Assessing the Impact of Ozone on Forest Trees in An Integrative Perspective: Are Foliar Visible Symptoms Suitable Predictors for Growth Reduction? A Critical Review
Abstract
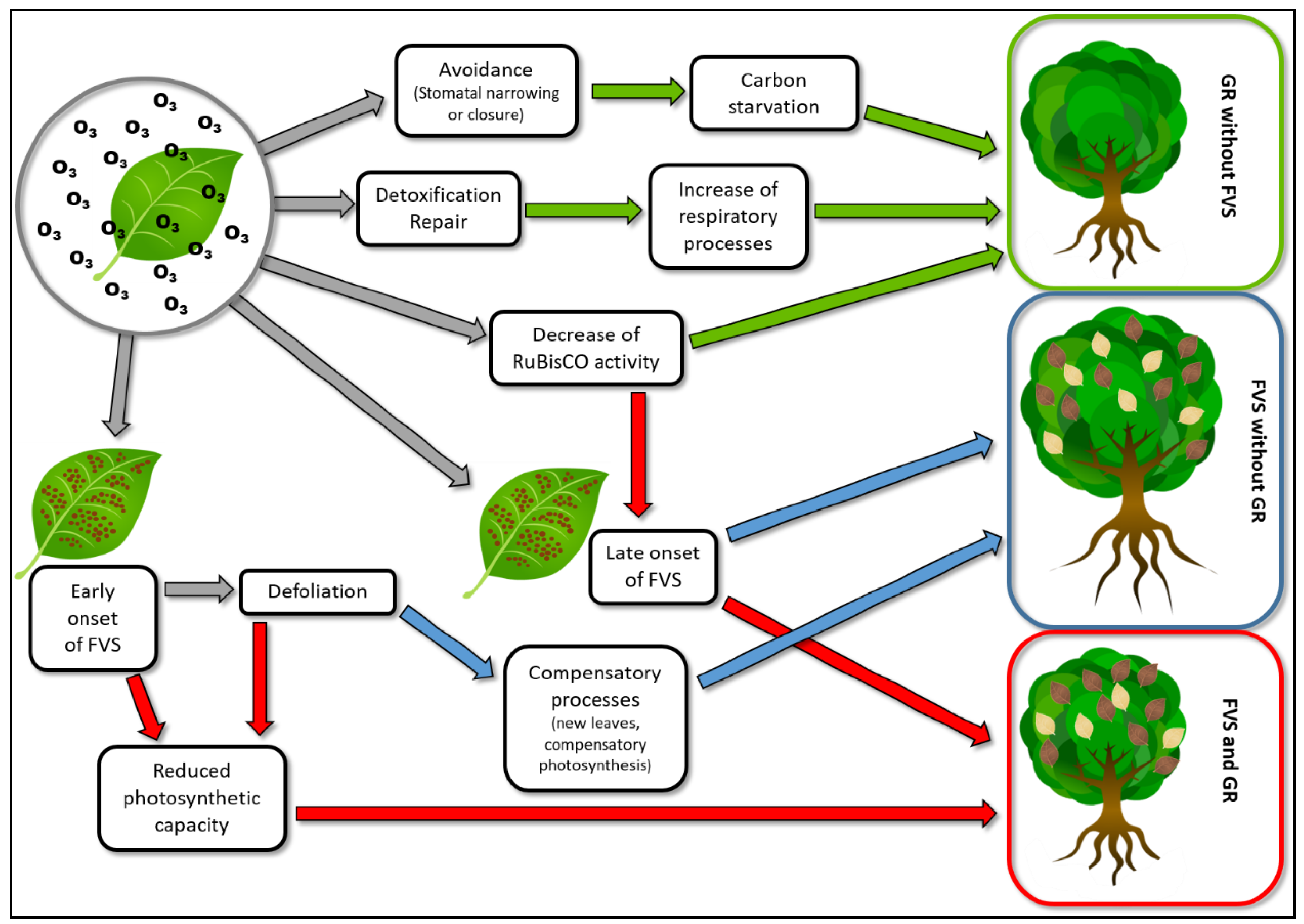
1. Introduction
2. Factors Implied in the Onset of Foliar Visible Symptoms
3. Factors Implied in Growth Reduction
4. Why Foliar Visible Symptoms and Growth Reduction Are Frequently Decoupled?
4.1. Compensatory Photosynthesis
4.2. Modest Amount of Injured Leaves
4.3. Late Season Onset of Foliar Visible Symptoms (After the End of the Growth Process of Plant)
5. Significance of Foliar Visible Symptoms Assessment in the Field
5.1. Variability of the Symptomatology
5.2. Variability of O3 Sensitivity within the Same Species
5.3. Variability in Plant Communities
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Todd, G.W. Effect of ozone and ozonated 1-hexene on respiration and photosynthesis of leaves. Plant Physiol. 1958, 33, 416. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, J.; Skärby, L.; Ashmore, M.R. Critical levels for ozone effects on vegetation in Europe. Environ. Pollut. 1997, 97, 91–106. [Google Scholar] [CrossRef]
- Büker, P.; Feng, Z.; Uddling, J.; Briolat, A.; Alonso, R.; Braun, S.; Elvira, S.; Gerosa, G.; Karlsson, P.E.; Le Thiec, D.; et al. New flux based dose-response relationships for ozone for European forest tree species. Environ. Pollut. 2015, 206, 163–174. [Google Scholar] [CrossRef] [PubMed]
- CLRTAP, Chapter III of Manual on methodologies and criteria for modelling and mapping critical loads and levels and air pollution effects, risks and trends. In Mapping Critical Levels for Vegetation. UNECE Convention on Long-Range Transboundary Air Pollution; Umwelbundesamt: Berlin, Germany, 2017; Available online: https://www.umweltbundesamt.de/sites/default/files/medien/4292/dokumente/ch3-mapman-2017-10.pdf (accessed on 2 October 2019).
