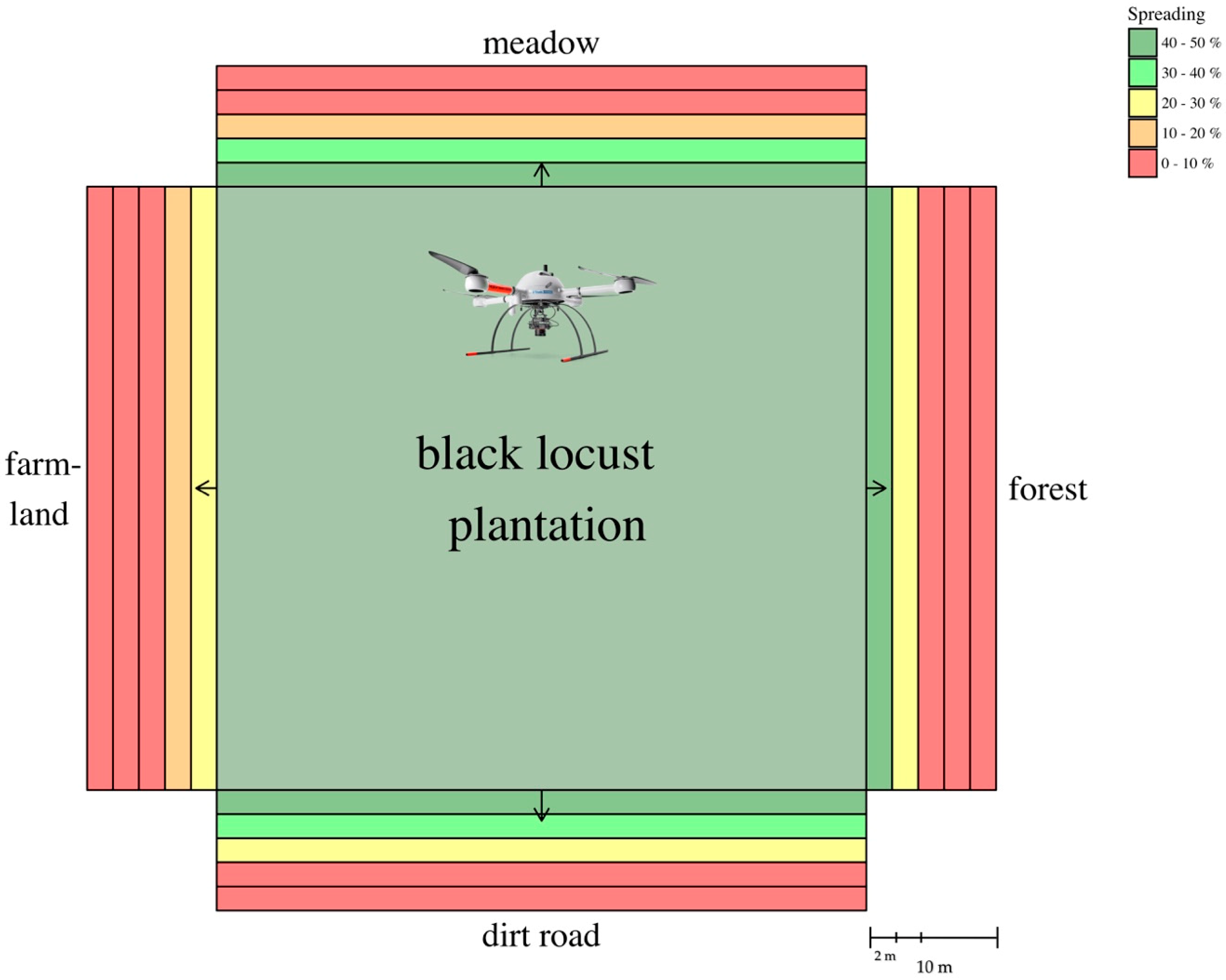
3.3. Root Suckering (Spreading) via OBIA
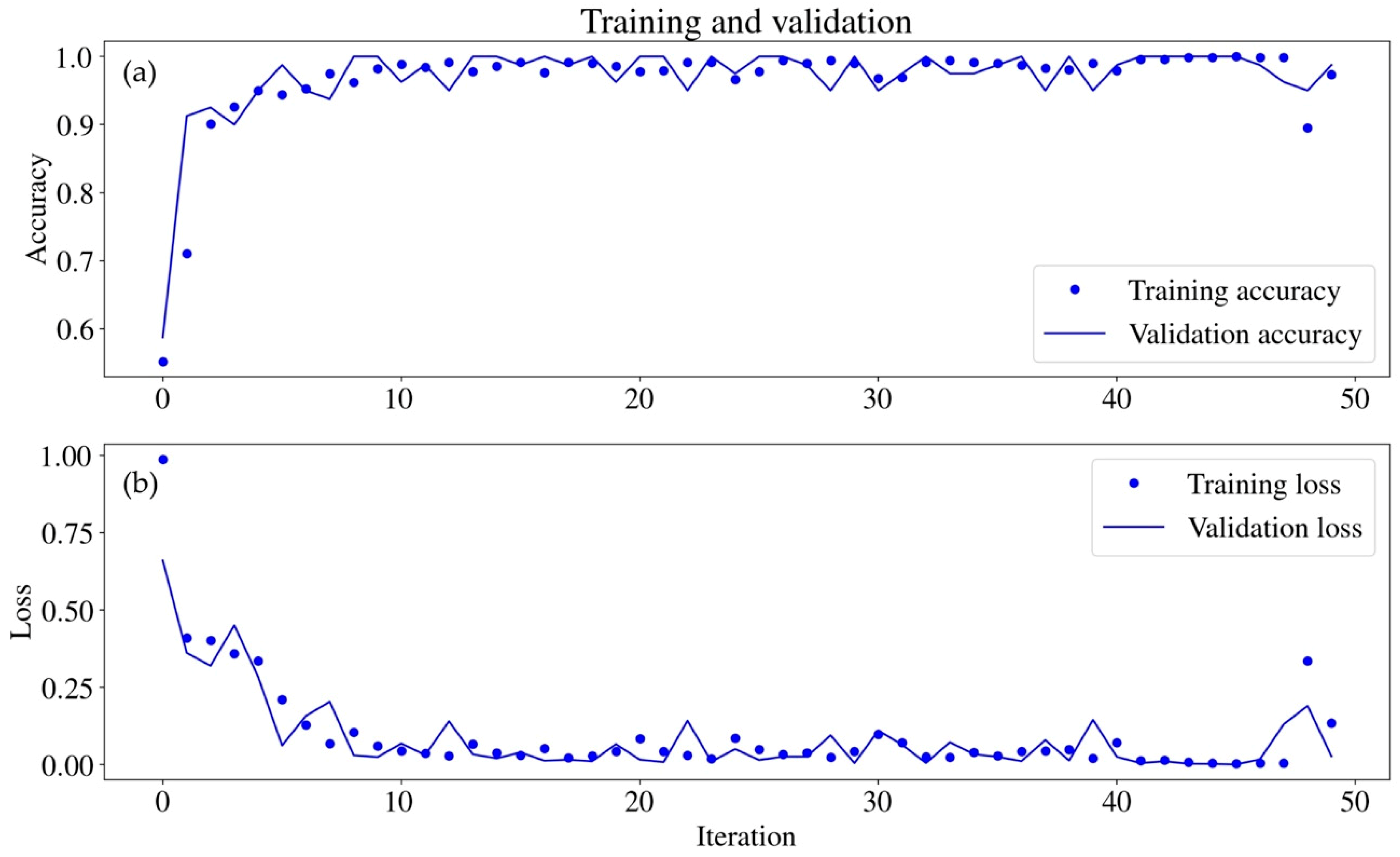
3.4. Classification via CNN
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Adam | Adaptive moment estimation |
| ANOVA | Analysis of Variance |
| CI | Confidence Interval |
| CNN | Convolutional Neural Network(s) |
| h | Hours |
| KIA | Kappa Index of Agreement |
| LiDAR | Light Detection and Ranging |
| NIR | Near-InfraRed |
| OBIA | Object-Based Image Analysis |
| QGIS | Quantum GIS |
| RGB | Red-Green-Blue |
| SD | Standard Deviation |
| SE | Standard Error of the Mean |
| SfM | Structure from Motion |
| UAS | Unmanned Aerial System(s) |
References
- Little, E.L. Atlas of United States Trees; Conifers and Important Hardwoods, US Department of Agriculture, Forest Service: Washington, DC, USA, 1971. [Google Scholar]
- Huntley, J.C. Robinia pseudoacacia L. In Silvics of North America, Vol. 2, Hardwoods; Burns, R.M., Honkala, B.H., Eds.; USDA Foreign Agricultural Service Handbook 654: Washington, DC, USA, 1990; pp. 755–761. [Google Scholar]
- Vadas, E. Das Lehrrevier und der botanische Garten der königl. ung. forstl. Hochschule als Versuchsfeld; Joerges: Selmecbánya, Slovakia, 1914; pp. 1–25. [Google Scholar]
- Ernyey, J. Die Wanderwege der Robinie und ihre Ansiedlung in Ungarn. Magy. Botan. Lapok 1927, 25, 161–191. [Google Scholar]
- Kolbek, J.; Vítková, M.; Vetvicka, V. Z historie stredoevropsky’ ch akátin a jejich spolecenstev. From history of Central European Robinia growths and its communities. Zpr. Ces. Bot. Spolec. 2004, 39, 287–298. [Google Scholar]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Wüstenhagen, R.; Bilharz, M. Green energy market development in Germany: Effective public policy and emerging customer demand. Energy Policy 2006, 34, 1681–1696. [Google Scholar] [CrossRef]
- Bielefeldt, J.; Bolte, A.; Busch, G.; Dohrenbusch, A.; Kroiher, F.; Lamersdorf, N.; Schulz, U.; Stoll, B. Energieholzproduktion in der Landwirtschaft. Chancen und Risiken aus Sicht der Natur-und Umweltschutzes; NABU Bundesverb, 2008; pp. 17–19. Available online: https://www.nabu.de/imperia/md/content/nabude/energie/biomasse/nabu-studie_energieholz.pdf (accessed on 12 January 2017).
- Bemmann, A.; Butler Manning, D. Energieholzplantagen in der Landwirtschaft; Agrimedia: Hannover, Germany, 2013; ISBN 978-3-86263-081-3. [Google Scholar]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Staska, B.; Essl, F.; Samimi, C. Density and age of invasive Robinia pseudoacacia modulate its impact on floodplain forests. Basic Appl. Ecol. 2014, 15, 551–558. [Google Scholar] [CrossRef]
- Vor, T.; Bolte, A.; Spellmann, H.; Ammer, C. Potenziale und Risiken eingeführter Baumarten—Baumartenportraits mit naturschutzfachlicher Bewertung; Universitätsverlag Göttingen: Göttingen, Germany, 2015; pp. 277–292. ISBN 978-3-86395-240-2. [Google Scholar]
- Willoughby, I.; Stokes, V.; Poole, J.; White, J.E.; Hodge, S.J. The potential of 44 native and non-native tree species for woodland creation on a range of contrasting sites in lowland Britain. Forestry 2007, 80, 531–553. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Wilhelm, G.J.; Thomsen, I.M.; Metzler, B.; Kirisits, T.; Havrdová, L.; Enderle, R.; Dobrowolska, D.; Cleary, M.; Clark, J. Silvicultural strategies for Fraxinus excelsior in response to dieback caused by Hymenoscyphus fraxineus. Forestry 2017, 90, 455–472. [Google Scholar] [CrossRef]
- Hoffmann, G. Die Stickstoffbindung der Robinie (Robinia pseudoacacia L.). Archiv für Forstwesen 1961, 10, 627–632. [Google Scholar]
