Carbon Limitation and Drought Sensitivity at Contrasting Elevation and Competition of Abies pinsapo Forests. Does Experimental Thinning Enhance Water Supply and Carbohydrates?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Environmental Monitoring
2.2. Hydraulic Conductivity and Cavitation Vulnerability Curves
2.3. SAP Flow Measurements
2.4. Secondary Growth, Gas Exchange Variables, and Xylem Water Potential
2.5. Non-Structural Carbohydrates
2.6. Statistical Analyses
3. Results
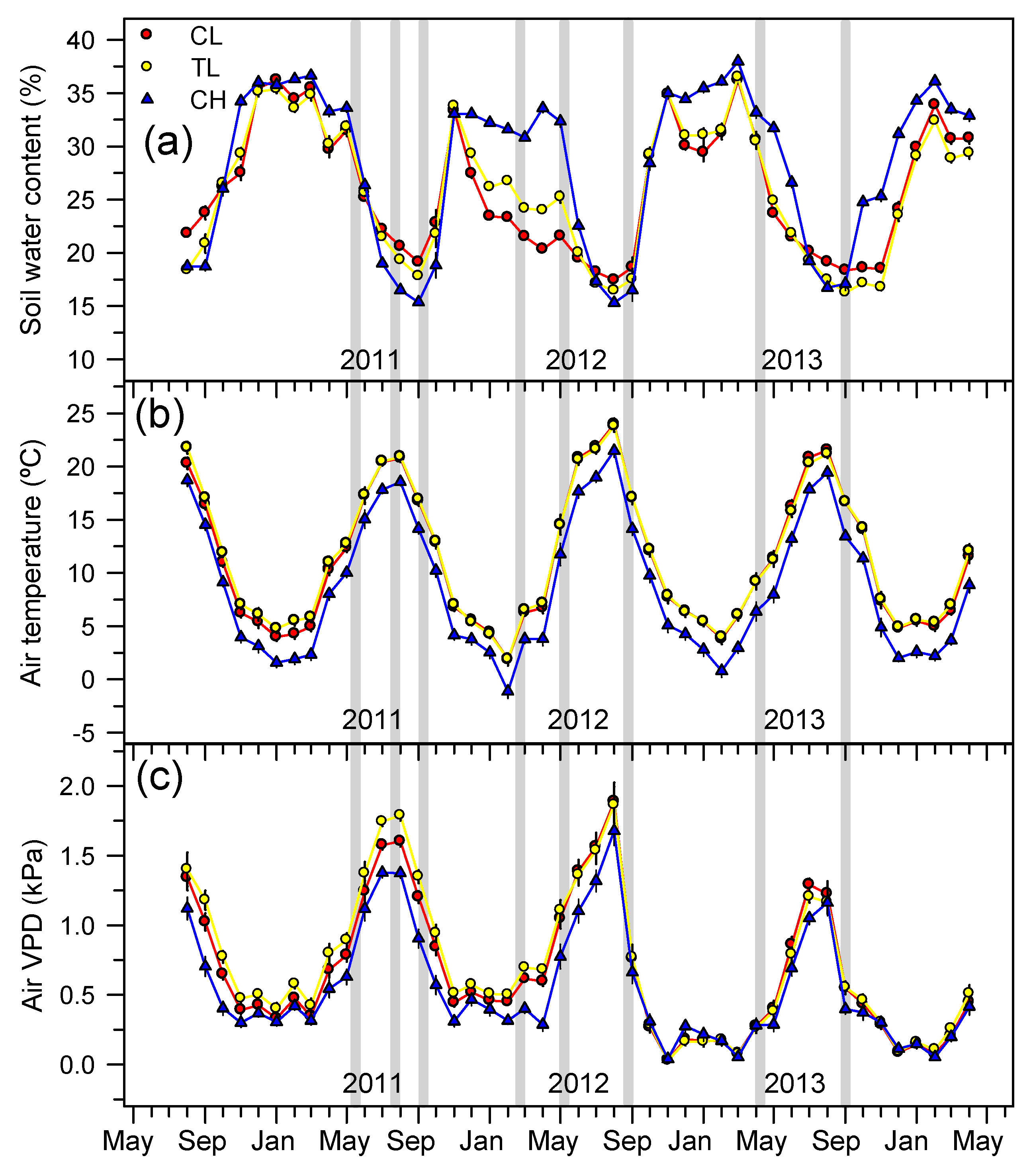
3.1. Microclimatic Conditions
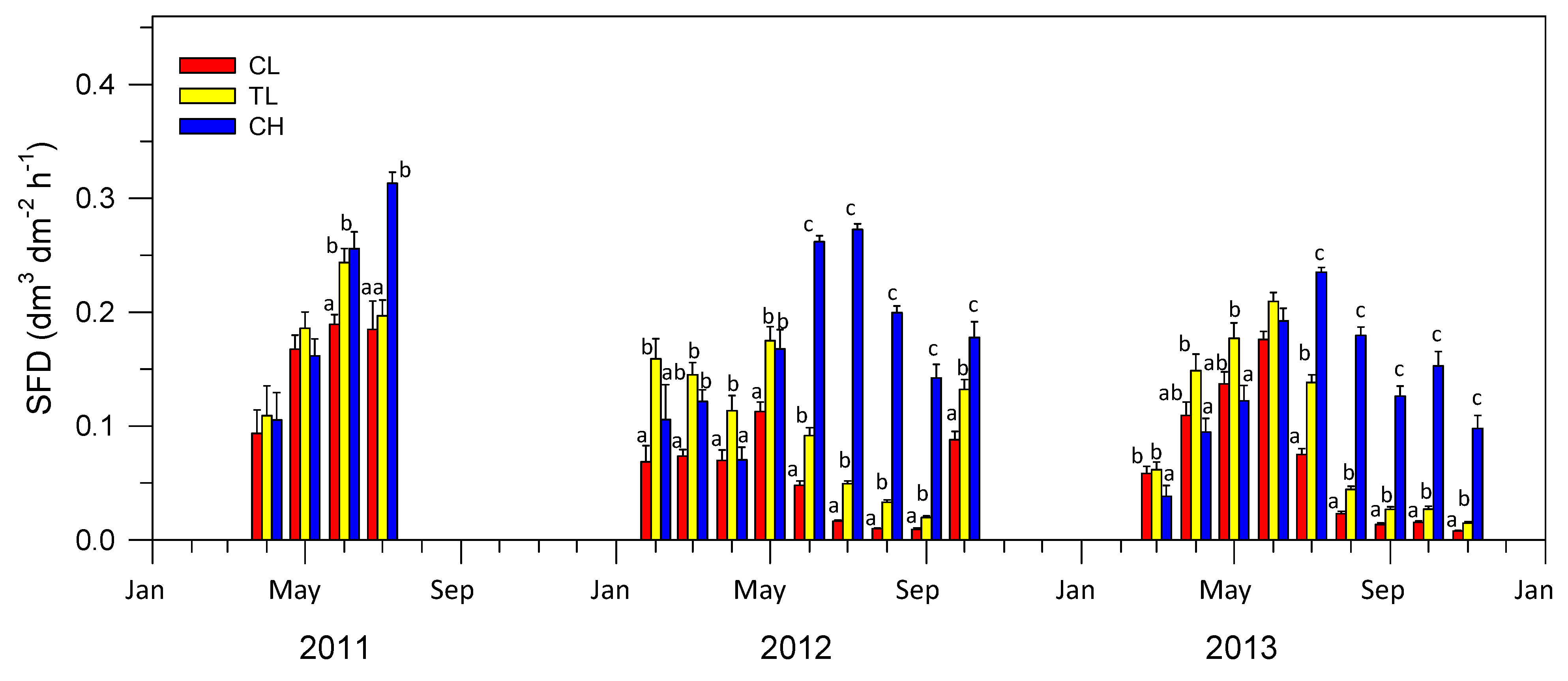
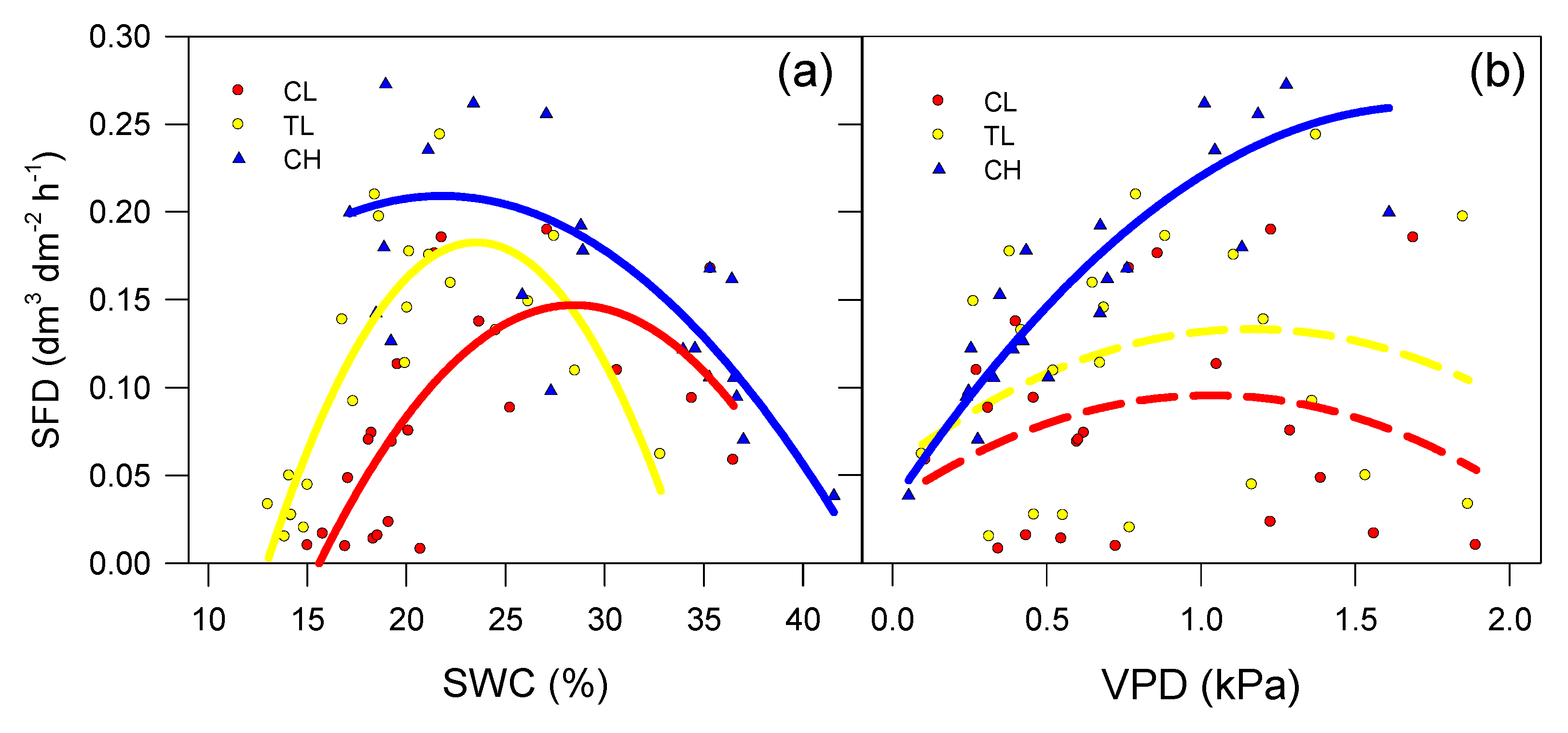
3.2. SAP Flow Measurements
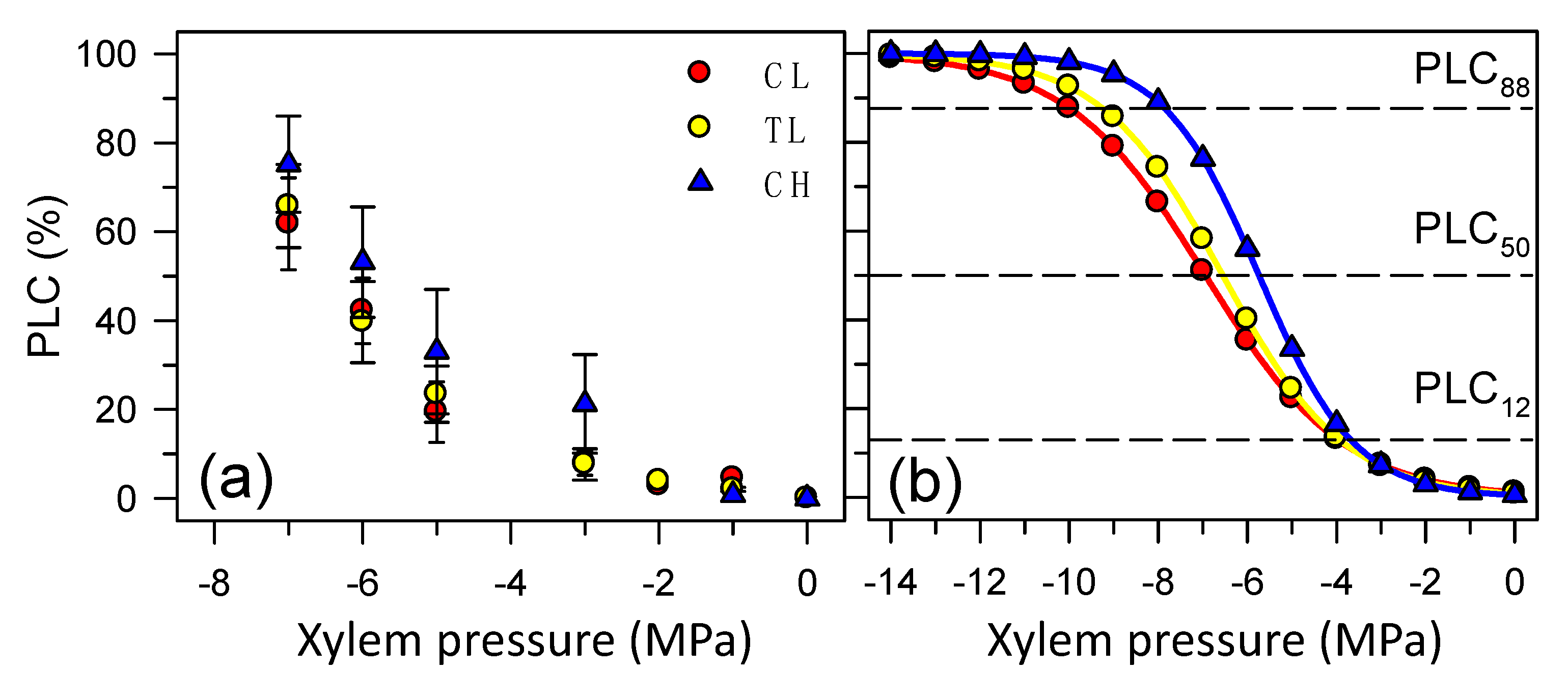
3.3. Hydraulic Conductivity and Cavitation Vulnerability Curves