- Ashmore, M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- Guderian, R. Air pollution—Phytotoxicity of acidic gases and its significance in air pollution control. In Ecological Studies; Billings, W.D., Golley, F., Lange, O.L., Olson, J.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; Volume 22, pp. 11–13. [Google Scholar]
- Grünhage, L.; Jäger, H. From critical levels to critical loads for ozone: A discussion of a new experimental and modelling approach for establishing flux-response relationships for agricultural crops and native plant species. Environ. Pollut. 2003, 125, 99–110. [Google Scholar] [CrossRef]
- De Marco, A.; Vitale, M.; Popa, I.; Anav, A.; Badea, O.; Silaghi, D.; Leca, S.; Screpanti, A.; Paoletti, E. Ozone exposure affects tree defoliation in a continental climate. Sci. Total Environ. 2017, 596, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; De Marco, A.; Dalstein-Richier, L.; Tagliaferro, F.; Renou, C.; Paoletti, E. An epidemiological assessment of stomatal ozone flux-based critical levels for visible ozone injury in Southern European forests. Sci. Total Environ. 2016, 541, 729–741. [Google Scholar] [CrossRef]
- Paoletti, E.; Alivernini, A.; Anav, A.; Badea, O.; Carrari, E.; Chivulescu, S.; Conte, A.; Ciriani, M.; Dalstein-Richier, L.; De Marco, A. Toward stomatal–flux based forest protection against ozone: The MOTTLES approach. Sci. Total Environ. 2019, 691, 516–527. [Google Scholar] [CrossRef]
- Gerosa, G.; Ferretti, M.; Bussotti, F.; Rocchini, D. Estimates of AOT40 from passive sampling in forest sites in South Western Europe. Environ. Pollut. 2007, 145, 629–635. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). Air Quality in Europe—2019 Report. EEA Report No 10/2019. Available online: https://www.eea.europa.eu//publications/air-quality-in-europe-2019 (accessed on 20 October 2019).
- Eichhorn, J.; Roskams, P.; Potocic, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletkovic, I.; Schroeck, H.-W.; et al. Part IV: Visual assessment of crown condition and damaging agents. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Coordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; 54p, Available online: http://www.icp-forests.org/Manual.htm (accessed on 15 July 2019).
- Ferretti, M.; Calderisi, M.; Bussotti, F. Ozone exposure, defoliation of beech (Fagus sylvatica L.) and visible foliar symptoms on native plants in selected plots of South-Western Europe. Environ. Pollut. 2007, 145, 644–651. [Google Scholar] [CrossRef]
- Ferretti, M.; Bacaro, G.; Brunialti, G.; Confalonieri, M.; Cristofolini, F.; Cristofori, A.; Frati, L.; Finco, A.; Gerosa, G.; Maccherini, S. Scarce evidence of ozone effect on recent health and productivity of alpine forests—A case study in Trentino, N. Italy. Environ. Sci. Pollut. Res. 2018, 25, 8217–8232. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Ferretti, M. Visible injury, crown condition, and growth responses of selected Italian forests in relation to ozone exposure. Environ. Pollut. 2009, 157, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Ferretti, M. In search for evidence: Combining ad hoc survey, monitoring, and modeling to estimate the potential and actual impact of ground level ozone on forests in Trentino (Northern Italy). Environ. Sci. Pollut. Res. 2018, 25, 8206–8216. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; De Marco, A.; Anav, A.; Gasparini, P.; Pompei, E. Five-year volume growth of European beech does not respond to ozone pollution in Italy. Environ. Sci. Pollut. Res. 2018, 25, 8233–8239. [Google Scholar] [CrossRef]
- Cailleret, M.; Ferretti, M.; Gessler, A.; Rigling, A.; Schaub, M. Ozone effects on European forest growth—Towards an integrative approach. J. Ecol. 2018, 106, 1377–1389. [Google Scholar] [CrossRef]
- Vollenweider, P.; Ottiger, M.; Günthardt-Goerg, M.S. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environ. Pollut. 2003, 124, 101–118. [Google Scholar] [CrossRef]
- Davison, A.; Neufeld, H.; Chappelka, A.; Wolff, K.; Finkelstein, P. Interpreting spatial variation in ozone symptoms shown by cutleaf cone flower, Rudbeckia laciniata L. Environ. Pollut. 2003, 125, 61–70. [Google Scholar] [CrossRef]
- Cascio, C.; Schaub, M.; Novak, K.; Desotgiu, R.; Bussotti, F.; Strasser, R.J. Foliar responses to ozone of Fagus sylvatica L. seedlings grown in shaded and in full sunlight conditions. Environ. Exp. Bot. 2010, 68, 188–197. [Google Scholar] [CrossRef]
- Gollan, P.J.; Tikkanen, M.; Aro, E.-M. Photosynthetic light reactions: Integral to chloroplast retrograde signalling. Curr. Opin. Plant Biol. 2015, 27, 180–191. [Google Scholar] [CrossRef]
- Innes, J.; Skelly, J.; Schaub, M. Ozone and braodleaved species. In A guide to the Identification of Ozone-induced Foliar Injury; Eidgenössische Forschungsanstalt WSL, Haupt Verlag: Bern, Switzerland, 2001; 136p. [Google Scholar]
- Bussotti, F.; Desotgiu, R.; Cascio, C.; Strasser, R.J. Photosynthesis responses to ozone in young trees of three species with different sensitivities, in a 2-year open-top chamber experiment (Curno, Italy). Physiol. Plant. 2007, 130, 122–135. [Google Scholar] [CrossRef]