- Hoffmann, G. Effektivität und Wirtsspezifität der Knöllchenbakterien von Robinia pseudoacacia L. Archiv für Forstwesen 1964, 13, 563–574. [Google Scholar]
- Rédei, K. Black Locust (Robinia pseudoacacia L.) Growing in Hungary; Hungarian Forest Research Institute: Sarvar, Hungary, 2013. [Google Scholar]
- Carl, C.; Landgraf, D.; van der Maaten-Theunissen, M.; Biber, P.; Pretzsch, H. Robinia pseudoacacia L. Flower Analyzed by Using Unmanned Aerial Vehicle (UAV). Remote Sens. 2017, 9, 1091. [Google Scholar] [CrossRef]
- Marjai, Z. Az akác-magbank. Erdészeti Lapok 1995, 130, 311–313. [Google Scholar]
- Farrar, J.L. Trees of the Northern United States and Canada; Blackwell Publishing: Ames, IA, USA, 1995; p. 502. [Google Scholar]
- Schubert, J. Lagerung und Vorbehandlung von Saatgut wichtiger Baum-und Straucharten; LÖBF: Eberswalde-Finow, Germany, 1998. [Google Scholar]
- Vines, R.A. Trees, Shrubs, and Woody Vines of the Southwest; University of Texas Press: Austin, TX, USA, 1960; Volume 1104, p. 7707. [Google Scholar]
- Rédei, K.; Csiha, I.; Keseru, Z.; Gál, J. Influence of regeneration method on the yield and stem quality of Black locust (Robinia pseudoacacia L.) stands: A case study. Acta Silv. Lign. Hung. 2012, 8, 103–112. [Google Scholar] [CrossRef]
- Bogoroditskii, I.I.; Sholokhov, L.V. German: Feuchtigkeitsregime von Robinia pseudoacacia Samen, vorbereitet für die Saat durch Vakuum-Wasser-Sättigungsmethode und durch Brühen in kochendem Wasser. Tr. Novocherkas. Inzh.-Melior. Inta 1975, 16, 115–118, (Original in Russian). [Google Scholar]
- Velkov, D. Influence of high temperatures on the water regime and viability of black locust (Robinia pseudoacacia L.) seeds. In Proceedings of the International Symposium on Seed Physiology of Woody Plants at Kornik, Panstwowe, Wydawnictwo Naukowe, Warszawa-Poznan, Poland, 3–8 September 1968; pp. 111–119. [Google Scholar]
- Hull, J.C.; Scott, R.C. Plant Succession on Debris Avalanches of Nelson County, Virginia. Castanea 1982, 47, 158–176. [Google Scholar]
- Martin, W.H. The Role and History of Fire in the Daniel Boone National Forest; Final Report; U.S. Department of Agriculture, Forest Service, Daniel Boone National Forest: Winchester, KY, USA, 1990; p. 131. [Google Scholar]
- Keresztesi, B. The Black Locust; Akadémiai Kiadó: Budapest, Hungary, 1988. [Google Scholar]
- Voss, E.G. Michigan Flora. Part II. Dicots (Saururaceae–Cornaceae); Bull. 59; Cranbrook Institute of Science: Bloomfield Hills, MI, USA; University of Michigan Herbarium: Ann Arbor, MI, USA, 1985; p. 724. [Google Scholar]
- Harrod, J.C.; Harmon, M.E.; White, P.S. Post-fire succession and 20th century reduction in fire frequency on xeric southern Appalachian sites. J. Veg. Sci. 2000, 11, 465–472. [Google Scholar] [CrossRef]
- Elliott, K.J.; Vose, J.M.; Clinton, B.D.; Knoepp, J.D. Effects of understory burning in a mesic mixed-oak forest of the southern Appalachians. In Fire in Temperate, Boreal, and Montane Ecosystems: Proceedings of the 22nd Tall Timbers Fire Ecology Conference: An International Symposium, Kananaskis Village, AB, Canada, 15–18 October 2001; Tall Timbers Research: Tallahassee, FL, USA, 2004; pp. 272–283. [Google Scholar]
- Stone, K.R. Robinia pseudoacacia. In Fire Effects Information System; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer), 2009; Available online: https://www.fs.fed.us/database/feis/plants/tree/robpse/all.html (accessed on 11 December 2018).
- Carl, C.; Biber, P.; Landgraf, D.; Buras, A.; Pretzsch, H. Allometric Models to Predict Aboveground Woody Biomass of Black Locust (Robinia pseudoacacia L.) in Short Rotation Coppice in Previous Mining and Agricultural Areas in Germany. Forests 2017, 8, 328. [Google Scholar] [CrossRef]