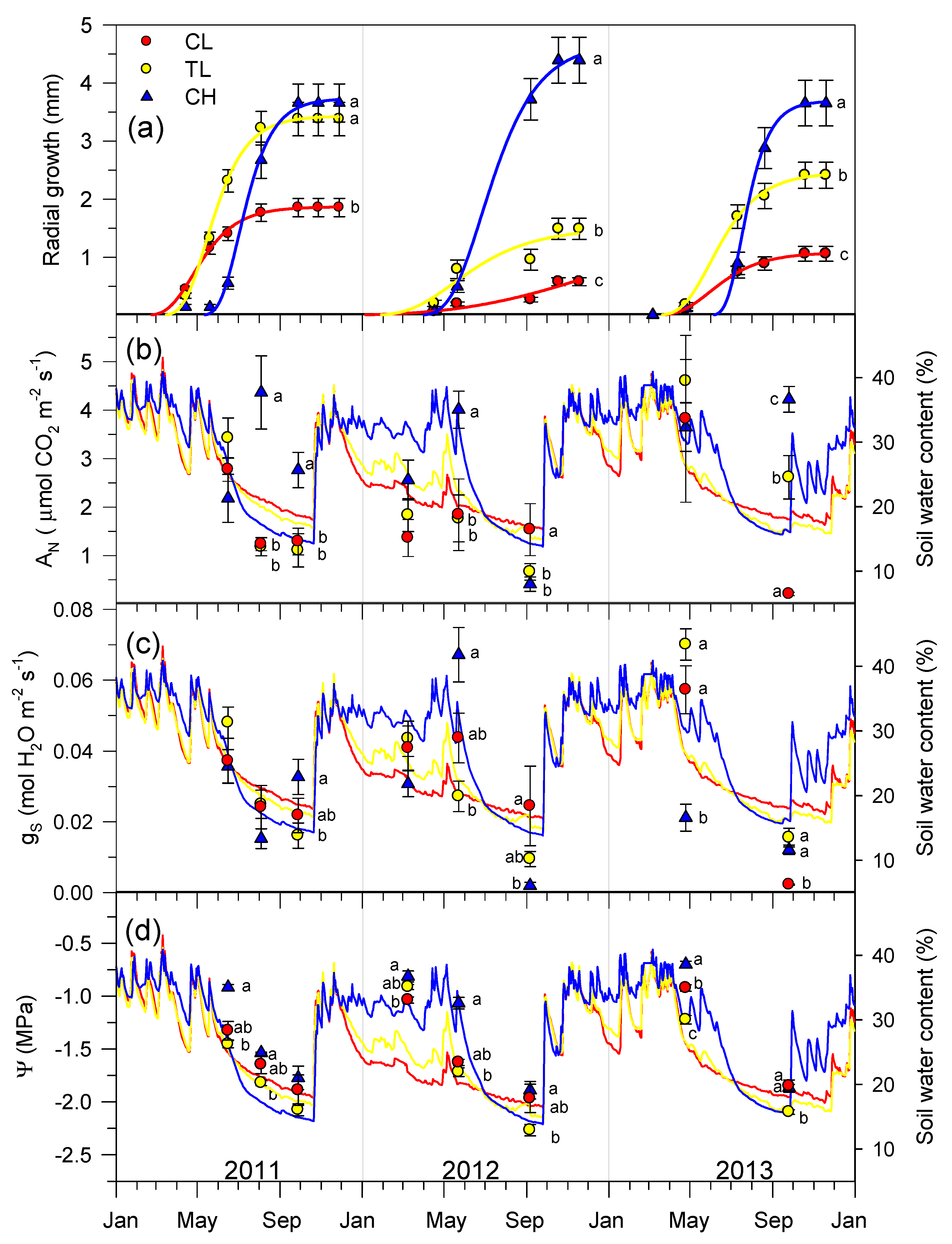
3.4. Secondary Growth, Gas Exchange, and Xylem Water Potential
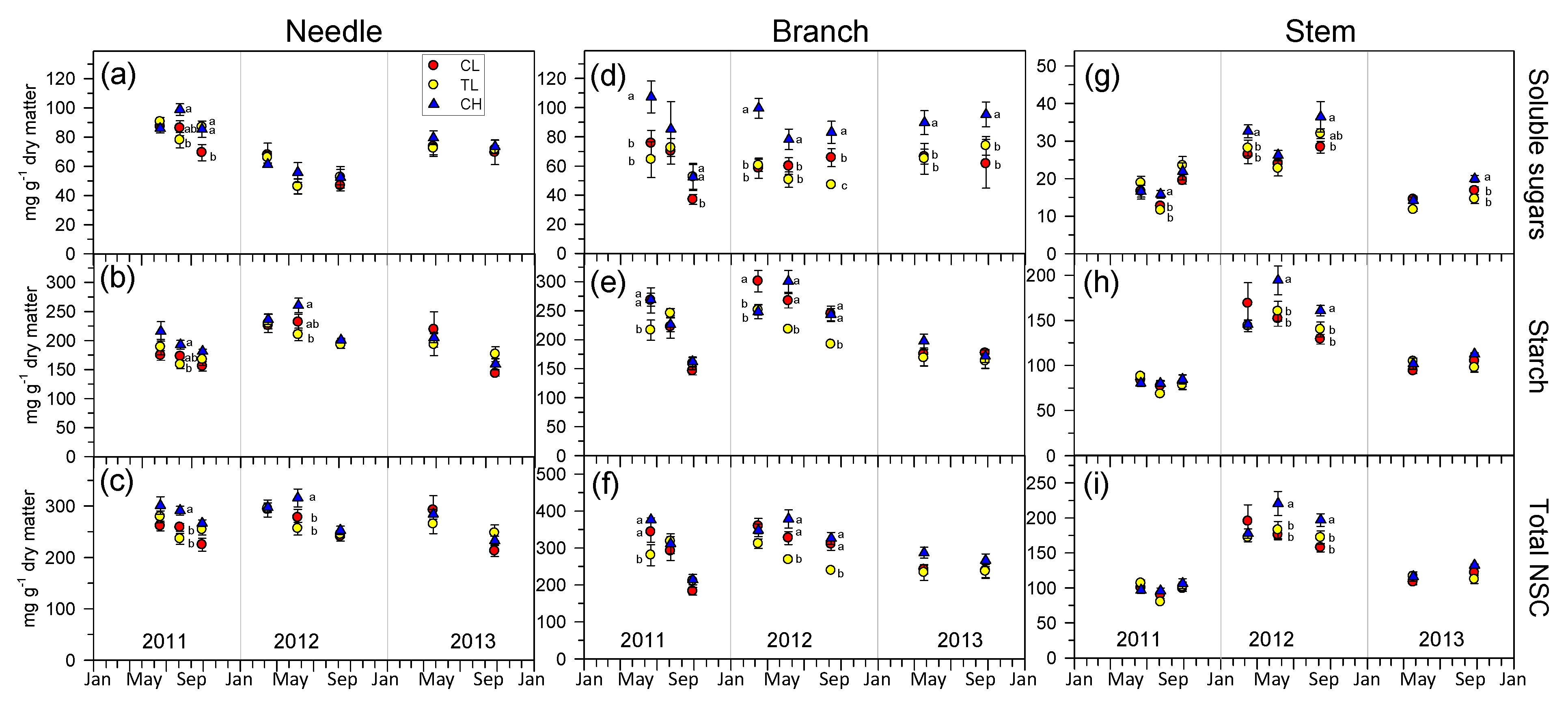
3.5. Non-Structural Carbohydrates Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gómez-Aparicio, L.; García-Valdés, R.; Ruíz-Benito, P.; Zavala, M.A. Disentangling the relative importance of climate, size and competition on tree growth in Iberian forests: Implications for forest management under global change. Glob. Change Biol. 2011, 17, 2400–2414. [Google Scholar] [CrossRef]
- Carnwath, G.C.; Nelson, C.R. The effect of competition on responses to drought and interannual climate variability of a dominant conifer tree of western North America. J. Ecol. 2016, 104, 1421–1431. [Google Scholar] [CrossRef]