- Marzuoli, R.; Gerosa, G.; Desotgiu, R.; Bussotti, F.; Ballarin-Denti, A. Ozone fluxes and foliar injury development in the ozone-sensitive poplar clone Oxford (Populus maximowiczii × Populus berolinensis): A dose-response analysis. Tree Physiol. 2009, 29, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Craker, L.E. Ethylene production from ozone injured plants. Environ. Pollut. 1971, 1, 299–304. [Google Scholar] [CrossRef]
- Vollenweider, P.; Woodcock, H.; Kelty, M.; Hofer, R.-M. Reduction of stem growth and site dependency of leaf injury in Massachusetts black cherries exhibiting ozone symptoms. Environ. Pollut. 2003, 125, 467–480. [Google Scholar] [CrossRef]
- Wittig, V.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Fontaine, V.; Cabané, M.; Dizengremel, P. Regulation of phosphoenolpyruvate carboxylase in Pinus halepensis needles submitted to ozone and water stress. Physiol. Plant. 2003, 117, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Desotgiu, R.; Cascio, C.; Pollastrini, M.; Gravano, E.; Gerosa, G.; Marzuoli, R.; Nali, C.; Lorenzini, G.; Salvatori, E.; et al. Ozone stress in woody plants assessed with chlorophyll a fluorescence. A critical reassessment of existing data. Environ. Exp. Bot. 2011, 73, 19–30. [Google Scholar] [CrossRef]
- Kitao, M.; Winkler, J.B.; Löw, M.; Nunn, A.J.; Kuptz, D.; Häberle, K.-H.; Reiter, I.M.; Matyssek, R. How closely does stem growth of adult beech (Fagus sylvatica) relate to net carbon gain under experimentally enhanced ozone stress? Environ. Pollut. 2012, 166, 108–115. [Google Scholar] [CrossRef]
- Hoshika, Y.; Watanabe, M.; Inada, N.; Koike, T. Model-based analysis of avoidance of ozone stress by stomatal closure in Siebold’s beech (Fagus crenata). Ann. Bot. 2013, 112, 1149–1158. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree responses to drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef]
- Dizengremel, P. Effects of ozone on the carbon metabolism of forest trees. Plant Physiol. Biochem. 2001, 39, 729–742. [Google Scholar] [CrossRef]
- Matyssek, R. Linking ozone uptake and defense towards a mechanistic risk assessment for forest trees. New Phytol. 2007, 174, 7–9. [Google Scholar]
- Marzuoli, R.; Bussotti, F.; Calatayud, V.; Calvo, E.; Alonso, R.; Bermejo, V.; Pollastrini, M.; Monga, R.; Gerosa, G. Dose-response relationships for ozone effect on the growth of deciduous broadleaf oaks in mediterranean environment. Atmos. Environ. 2018, 190, 331–341. [Google Scholar] [CrossRef]
- Braun, S.; Schindler, C.; Rihm, B. Growth losses in Swiss forests caused by ozone: Epidemiological data analysis of stem increment of Fagus sylvatica L. and Picea abies Karst. Environ. Pollut. 2014, 192, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Pollastrini, M.; Gessler, A.; Luo, Z.-B. Experiments with trees: From seedlings to ecosystems. Environ. Exp. Bot. 2018, 152, 1–6. [Google Scholar] [CrossRef]
- Davis, D.D.; Skelly, J. Foliar sensitivity of eight eastern hardwood tree species to ozone. Water Air Soil Pollut. 1992, 62, 269–277. [Google Scholar] [CrossRef]
- Ribas, A.; Peñuelas, J.; Elvira, S.; Gimeno, B. Contrasting effects of ozone under different water supplies in two Mediterranean tree species. Atmos. Environ. 2005, 39, 685–693. [Google Scholar] [CrossRef]
- Ribas, A.; Peñuelas, J.; Elvira, S.; Gimeno, B.S. Ozone exposure induces the activation of leaf senescence-related processes and morphological and growth changes in seedlings of Mediterranean tree species. Environ. Pollut. 2005, 134, 291–300. [Google Scholar] [CrossRef]
- Calatayud, V.; Cervero, J.; Calvo, E.; García-Breijo, F.-J.; Reig-Armiñana, J.; Sanz, M.J. Responses of evergreen and deciduous Quercus species to enhanced ozone levels. Environ. Pollut. 2011, 159, 55–63. [Google Scholar] [CrossRef]
- Gerosa, G.; Fusaro, L.; Monga, R.; Finco, A.; Fares, S.; Manes, F.; Marzuoli, R. A flux-based assessment of above and below ground biomass of Holm oak (Quercus ilex L.) seedlings after one season of exposure to high ozone concentrations. Atmos. Environ. 2015, 113, 41–49. [Google Scholar] [CrossRef]
- Díaz-de-Quijano, M.; Schaub, M.; Bassin, S.; Volk, M.; Peñuelas, J. Ozone visible symptoms and reduced root biomass in the subalpine species Pinus uncinata after two years of free-air ozone fumigation. Environ. Pollut. 2012, 169, 250–257. [Google Scholar] [CrossRef]
- Marzuoli, R.; Monga, R.; Finco, A.; Gerosa, G. Biomass and physiological responses of Quercus robur (L.) young trees during 2 years of treatments with different levels of ozone and nitrogen wet deposition. Trees-Struct. Funct. 2016, 30, 1995–2010. [Google Scholar] [CrossRef]
- Andersen, C.P.; Wilson, R.; Plocher, M.; Hogsett, W.E. Carry-over effects of ozone on root growth and carbohydrate concentrations of ponderosa pine seedlings. Tree Physiol. 1997, 17, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Hogsett, W.; Plocher, M.; Wildman, V.; Tingey, D.; Bennett, J. Growth response of two varieties of slash pine seedlings to chronic ozone exposures. Can. J. Bot. 1985, 63, 2369–2376. [Google Scholar] [CrossRef]