- Bernetti, G. Macchia coppices with prevalent Quercus ilex in Tuscany. In L’auxometria dei boschi cedui Italiani; Forestale e Montane: Tuscany, Italy, 1990; Volume 35, pp. 1–24. [Google Scholar]
- Reich, P.B.; Teskey, R.O.; Johnson, P.S.; Hinckley, T.M. Periodic root and shoot growth in oak. For. Sci. 1980, 26, 590–598. [Google Scholar] [CrossRef]
- Cobb, S.W.; Miller, A.E.; Zahner, R. Recurrent shoot flushes in scarlet oak stump sprouts. For. Sci. 1985, 31, 725–730. [Google Scholar] [CrossRef]
- Dimitrov, E.P.; Stiptsov, V. Yield table for coppice stands of Quercus cerris in Bulgaria. Gorsko Stopanstvo 1991, 47, 13–14. [Google Scholar]
- Beky, A. Yield of sessile oak coppice stands (Quercus petraea). Erdeszeti-Kutatasok 1991, 82–83, 176–192. [Google Scholar]
- Tatoni, T.; Roche, P. Comparison of old-field and forest revegetation dynamics in Provence. J. Veg. Sci. 1994, 5, 295–302. [Google Scholar] [CrossRef]
- Cañellas, I.; Montero, G.; Bachiller, A. Transformation of quejigo oak (Quercus faginea Lam.) coppice forest into high forest by thinning. Ann. Ist. Sper. Selvic. 1996, 27, 143–147. [Google Scholar]
- Cinnirella, S.; Iovino, F.; Porto, P.; Ferro, V. Anti-erosive effectiveness of Eucalyptus coppices through the cover management factor estimate. Hydrol. Process 1998, 12, 635–649. [Google Scholar] [CrossRef]
- Chatziphilippidis, G.; Spyroglou, G. Modelling the Growth of Quercus frainetto in Greece. In Sustainable Forest Management–Growth Models for Europe; Springer: Berlin/Heidelberg, Germany, 2006; pp. 373–393. [Google Scholar]
- Fonti, P.; Cherubini, P.; Rigling, A.; Weber, P.; Biging, G. Tree rings show competition dynamics in abandoned Castanea sativa coppices after land-use changes. J. Veg. Sci. 2006, 17, 103–112. [Google Scholar] [CrossRef]
- Salazar-García, S.; Cossio-Vargas, L.E.; Lovatt, C.J.; González-Durán, I.J.; Pérez-Barraza, M.H. Crop Load Affects Vegetative Growth Flushes and Shoot Age Influences Irreversible Commitment to Flowering of ‘Hass’ Avocado. HortScience 2006, 41, 1541–1546. [Google Scholar]
- Kneifl, M.; Kadavý, J.; Knott, R. Gross value yield potential of coppice, high forest and model conversion of high forest to coppice on best sites. J. For. Sci. 2011, 57, 536–546. [Google Scholar] [CrossRef]
- Razakamanarivo, R.H.; Razakavololona, A.; Razafindrakoto, M.A.; Vieilledent, G.; Albrecht, A. Below-ground biomass production and allometric relationships of eucalyptus coppice plantation in the central highlands of Madagascar. Biomass Bioenergy 2012, 45, 1–10. [Google Scholar] [CrossRef]
- Zeckel, C. Betrachtung des Ertragspotenzials von Stockausschlägen der Robinie (Robinia pseudoacacia L.) von verschiedenen Waldstandorten geogenen und anthorpogenen Ausgangssubstrates in der Niederlausitz unter Berücksichtigung ihrer energetischen Nutzung. Diploma Thesis, Brandenburg University of Technology, Senftenberg, Germany, 2007; pp. 38–39. [Google Scholar]
- Ertle, C.; Böcker, L.; Landgraf, D. Wuchspotenzial von Stockausschlägen der Robinie. AFZ-Der Wald 2008, 63, 994–995. [Google Scholar]
- Dooley, T. Lessons learned from eleven years of prescribed fire at the Albany Pine Bush Preserve. In Using Fire to Control Invasive Plants: What’s New, What Works in the Northeast?—2003 Workshop Proceedings; University of New Hampshire, Cooperative Extension: Portsmouth, NH, USA; Durham, NH, USA, 2003; pp. 7–10. Available online: http://extension.unh.edu/resources/files/Resource000412_Rep434.pdf (accessed on 10 June 2016).