- Fernández-de-Uña, L.; McDowell, N.G.; Canellas, I.; Gea-Izquierdo, G. Disentangling the effect of competition, CO2 and climate on intrinsic water-use efficiency and tree growth. J. Ecol. 2016, 104, 678–690. [Google Scholar] [CrossRef]
- Gleason, K.E.; Bradford, J.B.; Bottero, A.; D’Amato, A.W.; Fraver, S.; Palik, B.J.; Battaglia, M.A.; Iverson, L.; Kenefic, L.; Kern, C.C. Competition amplifies drought stress in forests across broad climatic and compositional gradients. Ecosphere 2017, 8, 7. [Google Scholar] [CrossRef]
- Lu, K.; Chen, N.; Zhang, C.; Dong, X.; Zhao, C. Drought Enhances the Role of Competition in Mediating the Relationship between Tree Growth and Climate in Semi-Arid Areas of Northwest China. Forests 2019, 10, 804. [Google Scholar] [CrossRef]
- Canham, C.D.; Papaik, M.J.; Uriarte, M.; McWilliams, W.H.; Jenkins, J.C.; Twery, M.J. Neighborhood analyses of canopy tree competition along environmental gradients in New England forests. Ecol. Appl. 2006, 16, 540–554. [Google Scholar] [CrossRef]
- Ruíz-Benito, P.; Lines, E.; Gómez-Aparicio, L.; Coomes, D.; Zavala, M.A. Climatic effects on tree mortality are amplified by competition in Mediterranean forests. PLoS ONE 2013, e56843. [Google Scholar] [CrossRef]
- Fernandez-de-Uña, L.; Cañellas, I.; Gea-Izquierdo, G. Stand competition determines how different tree species will cope with a warming climate. PLoS ONE 2015, 10, e0122255. [Google Scholar] [CrossRef]
- Ford, K.R.; Breckheimer, I.K.; Franklin, J.F.; Freund, J.A.; Kroiss, S.J.; Larson, A.J.; Theobald, E.J.; Hille Ris