- Wang, D.; Karnosky, D.F.; Bormann, F.H. Effects of ambient ozone on the productivity of Populus tremuloides Michx. grown under field conditions. Can. J. For. Res. 1986, 16, 47–55. [Google Scholar] [CrossRef]
- Wang, D.; Bormann, F.H.; Karnosky, D.F. Regional tree growth reductions due to ambient ozone: Evidence from field experiments. Environ. Sci. Technol. 1986, 20, 1122–1125. [Google Scholar] [CrossRef]
- Temple, P.J. Injury and growth of Jeffrey pine and giant sequoia in response to ozone and acidic mist. Environ. Exp. Bot. 1988, 28, 323–333. [Google Scholar] [CrossRef]
- Karnosky, D.; Gagnon, Z.; Reed, D.; Witter, J. Growth and biomass allocation of symptomatic and asymptomatic Populus tremuloides clones in response to seasonal ozone exposures. Can. J. For. Res. 1992, 22, 1785–1788. [Google Scholar] [CrossRef]
- Karnosky, D.; Gagnon, Z.; Reed, D.; Witter, J.A. Effects of genotype on the response of Populus tremuloides Michx. to ozone and nitrogen deposition. Water Air Soil Pollut. 1992, 62, 189–199. [Google Scholar] [CrossRef][Green Version]
- Pääkkönen, E.; Paasisalo, S.; Holopainen, T.; Kärenlamp, L. Growth and stomatal responses of birch (Betula pendula Roth.) clones to ozone in open-air and chamber fumigations. New Phytol. 1993, 125, 615–623. [Google Scholar] [CrossRef]
- Shimizu, H.; Fujinuma, Y.; Kubota, K.; Totsuka, T.; Omasa, K. Effects of low concentrations of ozone (O3) on the growth of several woody plants. J. Agric. Meteorol. 1993, 48, 723–726. [Google Scholar] [CrossRef]
- Temple, P.J.; Miller, P.R. Foliar ozone injury and radial growth of ponderosa pine. Can. J. For. Res. 1994, 24, 1877–1882. [Google Scholar] [CrossRef]
- Samuelson, L. Ozone-exposure responses of black cherry and red maple seedlings. Environ. Exp. Bot. 1994, 34, 355–362. [Google Scholar] [CrossRef]
- Matyssek, R.; Günthardt-Goerg, M.S.; Maurer, S.; Keller, T. Nighttime exposure to ozone reduces whole-plant production in Betula pendula. Tree Physiol. 1995, 15, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Karnosky, D.; Gagnon, Z.E.; Dickson, R.E.; Coleman, M.D.; Lee, E.H.; Isebrands, J.G. Changes in growth, leaf abscission, and biomass associated with seasonal tropospheric ozone exposures of Populus tremuloides clones and seedlings. Can. J. For. Res. 1996, 26, 23–37. [Google Scholar] [CrossRef]
- Pääkkönen, E.; Holopainen, T.; Kärenlampi, L. Differences in growth, leaf senescence and injury, and stomatal density in birch (Betula pendula Roth.) in relation to ambient levels of ozone in Finland. Environ. Pollut. 1997, 96, 117–127. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; Maurer, S.; Bolliger, J.; Clark, A.J.; Landolt, W.; Bucher, J.B. Responses of young trees (five species in a chamber exposure) to near-ambient Ozone concentrations. Water Air Soil Pollut. 1999, 116, 323–332. [Google Scholar] [CrossRef]
- Günthardt-Goerg, M.S.; McQuattie, C.; Maurer, S.; Frey, B. Visible and microscopic injury in leaves of five deciduous tree species related to current critical ozone levels. Environ. Pollut. 2000, 109, 489–500. [Google Scholar] [CrossRef]
- Karnosky, D.; Mankovska, B.; Percy, K.; Dickson, R.; Podila, G.; Sober, J.; Noormets, A.; Hendrey, G.; Coleman, M.D.; Kubiske, M. Effects of tropospheric O3 on trembling aspen and interaction with CO2: Results from an O3-gradient and a FACE experiment. Water Air Soil Pollut. 1999, 116, 311–322. [Google Scholar] [CrossRef]
- Karnosky, D.; Percy, K.; Mankovska, B.; Prichard, T.; Noormets, A.; Dickson, R.; Jepsen, E.; Isebrands, J. Ozone affects the fitness of trembling aspen. Dev. Environ. Sci. 2003, 3, 199–209. [Google Scholar]
- Inclan, R.; Ribas, A.; Peñuelas, J.; Gimeno, B.S. The relative sensitivity of different mediterranean plant species to Ozone exposure. Water Air Soil Pollut. 1999, 116, 273–277. [Google Scholar] [CrossRef]
- Yun, S.-C.; Laurence, J.A. The response of clones of Populus tremuloides differing in sensitivity to ozone in the field. New Phytol. 1999, 141, 411–421. [Google Scholar] [CrossRef]
- Paludan-Müller, G.; Saxe, H.; Leverenz, J. Responses to ozone in 12 provenances of European beech (Fagus sylvatica): Genotypic variation and chamber effects on photosynthesis and dry-matter partitioning. New Phytol. 1999, 144, 261–273. [Google Scholar] [CrossRef]
- Thomas, V.F.D.; Braun, S.; Flückiger, W. Effects of simultaneous ozone exposure and nitrogen loads on carbohydrate concentrations, biomass, growth, and nutrient concentrations of young beech trees (Fagus sylvatica). Environ. Pollut. 2005, 143, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, V.; Cerveró, J.; Sanz, M.J. Foliar, physiologial and growth responses of four maple species exposed to Ozone. Water Air Soil Pollut. 2007, 185, 239–254. [Google Scholar] [CrossRef]
- Novak, K.; Cherubini, P.; Saurer, M.; Fuhrer, J.; Skelly, J.M.; Kräuchi, N.; Schaub, M. Ozone air pollution effects on tree-ring growth, δ13C, visible foliar injury and leaf gas exchange in three ozone-sensitive woody plant species. Tree Physiol. 2007, 27, 941–949. [Google Scholar] [CrossRef]