- Crosti, R.; Agrillo, E.; Ciccarese, L.; Guarino, R.; Paris, P.; Testi, A. Assessing escapes from short rotation plantations of the invasive tree species Robinia pseudoacacia L. in Mediterranean ecosystems: A study in central Italy. IFOREST 2016, e1–e8. [Google Scholar] [CrossRef]
- Ustin, S.L.; DiPietro, D.; Olmstead, K.; Underwood, E.; Scheer, G.J. Hyperspectral remote sensing for invasive species detection and mapping. In Proceedings of the 2002 IEEE International Geoscience and Remote Sensing Symposium, IGARSS’02, Toronto, ON, Canada, 24–28 June 2002; Volume 3, pp. 1658–1660. [Google Scholar]
- Zhao, Q.; Wang, F.; Zhao, J.; Zhou, J.; Yu, S.; Zhao, Z. Estimating Forest Canopy Cover in Black Locust (Robinia pseudoacacia L.) Plantations on the Loess Plateau Using Random Forest. Forests 2018, 9, 623. [Google Scholar] [CrossRef]
- Wallace, L.; Lucieer, A.; Malenovský, Z.; Turner, D.; Vopěnka, P. Assessment of forest structure using two UAV techniques: A comparison of airborne laser scanning and structure from motion (SfM) point clouds. Forests 2016, 7, 62. [Google Scholar] [CrossRef]
- Ota, T.; Ogawa, M.; Mizoue, N.; Fukumoto, K.; Yoshida, S. Forest Structure Estimation from a UAV-Based Photogrammetric Point Cloud in Managed Temperate Coniferous Forests. Forests 2017, 8, 343. [Google Scholar] [CrossRef]
- Mlambo, R.; Woodhouse, I.H.; Gerard, F.; Anderson, K. Structure from Motion (SfM) photogrammetry with drone data: A low cost method for monitoring greenhouse gas emissions from forests in developing countries. Forests 2017, 8, 68. [Google Scholar] [CrossRef]
- Mohan, M.; Silva, C.A.; Klauberg, C.; Jat, P.; Catts, G.; Cardil, A.; Hudak, A.T.; Dia, M. Individual tree detection from unmanned aerial vehicle (UAV) derived canopy height model in an open canopy mixed conifer forest. Forests 2017, 8, 340. [Google Scholar] [CrossRef]
- Guerra-Hernández, J.; González-Ferreiro, E.; Monleón, V.J.; Faias, S.P.; Tomé, M.; Díaz-Varela, R.A. Use of Multi-Temporal UAV-Derived Imagery for Estimating Individual Tree Growth in Pinus pinea Stands. Forests 2017, 8, 300. [Google Scholar] [CrossRef]
- Dempewolf, J.; Nagol, J.; Hein, S.; Thiel, C.; Zimmermann, R. Measurement of within-season tree height growth in a mixed forest stand using UAV imagery. Forests 2017, 8, 231. [Google Scholar] [CrossRef]
- Qiu, Z.; Feng, Z.K.; Wang, M.; Li, Z.; Lu, C. Application of UAV Photogrammetric System for Monitoring Ancient Tree Communities in Beijing. Forests 2018, 9, 735. [Google Scholar] [CrossRef]
- Alonzo, M.; Andersen, H.E.; Morton, D.C.; Cook, B.D. Quantifying Boreal Forest Structure and Composition Using UAV Structure from Motion. Forests 2018, 9, 119. [Google Scholar] [CrossRef]
- Feduck, C.; McDermid, G.; Castilla, G. Detection of coniferous seedlings in UAV imagery. Forests 2018, 9, 432. [Google Scholar] [CrossRef]
- Morales, G.; Kemper, G.; Sevillano, G.; Arteaga, D.; Ortega, I.; Telles, J. Automatic Segmentation of Mauritia flexuosa in Unmanned Aerial Vehicle (UAV) Imagery Using Deep Learning. Forests 2018, 9, 736. [Google Scholar] [CrossRef]
- Fraser, B.; Congalton, R. Evaluating the Effectiveness of Unmanned Aerial Systems (UAS) for Collecting Thematic Map Accuracy Assessment Reference Data in New England Forests. Forests 2019, 10, 24. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rogan, J.; Franklin, J.; Roberts, D.A. A comparison of methods for monitoring multitemporal vegetation change using Thematic Mapper imagery. Remote Sens. Environ. 2002, 80, 143–156. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Tang, L.; Shao, G. Drone remote sensing for forestry research and practices. J. For. Res. 2015, 26, 791–797. [Google Scholar] [CrossRef]
- Lehmann, J.R.; Prinz, T.; Ziller, S.R.; Thiele, J.; Heringer, G.; Meira-Neto, J.A.; Buttschardt, T.K. Open-source processing and analysis of aerial imagery acquired with a low-cost unmanned aerial system to support invasive plant management. Front. Environ. Sci. 2017, 5, 44. [Google Scholar] [CrossRef]
- Müllerová, J.; Bartaloš, T.; Brůna, J.; Dvořák, P.; Vítková, M. Unmanned aircraft in nature conservation: An example from plant invasions. Int. J. Remote Sens. 2017, 38, 2177–2198. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Katsanevakis, S.; Moustakas, A. Spatial downscaling of alien species presences using machine learning. Front. Earth Sci. 2017, 5, 60. [Google Scholar] [CrossRef]