- Novak, K.; Schaub, M.; Fuhrer, J.; Skelly, J.M.; Frey, B. Ozone effects on visible foliar injury and growth of Fagus sylvatica and Viburnum lantana seedlings grown in monocolture or in mixture. Environ. Exp. Bot. 2008, 62, 212–220. [Google Scholar] [CrossRef]
- Nikula, S.; Percy, K.; Oksanen, E.; Holopainen, T.; Manninen, S. Effects of elevated ozone on growth and foliar traits of European and hybrid aspen. Boreal Environ. Res. 2009, 14 (Suppl. A), 29–47. [Google Scholar]
- Gerosa, G.; Marzuoli, R.; Desotgiu, R.; Bussotti, F.; Ballarin-Denti, A. Visible leaf injury in young trees of Fagus sylvatica L. and Quercus robur L. in relation to ozone uptake and ozone exposure. An open-top chambers experiment in South Alpine environmental conditions. Environ. Pollut. 2008, 152, 274–284. [Google Scholar] [CrossRef]
- Pollastrini, M.; Desotgiu, R.; Cascio, C.; Bussotti, F.; Cherubini, P.; Saurer, M.; Gerosa, G.; Marzuoli, R. Growth and physiological responses to ozone and mild drought stress of tree species with different ecological requirements. Trees Struct. Funct. 2010, 24, 695–704. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Z.; Wang, X.; Niu, J. Responses of native broadleaved woody species to elevated ozone in subtropical China. Environ. Pollut. 2012, 163, 149–157. [Google Scholar] [CrossRef]
- Gravano, E.; Ferretti, M.; Bussotti, F.; Grossoni, P. Foliar symptoms and growth reduction of Ailanthus altissima Desf. in an area with high ozone and acidic deposition in Italy. In Forest Growth Responses to the Pollution Climate of the 21st Century; Sheppard, L.J., Cape, J.N., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 267–272. [Google Scholar]
- Peterson, D.L.; Arbaugh, M.J.; Wakefield, V.A.; Miller, P.R. Evidence of growth reduction in ozone-injured Jeffrey pine (Pinus jeffreyi Grev. and Balf.) in Sequoia and Kings Canyon National Parks. JAPCA 1987, 37, 906–912. [Google Scholar] [CrossRef]
- Somers, G.; Chappelka, A.; Rosseau, P.; Renfro, J. Empirical evidence of growth decline related to visible ozone injury. For. Ecol. Manag. 1998, 104, 129–137. [Google Scholar] [CrossRef]
- Peterson, D.L.; Arbaugh, M.J. An evaluation of the effects of ozone injury on radial growth of ponderosa pine (Pinus ponderosa) in the southern Sierra Nevada. JAPCA 1988, 38, 921–927. [Google Scholar] [CrossRef]
- Peterson, D.L.; Arbaugh, M.J.; Robinson, L.J. Regional growth changes in ozone-stressed ponderosa pine (Pinus ponderosa) in the Sierra Nevada, California, USA. Holocene 1991, 1, 50–61. [Google Scholar] [CrossRef]
- de Bauer, M.L.; Hernández-Tejeda, T. A review of ozone-induced effects on the forests of central Mexico. Environ. Pollut. 2007, 147, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Karnosky, D.F.; Skelly, J.M.; Percy, K.E.; Chappelka, A.H. Perspectives regarding 50 years of research on effects of tropospheric ozone air pollution on US forests. Environ. Pollut. 2007, 147, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, P.; Günthardt-Goerg, M.S.; Menard, T.; Baumgarten, M.; Matyssek, R.; Schaub, M. Macro-and microscopic leaf injury triggered by ozone stress in beech foliage (Fagus sylvatica L.). Ann. For. Sci. 2019, 76, 71. [Google Scholar] [CrossRef]
- Zona, D.; Gioli, B.; Fares, S.; De Groote, T.; Pilegaard, K.; Ibrom, A.; Ceulemans, R. Environmental controls on ozone fluxes in a poplar plantation in Western Europe. Environ. Pollut. 2014, 184, 201–210. [Google Scholar] [CrossRef]
- Nowak, R.; Caldwell, M. A test of compensatory photosynthesis in the field: Implications for herbivory tolerance. Oecologia 1984, 61, 311–318. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Swarbrick, P.J.; Scholes, J.D.; Rolfe, S.A. Imaging photosynthesis in wounded leaves of Arabidopsis thaliana. J. Exp. Bot. 2005, 57, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Hamilton, J.G.; Kessler, A. Herbivore-specific elicitation of photosynthesis by mirid bug salivary secretions in the wild tobacco Nicotiana attenuata. New Phytol. 2011, 191, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G. Pattern of defoliation and its effect on photosynthesis and growth of goldenrod. Funct. Ecol. 1998, 12, 270–279. [Google Scholar] [CrossRef]
- McNickle, G.G.; Evans, W.D. Toleration games: Compensatory growth by plants in response to enemy attack is an evolutionarily stable strategy. AoB Plants 2018, 10, ply035. [Google Scholar] [CrossRef]
- Pell, E.; Temple, P.; Friend, A.; Mooney, H.; Winner, W. Compensation as a plant response to ozone and associated stresses: An analysis of ROPIS experiments. J. Environ. Qual. 1994, 23, 429–436. [Google Scholar] [CrossRef]
- Desotgiu, R.; Pollastrini, M.; Cascio, C.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Chlorophyll a fluorescence analysis along a vertical gradient of the crown in a poplar (Oxford clone) subjected to ozone and water stress. Tree Physiol. 2012, 32, 976–986. [Google Scholar] [CrossRef]
- Schaub, M.; Calatayud, V.; Ferretti, M.; Brunialti, G.; Lövblad, G.; Krause, G.; Sanz, M. Part VIII: Assessment of Ozone injury. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; 21p, Available online: http://www.icp-forests.org/Manual.htm (accessed on 15 July 2019).
- du Cros, T.E.; Le Tacon, F.; Nepveu, G.; Pardé, J.; Perrin, R.; Timbal, J. Le hêtre; Institut Nationale de la Recerche Agronomique: Paris, France, 1981; p. 610. [Google Scholar]
- Gerosa, G.; Marzuoli, R.; Desotgiu, R.; Bussotti, F.; Ballarin-Denti, A. Validation of the stomatal flux approach for the assessment of ozone visible injury in young forest trees. Results from the TOP (transboundary ozone pollution) experiment at Curno, Italy. Environ. Pollut. 2009, 157, 1497–1505. [Google Scholar] [CrossRef]
- Gielen, B.; Löw, M.; Deckmyn, G.; Metzger, U.; Franck, F.; Heerdt, C.; Matyssek, R.; Valcke, R.; Ceulemans, R. Chronic ozone exposure affects leaf senescence of adult beech trees: A chlorophyll fluorescence approach. J. Exp. Bot. 2006, 58, 785–795. [Google Scholar] [CrossRef]
- Skärby, L.; Ro-Poulsen, H.; Wellburn, F.A.M.; Sheppard, L. Impacts of ozone on forests: A European perspective. New Phytol. 1998, 139, 109–122. [Google Scholar] [CrossRef]
- Tuovinen, J.-P.; Emberson, L.; Simpson, D. Modelling ozone fluxes to forests for risk assessment: Status and prospects. Ann. For. Sci. 2009, 66, 1–14. [Google Scholar] [CrossRef]
- Bussotti, F.; Schaub, M.; Cozzi, A.; Kräuchi, N.; Ferretti, M.; Novak, K.; Skelly, J.M. Assessment of ozone visible symptoms in the field: Perspectives of quality control. Environ. Pollut. 2003, 125, 81–89. [Google Scholar] [CrossRef]
- Bussotti, F.; Schaub, M.; Cozzi, A.; Gerosa, G.; Novak, K.; Hug, C. Sources of errors in assessing ozone visible symptoms on native vegetation. Environ. Pollut. 2006, 140, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Pollastrini, M. Field surveys of ozone symptoms in Europe. Problems, reliability and significance for ecosystems. Ann. Bot. 2015, 5, 45–51. [Google Scholar]
- Walter, J.; Jentsch, A.; Beierkuhnlein, C.; Kreyling, J. Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environ. Exp. Bot. 2013, 94, 3–8. [Google Scholar] [CrossRef]
- Ladd, I.; Skelly, J.; Pippin, M.; Fishman, J. Ozone-induced foliar injury. In Field Guide; National Aeronautics and Space Administration, Ed.; Langley Research Center: Hampton, VA, USA, 2011; p. 142. [Google Scholar]
- Ballach, H. Suitability and use of poplars as bioindicators—A new concept. Environ. Sci. Pollut. Res. Int. 1997, 4, 37–45. [Google Scholar] [CrossRef]
- Jansson, S.; Douglas, C.J. Populus: A model system for plant biology. Annu. Rev. Plant Biol. 2007, 58, 435–458. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofori, A.; Cristofolini, F.; Nali, C.; Pellegrini, E.; Bussotti, F.; Ferretti, M. Chlorophyll-related indicators are linked to visible ozone symptoms: Evidence from a field study on native Viburnum lantana L. plants in northern Italy. Ecol. Indic. 2014, 39, 65–74. [Google Scholar] [CrossRef]
- Sanz, M.; Calatayud, V.; Calvo, E. Spatial pattern of ozone injury in Aleppo pine related to air pollution dynamics in a coastal–mountain region of eastern Spain. Environ. Pollut. 2000, 108, 239–247. [Google Scholar] [CrossRef]
- Huston, M.; Smith, T. Plant succession: Life history and competition. Am. Nat. 1987, 130, 168–198. [Google Scholar] [CrossRef]
- Bussotti, F. Functional leaf traits, plant communities and acclimation processes in relation to oxidative stress in trees: A critical overview. Glob. Chang. Biol. 2008, 14, 2727–2739. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2006; p. 419. [Google Scholar]
- Körner, C. Concepts in empirical plant ecology. Plant Ecol. Divers. 2018, 11, 405–428. [Google Scholar] [CrossRef]
- Fuhrer, J.; Val Martin, M.; Mills, G.; Heald, C.L.; Harmens, H.; Hayes, F.; Sharps, K.; Bender, J.; Ashmore, M.R. Current and future ozone risks to global terrestrial biodiversity and ecosystem processes. Ecol. Evol. 2016, 6, 8785–8799. [Google Scholar] [CrossRef]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The challenge of Mediterranean sclerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Jansen, M.A. Different stresses, similar morphogenic responses: Integrating a plethora of pathways. Plant Cell Environ. 2009, 32, 158–169. [Google Scholar] [CrossRef]
- Strasser, R.; Tsimilli-Michael, M. Stress in plants, from daily rhythm to global changes, detected and quantified by the JIP-test. Chim. Nouv. (SRC) 2001, 75, 3321–3326. [Google Scholar]
- Millar, C.I.; Stephenson, N.L. Temperate forest health in an era of emerging megadisturbance. Science 2015, 349, 823–826. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Observing climate change impacts on European forests: What works and what does not in ongoing long-term monitoring networks. Front. Plant Sci. 2017, 8, 629. [Google Scholar] [CrossRef]