- Li, W.; Dong, R.; Fu, H. Large-Scale Oil Palm Tree Detection from High-Resolution Satellite Images Using Two-Stage Convolutional Neural Networks. Remote Sens. 2019, 11, 11. [Google Scholar] [CrossRef]
- Denize, J.; Hubert-Moy, L.; Betbeder, J.; Corgne, S.; Baudry, J.; Pottier, E. Evaluation of Using Sentinel-1 and-2 Time-Series to Identify Winter Land Use in Agricultural Landscapes. Remote Sens. 2019, 11, 37. [Google Scholar] [CrossRef]
- Zhou, K.; Lindenbergh, R.; Gorte, B. Automatic Shadow Detection in Urban Very-High-Resolution Images Using Existing 3D Models for Free Training. Remote Sens. 2019, 11, 72. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, H.; Wang, C.; Wang, Y.; Xu, L. Multi-Temporal SAR Data Large-Scale Crop Mapping Based on U-Net Model. Remote Sens. 2019, 11, 68. [Google Scholar] [CrossRef]
- Duarte-Carvajalino, J.; Alzate, D.; Ramirez, A.; Santa-Sepulveda, J.; Fajardo-Rojas, A.; Soto-Suárez, M. Evaluating Late Blight Severity in Potato Crops Using Unmanned Aerial Vehicles and Machine Learning Algorithms. Remote Sens. 2018, 10, 1513. [Google Scholar] [CrossRef]
- Duarte, D.; Nex, F.; Kerle, N.; Vosselman, G. Multi-Resolution Feature Fusion for Image Classification of Building Damages with Convolutional Neural Networks. Remote Sens. 2018, 10, 1636. [Google Scholar] [CrossRef]
- Lu, T.; Ming, D.; Lin, X.; Hong, Z.; Bai, X.; Fang, J. Detecting building edges from high spatial resolution remote sensing imagery using richer convolution features network. Remote Sens. 2018, 10, 1496. [Google Scholar] [CrossRef]
- DWD. Deutscher Wetterdienst Archiv Monats- und Tageswerte. 2018. Available online: http://www.dwd.de (accessed on 10 June 2018).
- ESRI. ArcGIS 9.2. © Environmental Systems Research Institute. 2007. Available online: http://www.esri.com/software/arcgis/eval-help/arcgis-92 (accessed on 19 March 2017).
- Microdrones. MD4-1000. 2018. Available online: https://www.microdrones.com/de/integrated-systems/mdmapper1000dg/ (accessed on 1 October 2018).
- R Core Team. R: A Language and Environment for Statistical Computing—Version R 3.3.2 GUI 1.68. Available online: https://www.R-project.org/ (accessed on 2 November 2016).
- Hain, J. Statistik mit R: Grundlagen der Datenanalyse; RRZN-Handbook, Regionales Rechenzentrum für Niedersachsen/Leipnitz Universität Hannover/Lehrstuhl für Mathematik VIII (Statistik) der Universität Würzburg: Würzburg/Hannover, Germany, 2011; pp. 176–187. [Google Scholar]
- SZ DJI Technology Co., Ltd. DJI MAVIC Pro. 2018. Available online: https://www.dji.com/de/mavic (accessed on 10 October 2018).
- Mapir Camera. Mapir Survey 3 Camera. 2018. Available online: https://www.mapir.camera/collections/survey3 (accessed on 12 June 2018).
- Sony Europe Limited. SONY-ILCE-5100. 2018. Available online: https://www.sony.de/electronics/wechselobjektivkameras/ilce-5100-body-kit (accessed on 12 August 2018).
- Trimble. DGPS—Trimble. 2018. Available online: https://www.trimble.com/gps_tutorial/dgps.aspx (accessed on 10 June 2018).
- Dandois, J.P.; Olano, M.; Ellis, E.C. Optimal altitude, overlap, and weather conditions for computer vision UAV estimates of forest structure. Remote Sens. 2015, 7, 13895–13920. [Google Scholar] [CrossRef]
- Pix4D S.A. Pix4D Mapper. 2018. Available online: https://cloud.pix4d.com/store/?=&solution=pro#solution_pro (accessed on 1 October 2018).
- Trimble. eCognition Developer Software. 2018. Available online: http://www.ecognition.com/suite/ecognition-developer (accessed on 1 May 2018).
- Blaschke, T. Object based image analysis for remote sensing. ISPRS 2010, 65, 2–16. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation 2018. Available online: http://qgis.osgeo.org (accessed on 8 May 2018).
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data—Principles and Practices, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Python Software Foundation. Python Language Reference, Version 3.7.1. 2018. Available online: http://www.python.org (accessed on 10 August 2018).
- Kluyver, T.; Ragan-Kelley, B.; Pérez, F.; Granger, B.E.; Bussonnier, M.; Frederic, J.; Kelly, K.; Hamrick, J.; Grout, J.; Corlay, S.; et al. Jupyter Development Team. Jupyter Notebooks—A publishing format for reproducible computational workflows. ELPUB 2016, 87–90. [Google Scholar] [CrossRef]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-scale Machine Learning on Heterogeneous Systems. 2015. Available online: Tensorflow.org (accessed on 10 August 2018).
- Chollet, F. Keras: The Python Deep Learning Library. 2015. Available online: https://keras.io (accessed on 10 August 2018).