| Authors | Species | Plant Age | Experimental Set up | O3 Treat. Duration | Detection of FVS | Detection of Significant GR | Notes |
|---|---|---|---|---|---|---|---|
| Hogsett et al. [48] | Pinus elliottii | Seedlings | O3 fumigation chambers | 16 weeks | Yes | Yes | |
| Wang et al. [49] | Populus tremuloides (O3-tolerant and O3-sensitive clones) | Saplings | Open-top chambers | 2.5 growing seasons | Yes | Yes | |
| Wang et al. [50] | Populus hybrid; Populus deltoides; Robinia pseudoacacia | Seedlings | Open-top chambers | 1 growing season | No | Yes (Populus hybrid) | Only the above-ground biomass of Populus hybrid was significantly reduced |
| Temple [51] | Pinus jefferyi; Sequoiadendron giganteum | 2-year old seedlings | O3 fumigation chambers | 2 months for 2 growing seasons | Yes (P. jefferyi) No (S. giganteum) | No | |
| Karnosky et al. [52] | Populus tremuloides (various O3 tolerant and O3 sensitive clones) | Rooted cuttings | Open-top chambers | 1 growing season | Yes | Yes | Only the O3 sensitive clones showed FVS and GR of the above-ground biomass |
| Karnosky et al. [53] | Populus tremuloides (2 O3 sensitive clones) | Rooted cuttings | Open-top chambers | 1 growing season | Yes | No | |
| Pääkkönen et al. [54] | Betula pendula | 2-year old seedlings | Open field O3 fumigation and O3 fumigation chambers | 2 growing seasons | Yes | Yes | |
| Shimizu et al. [55] | Populus hybrids; Abies firma; Quercus acutissima; Cryptomeria japonica; Viburnum odoratissimum | 2-year old seedlings; cuttings | O3 fumigation chambers | 3-4 weeks | Yes (Populus hybrids; V. odoratissimum) No (C. japonica; A. firma; Q. acutissima) | Yes (one Populus hybrid; C. japonica; V. odoratissimum) No (one Populus hybrid; A. firma; Q. acutissima) | |
| Temple and Miller [56] | Pinus ponderosa | Seedlings | Open-top chambers | 3 growing seasons | Yes | Yes | Only stem diameter was measured |
| Samuelson [57] | Prunus serotina; Acer rubrum | 1-year old seedlings | Open-top chambers | 1 growing season | Yes | Yes (P. serotina) No (A. rubrum) | Only plant height and root/shoot ratio of Prunus serotina were significantly reduced |
| Matyssek et al. [58] | Betula pendula | Rooted cuttings | Open-top chambers | 1 growing season | Yes | Yes | No information on the statistical significance of the GR |
| Karnosky et al. [59] | Populus tremuloides | Rooted cuttings | Open-top chambers | 2 growing seasons | Yes | Yes | |
| Pääkkönen et al. [60] | Betula pendula (4 clones) | 2-year old seedlings | Open field growing in 3 different sites and open field O3 fumigation | 2 growing seasons | Yes | Yes | Only plant height was significantly reduced and only after the 2nd growing season |
| Günthardt-Goerg et al. [61,62] | Fagus sylvatica; Prunus serotina; Carpinus betulus; Fraxinus excelsior; Sorbus aucuparia | Rooted cuttings; seedlings | O3 fumigation Chambers | 1 growing season | Yes | No | |
| Karnosky et al. [63,64] | Populus tremuloides (O3-tolerant and O3-sensitive clones) | Rooted cuttings | Open field growing in 3 sites with different ambient O3 levels | 5 growing seasons | Yes | Yes | Only volume growth was measured |
| Inclàn et al. [65] | Quercus ilex; Olea europea; Ceratonia siliqua; Arbutus unedo | 2-year old seedlings | Open-top chambers | 10 months | Yes (Q. ilex; A. unedo) No (O. europea; C. siliqua) | No (A. unedo) Yes (O. europea) | Only plant height and stem diameter were measured |
| Yun and Laurence [66] | Populus tremuloides (2 clones) | Rooted cuttings | Open-top chambers | 3 months | Yes | Yes | |
| Paludan-Müller et al. [67] | Fagus sylvatica | Seedlings | Open-top chambers | 2 growing seasons | No | Yes | Only the root/shoot ratio showed a significant reduction |
| Ribas et al. [41] | Quercus ilex; Ceratonia siliqua | 1-year old seedlings | Open-top chambers | 2 growing seasons | No | Yes | |
| Ribas et al. [42] | Quercus ilex; Quercus ballota; Ceratonia siliqua; Olea europea | 1-year old seedlings | Open-top chambers | 2 growing seasons | No | Yes (Q. ilex; Q. ballota; C. siliqua) No (O. europea) | Only the above-ground biomass was measured |
| Thomas et al. [68] | Picea abies | 2-year old seedlings | Open field O3 fumigation | 3 growing seasons | Yes | Yes | FVS and reduced shoot elongation starting from the 2nd growing season |
| Calatayud et al. [69] | Acer campestre; Acer opalus; Acer monspessulanum; Acer pseudoplatanus | 3-year old 4-year old seedlings; | Open-top chambers | 1 growing season | Yes | No | Only plant height was measured |
| Novak et al. [70] | Populus nigra; Viburnum lantana; Fraxinus excelsior | Seedings | Open-top chambers | 2 growing seasons | Yes | Yes (P. nigra) No (V. lantana; F. excelsior) | Only ring width was measured |