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Oliphant, T.E. A Guide to NumPy; Trelgol Publishing: Spanish Fork, UT, USA, 2006; Volume 1, p. 85. [Google Scholar]
- McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; Volume 445, pp. 51–56. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv, 2014; arXiv:1409.1556. [Google Scholar]
- Nguyen, C.N.; Zeigermann, O. Machine Learning, kurz und gut; O’Reillys Taschenbibliothek: Heidelberg, Germany, 2018; ISBN 978-3-96009-052-6. [Google Scholar]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. In Proceedings of the Advances in Neural Information Processing Systems, Lake Tahoe, NV, USA, 3–6 December 2012; pp. 1097–1105. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv, 2014; arXiv:1412.6980. [Google Scholar]
- Makkar, H.P.S.; Becker, K. Do tannins in leaves of trees and shrubs from African and Himalayan regions differ in level and activity? Agrofor. Syst. 1998, 40, 59–68. [Google Scholar] [CrossRef]
- Auld, B.; Morita, H.; Nishida, T.; Ito, M.; Michael, P. Shared exotica: Plant invasions of Japan and south eastern Australia. Cunninghamia 2003, 8, 147–152. [Google Scholar]
- Fuentes, N.; Ugarte, E.; Kühn, I.; Klotz, S. Alien plants in southern South America. A framework for evaluation and management of mutual risk of invasion between Chile and Argentina. Biol. Invas. 2010, 12, 3227–3236. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; Lippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Li, T.; Liu, G. Age-related changes of carbon accumulation and allocation in plants and soil of black locust forest on Loess Plateau in Ansai County, Shaanxi Province of China. Chin. Geogr. Sci. 2014, 24, 414–422. [Google Scholar] [CrossRef]
- Akatov, V.V.; Akatova, T.V.; Shadzhe, A.E. Robinia pseudoacacia L. in the Western Caucasus. Russ. J. Biol. Invas. 2016, 7, 105–118. [Google Scholar] [CrossRef]
- Lei, J.; Nan, L.; Bojie, F.; Guangyao, G.; Shuai, W.; Tiantian, J.; Liwei, Z.; Jianbo, L.; Di, Z. Comparison of transpiration between different aged black locust (Robinia pseudoacacia) trees on the semi-arid Loess Plateau, China. J. Arid. Land. 2016, 8, 604–617. [Google Scholar] [CrossRef]
- Tompa, K.; Szent-Istvany, A. German: Die Vorbereitung der Robiniensamen zur Saat mit Hilfe des Scheiben-Skarifikators; Erdömernöki Föiskola, Erdötelepites-Es Fasitastani Tanszek, Sopron, Sonderdr. O. J.: Sopron, Hungary, 1963; pp. 105–121. [Google Scholar]
- Czarapata, E.J. Invasive Plants of the Upper Midwest: An Illustrated Guide to Their Identification and Control; The University of Wisconsin Press: Madison, WI, USA, 2005; p. 215. [Google Scholar]
- Clark, F.B. Forest Planting on Strip-Mined Land; Technical Paper No. 141; U.S. Department of Agriculture, Forest Service, Central States Forest Experiment Station: Columbus, OH, USA, 1954; p. 33. [Google Scholar]
- Geyer, W.A. Biomass yield potential of short-rotation hardwoods in the Great Plains. Biomass 1989, 20, 167–175. [Google Scholar] [CrossRef]
- Carl, C.; Biber, P.; Veste, M.; Landgraf, D.; Pretzsch, H. Key drivers of competition and growth partitioning among Robinia pseudoacacia L. trees. For. Ecol. Manag. 2018, 430, 86–93. [Google Scholar] [CrossRef]
- Dünisch, O.; Koch, G.; Dreiner, K. Verunsicherung uber die Eigenschaften von Robinienholz. Holz-Zentralblatt 2007, 39, 1061–1062. [Google Scholar]
- Koch, G.; Dünisch, O. Juvenile wood in Robinie—Qualität von Robinienholz (Ro-binia pseudoacacia L.) und Folgerungen für Holzbearbeitung und Produktqualität; Abschlussbericht für das DGfH/AIF-Forschungsvorhaben, Fraunhofer IRB Verl.: Stuttgart, Germany, 2008. [Google Scholar]
- Dünisch, O.; Richter, H.-G.; Koch, G. Wood properties of juvenile and mature heartwood in Robinia pseudoacacia L. Wood Sci. Technol. 2010, 44, 301–313. [Google Scholar] [CrossRef]
- Latorraca, J.V.F.; Dünisch, O.; Koch, G. Chemical composition and natural durability of juvenile and mature heartwood of Robinia pseudoacacia L. Anais da Academia Brasileira de Ciências 2011, 83, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Guo, W.; Wang, R.; Xu, W.; Du, N.; Wang, Y. Leaf movement and photosynthetic plasticity of black locust (Robinia pseudoacacia) alleviate stress under different light and water conditions. Acta Physiol. Plant 2009, 31, 553–563. [Google Scholar] [CrossRef]
- Kowarik, I. Funktionen klonalen Wachstums von Bäumen bei der Brachflächen-Sukzession unter besonderer Beachtung von Robinia pseudoacacia. Verh. Ges. Ökologie 1996, 26, 173–181. [Google Scholar]
- Grese, R. The landscape architect and problem exotic plants. In Proceedings of the American Society of Landscape Architects’ Open Committee on Reclamation: Reclamation Diversity, San Diego, CA, USA, 29 October 1991; Burley, J.B., Ed.; American Society of Landscape Architects: Washington, DC, USA, 1991; pp. 7–15. [Google Scholar]
- Van Miegroet, H.; Cole, D.W. The impact of nitrification on soil acidification and cation leaching in a red alder ecosystem. J. Environ. Qual. 1984, 13, 586–590. [Google Scholar] [CrossRef]
- Montagnini, F.; Haines, B.; Swank, W.T. Soil-solution chemistry in black locust, pine mixed-hardwoods and oak hickory forest stands in the Southern Appalachians, USA. For. Ecol. Manag. 1991, 40, 199–208. [Google Scholar] [CrossRef]
- Malcolm, G.M.; Bush, D.S.; Rice, S.K. Soil nitrogen conditions approach preinvasion levels following restoration of nitrogen-fixing black locust (Robinia pseudoacacia) stands in a pine-oak ecosystem. Restor. Ecol. 2008, 16, 70–78. [Google Scholar] [CrossRef]
- Fischer, M.; Stöcklin, J. Local extinctions of plants in remnants of extensively used calcareous grasslands 1950–1985. Conserv. Biol. 1997, 11, 727–737. [Google Scholar] [CrossRef]
- de Sá, N.C.; Castro, P.; Carvalho, S.; Marchante, E.; López-Núñez, F.A.; Marchante, H. Mapping the Flowering of an Invasive Plant Using Unmanned Aerial Vehicles: Is There Potential for Biocontrol Monitoring? Front. Plant Sci. 2018, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Dubash, N.K. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Oblack, R. Nitrogen—Gases in the Atmosphere. ThoughtCo. 22 June 2018. Available online: Thoughtco.com/nitrogen-in-the-atmosphere-3444094 (accessed on 9 August 2018).
- Gordon, N.; Holland, E. Nitrogen in the Earth System—Background on the Science, People, and Issues Involved in Nitrogen Cycle Research. 2015. Available online: https://www2.ucar.edu/news/backgrounders/nitrogen-earth-system (accessed on 9 August 2018).
- Strode, D.D. Black locust/Robinia pseudoacacia L. In Woody Plants as Wildlife Food Species. SO-16; U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station: Atlanta, GA, USA, 1977; pp. 215–216. [Google Scholar]
- Bartha, D.; Csiszár, Á.; Zsigmond, V. Black locust (Robinia pseudoacacia L.). In The Most Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; Institute of Ecology and Botany, Hungarian Academy of Sciences: Vácrátót, Hungary, 2008; pp. 63–76. [Google Scholar]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Nevalainen, O.; Honkavaara, E.; Tuominen, S.; Viljanen, N.; Hakala, T.; Yu, X.; Hyyppä, J.; Heikki, S.; Pölönen, I.; Imai, N.N.; et al. Individual tree detection and classification with UAV-based photogrammetric point clouds and hyperspectral imaging. Remote Sens. 2017, 9, 185. [Google Scholar] [CrossRef]
- Wallace, L.; Lucieer, A.; Watson, C.; Turner, D. Development of a UAV-LiDAR system with application to forest inventory. Remote Sens. 2012, 4, 1519–1543. [Google Scholar] [CrossRef]
- Yan, G.; Mas, J.F.; Maathuis, B.H.P.; Xiangmin, Z.; Van Dijk, P.M. Comparison of pixel-based and object-oriented image classification approaches—A case study in a coal fire area, Wuda, Inner Mongolia, China. Int. J. Remote Sens. 2006, 27, 4039–4055. [Google Scholar] [CrossRef]
- Weih, R.C.; Riggan, N.D. Object-based classification vs. pixel-based classification: Comparative importance of multi-resolution imagery. The International Archives of the Photogrammetry. Remote Sens. Spat. Inf. Sci. 2010, 38, C7. [Google Scholar]
- Lehmann, J.R.; Münchberger, W.; Knoth, C.; Blodau, C.; Nieberding, F.; Prinz, T.; Pancotto, V.A.; Kleinebecker, T. High-resolution Classification of South Patagonian Peat Bog Microforms Reveals Potential Gaps in Up-scaled CH4 Fluxes by use of Unmanned Aerial System (UAS) and CIR Imagery. Remote Sens. 2016, 8, 173. [Google Scholar] [CrossRef]
- Ammour, N.; Alhichri, H.; Bazi, Y.; Benjdira, B.; Alajlan, N.; Zuair, M. Deep learning approach for car detection in UAV imagery. Remote Sens. 2017, 9, 312. [Google Scholar] [CrossRef]
- Cao, R.; Zhu, J.; Tu, W.; Li, Q.; Cao, J.; Liu, B.; Zhang, Q.; Qiu, G. Integrating Aerial and Street View Images for Urban Land Use Classification. Remote Sens. 2018, 10, 1553. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Z.; Feng, Y.; Chen, Z. Road Extraction from High-Resolution Remote Sensing Imagery Using Deep Learning. Remote Sens. 2018, 10, 1461. [Google Scholar] [CrossRef]
- Liu, T.; Abd-Elrahman, A. An Object-Based Image Analysis Method for Enhancing Classification of Land Covers Using Fully Convolutional Networks and Multi-View Images of Small Unmanned Aerial System. Remote Sens. 2018, 10, 457. [Google Scholar] [CrossRef]
- Diegues, A.; Pinto, J.; Ribeiro, P. Automatic Habitat Mapping using Convolutional Neural Networks. IEEE OES AUV 2018. Available online: https://www.researchgate.net/profile/Jose_Pinto17/publication/330449471_Automatic_Habitat_Mapping_using_Convolutional_Neural_Networks/links/5c408fc9458515a4c72d24b7/Automatic-Habitat-Mapping-using-Convolutional-Neural-Networks.pdf (accessed on 12 August 2018).
- Abrams, J.F.; Vashishtha, A.; Wong, S.T.; Nguyen, A.; Mohamed, A.; Wieser, S.; Kuijper, A.; Wilting, A.; Mukhopadhyay, A. Habitat-Net: Segmentation of habitat images using deep learning. bioRxiv 2018, 483222. [Google Scholar] [CrossRef]
- LeCun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Paszke, A.; Chaurasia, A.; Kim, S.; Culurciello, E. Enet: A deep neural network architecture for real-time semantic segmentation. arXiv, 2016; arXiv:1606.02147. [Google Scholar]