| Novak et al. [71] | Fagus sylvatica; Viburnum lantana | 2-year old seedlings | Open-top chambers | 2 growing seasons | Yes | No | |
| Nikula et al. [72] | Populus tremula Populus hybrid | 1-year old cuttings | Open field O3 fumigation | 2.5 months | Yes | No | |
| Gerosa et al. [73] Marzuoli et al. [26] Pollastrini et al. [74] | Populus hybrid; Fagus sylvatica; Quercus robur | 2-year old seedlings | Open-top chambers | 2 growing seasons | Yes (Populus hybrid; F. sylvatica) No (Q. robur) | Yes (Populus hybrid) No (F. sylvatica; Q. robur) | Only the above-ground biomass was measured |
| Calatayud et al. [43] | Quercus ilex; Quercus faginea, Quercus robur; Quercus pyrenaica | 2-year old seedlings | Open-top chambers | 2 growing seasons | Yes No (Q. ilex) | No Yes (Q. pyrenaica) | Only shoot/root ratio of Q. pyrenaica was affected. FVS were slight on Q. robur and Q. faginea |
| Dìaz-de-Quijano et al. [45] | Pinus uncinata | 7-year old saplings | Open field O3 fumigation | 2 growing seasons | Yes | Yes | Only the root biomass was significantly reduced |
| Zhang et al. [75] | Lyriodendron chinense; Liquidambar formosana; Cinnamomum camphora; Cyclobalanopsis clauca; Neolitsea sericea; Schima superba | 1-year old | Open-top chambers | 6-7 weeks | Yes No (N. sericea) | Yes No (N. sericea; C. clauca) | |
| Gerosa et al. [44] | Quercus ilex | 2-year old | Open-top chambers | 1 growing season | No | Yes |
| Authors | Species | Plant Age | Survey Methodology | GR Variables Considered | Main Results | Relationship between FVS and GR? |
|---|---|---|---|---|---|---|
| Peterson et al. [77] | Pinus jeffreyi | Mature forest | Comparison between 5 sites with symptomatic trees and 3 sites with asymptomatic trees | Growth index calculated from radial growth | Mean annual radial increment of trees with FVS was 11% less than trees at sites without injury | Yes |
| Peterson and Arbaugh [79] | Pinus ponderosa | Mature forest | Comparison between 5 sites with symptomatic trees and 2 sites with asymptomatic trees. | Growth index calculated from radial growth | No significant change in growth associated with FVS on trees | No |
| Peterson et al. [80] | Pinus ponderosa | Mature forest (>50 years) | Comparison between 4 stands from 7 sites with symptomatic trees and 4 stands from 7 sites with asymptomatic trees. | Radial growth | No evidence of widespread regional growth decreases during recent years, but in a few symptomatic sites, trees showed significant growth decreases | Contradictory results |
| Somers et al. [78] | Prunus serotina; Liriodendron tulipifera | Mature forest | Comparison between symptomatic and asymptomatic trees of the same species. Analysis on 30 trees for each species | Radial growth over 5- and 10-year periods | No evidence that GR in P. serotina was related to FVS. In L. tulipifera GR was significantly more intense in trees with FVS | Yes (L. tulipifera) No (Prunus serotina) |
| Gravano et al. [76] | Ailanthus altissima | 3–6 years old | Comparison between 2 groups of 5 ramets, with different exposure to ambient O3 (open field and protected below trees canopy) | Growth of the main stem, n of leaves | FVS and GR were stronger in plants of the open field site. | Yes |
| Vollenweider et al. [28] | Prunus serotina | 86-year old on average | Comparison between symptomatic and asymptomatic trees in the same site | Tree diameter and basal area | Over a 31-year period, trees with FVS had 28% lower stem growth rates than asymptomatic trees | Yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzuoli, R.; Gerosa, G.; Bussotti, F.; Pollastrini, M. Assessing the Impact of Ozone on Forest Trees in An Integrative Perspective: Are Foliar Visible Symptoms Suitable Predictors for Growth Reduction? A Critical Review. Forests 2019, 10, 1144. https://doi.org/10.3390/f10121144
Marzuoli R, Gerosa G, Bussotti F, Pollastrini M. Assessing the Impact of Ozone on Forest Trees in An Integrative Perspective: Are Foliar Visible Symptoms Suitable Predictors for Growth Reduction? A Critical Review. Forests. 2019; 10(12):1144. https://doi.org/10.3390/f10121144
Chicago/Turabian StyleMarzuoli, Riccardo, Giacomo Gerosa, Filippo Bussotti, and Martina Pollastrini. 2019. "Assessing the Impact of Ozone on Forest Trees in An Integrative Perspective: Are Foliar Visible Symptoms Suitable Predictors for Growth Reduction? A Critical Review" Forests 10, no. 12: 1144. https://doi.org/10.3390/f10121144
APA StyleMarzuoli, R., Gerosa, G., Bussotti, F., & Pollastrini, M. (2019). Assessing the Impact of Ozone on Forest Trees in An Integrative Perspective: Are Foliar Visible Symptoms Suitable Predictors for Growth Reduction? A Critical Review. Forests, 10(12), 1144. https://doi.org/10.3390/f10121144