- Garcia-Garcia, A.; Orts-Escolano, S.; Oprea, S.; Villena-Martinez, V.; Garcia-Rodriguez, J. A review on deep learning techniques applied to semantic segmentation. arXiv, 2017; arXiv:1704.06857. [Google Scholar]
- Flohr, F.; Gavrila, D. PedCut: An iterative framework for pedestrian segmentation combining shape models and multiple data cues. BMVC 2013. [Google Scholar] [CrossRef]
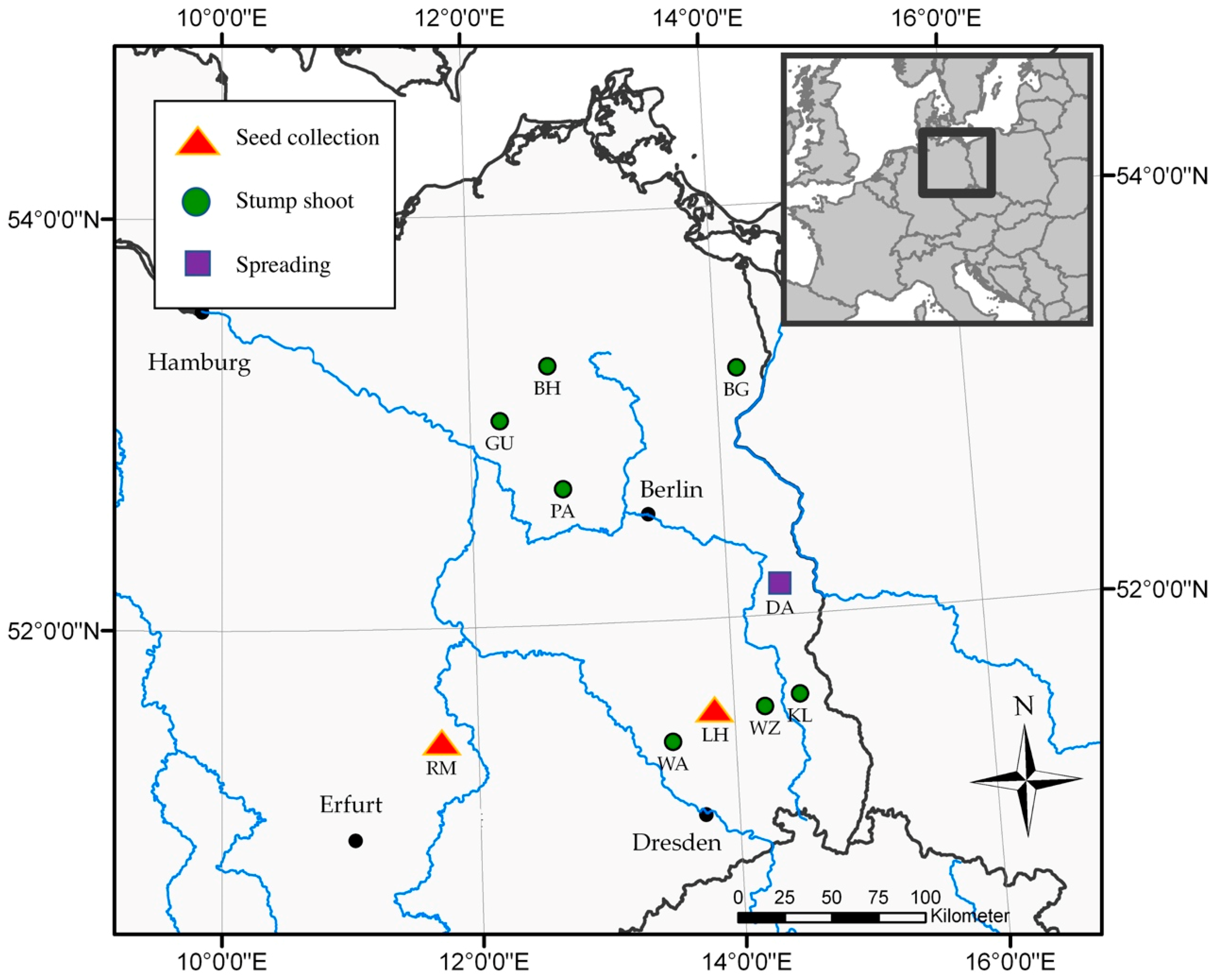
| Site Abbreviation | Location | Long (°E) | Lat (°N) | Tree/Shoot Age | Analysis |
|---|---|---|---|---|---|
| BG | Blumberg | 14°10′24″ | 53°12′25″ | 3 | Stump shoot |
| BH | Buchholz | 12°38′9″ | 53°15′34″ | 2 | Stump shoot |
| DA | Grunow-Dammendorf | 14°25′5″ | 52°8′26″ | 4 | Spreading |
| GU | Gumtow | 12°14′11″ | 52°59′46″ | 2 | Stump shoot |
| KL | Klein Loitz | 14°30′57″ | 51°36′35″ | 3 | Stump shoot |
| LH | Lauchhammer | 13°50′57″ | 51°32′20″ | 8 | Seed collection |
| PA | Paulinenaue | 12°43′52″ | 52°39′44″ | 3 | Stump shoot |
| RM | Röblingen | 11°42′42″ | 51°25′57″ | 8 | Seed collection |
| WA | Wainsdorf | 13°29′4″ | 51°24′50″ | 1 | Stump shoot |
| WZ | Welzow | 14°14′7″ | 51°33′32″ | 1 | Stump shoot |
| Treatment | I | II | III | IV | V |
|---|---|---|---|---|---|
| II | 0.3365 | ||||
| III | <0.001 | <0.001 | |||
| IV | <0.001 | <0.001 | <0.001 | ||
| V | 0.0086 | 0.009 | 0.015 | 0.750 | |
| VI | <0.001 | <0.001 | <0.001 | 0.011 | 0.274 |
| Site Abbreviation | Shoot Age | Plots | Data | Average Shoots | Shoots Per Age | SD | SE | CI | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| WA | 1 | 4 | 680 | 2.87 | 4.17 | 1.12 | 0.32 | 0.71 | <0.001 |
| WZ | 8 | 1331 | 4.82 | ||||||
| BH | 2 | 6 | 1000 | 3.98 | 3.61 | 0.61 | 0.16 | 0.35 | |
| GU | 8 | 1314 | 3.34 | ||||||
| BG | 3 | 3 | 441 | 1.56 | 2.18 | 1.19 | 0.45 | 1.10 | |
| KL | 3 | 478 | 3.38 | ||||||
| PA | 1 | 167 | 0.44 |
| Zone | 0–2 m (%) | 2–4 m (%) | 4–6 m (%) | 6–8 m (%) | 8–10 m (%) | Average Distance (m) |
|---|---|---|---|---|---|---|
| Meadow | 43.99 | 32.37 | 14.86 | 3.00 | 0.33 | 1.89 |
| Farmland | 27.45 | 19.60 | 9.78 | 0.91 | 0.07 | 1.16 |
| Dirt road | 41.96 | 37.81 | 26.07 | 6.38 | 0.58 | 2.26 |
| Forest | 40.23 | 25.42 | 7.79 | 1.14 | 0.002 | 1.49 |
| Actual | |||
|---|---|---|---|
| R. pseudoacacia | Non-R. pseudoacacia | ||
| Predicted | R. pseudoacacia | 177 | 7 |
| Non-R. pseudoacacia | 5 | 174 | |
| Producer Accuracy | 0.975 | 0.962 | |
| User Accuracy | 0.963 | 0.972 | |
| KIA Class | 0.942 | 0.928 | |
| Overall Accuracy | 0.966 | ||
| KIA Overall | 0.932 | ||
| (a) Robinia pseudoacacia L. | (b) Non Robinia pseudoacacia L. | ||
 | 1 True 1 Pred |  | 0 True 0 Pred |
 | 1 True 1 Pred |  | 0 True 0 Pred |
 | 1 True 1 Pred |  | 0 True 0 Pred |
 | 1 True 1 Pred |  | 0 True 0 Pred |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carl, C.; Lehmann, J.R.K.; Landgraf, D.; Pretzsch, H. Robinia pseudoacacia L. in Short Rotation Coppice: Seed and Stump Shoot Reproduction as well as UAS-based Spreading Analysis. Forests 2019, 10, 235. https://doi.org/10.3390/f10030235
Carl C, Lehmann JRK, Landgraf D, Pretzsch H. Robinia pseudoacacia L. in Short Rotation Coppice: Seed and Stump Shoot Reproduction as well as UAS-based Spreading Analysis. Forests. 2019; 10(3):235. https://doi.org/10.3390/f10030235
Chicago/Turabian StyleCarl, Christin, Jan R. K. Lehmann, Dirk Landgraf, and Hans Pretzsch. 2019. "Robinia pseudoacacia L. in Short Rotation Coppice: Seed and Stump Shoot Reproduction as well as UAS-based Spreading Analysis" Forests 10, no. 3: 235. https://doi.org/10.3390/f10030235
APA StyleCarl, C., Lehmann, J. R. K., Landgraf, D., & Pretzsch, H. (2019). Robinia pseudoacacia L. in Short Rotation Coppice: Seed and Stump Shoot Reproduction as well as UAS-based Spreading Analysis. Forests, 10(3), 235. https://doi.org/10.3390/f10030235