Lambers, J. Competition alters tree growth responses to climate at individual and stand scales. Can. J. For. Res. 2017, 47, 53–62. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; Worledge, D.; Pinkard, E.A. Co-ordination of growth, gas exchange and hydraulics define the carbon margin in tree species with contrasting drought strategies. Tree Physiol. 2014, 34, 443–458. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant. Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant. Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Field, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Nardini, A.; Battistuzzo, M.; Savi, T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 2013, 200, 322–329. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010, 186, 264–266. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [CrossRef]
- Mencuccini, M.; Minunno, F.; Salmon, Y.; Martínez-Vilalta, J.; Hölttä, T. Coordination of physiological traits involved in drought-induced mortality of woody plants. New Phytol. 2015, 208, 96–409. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Ortíz, C.; Covelo, F.; Ochoa, V.; García-Ruíz, R.; Seco, J.; Carreira, J.A.; Merino, J.A.; Linares, J.C. Regulation of water use in the southernmost European fir (Abies pinsapo Boiss.): Drought avoidance matters. Forests 2015, 6, 2241–2260. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Flexas, J.; Galmés, J.; Niinemets, U.; Sancho-Knapik, D.; Barredo, G.; Villarroya, D.; Gil-Pelegrín, E. Leaf anatomical properties in relation to differences in mesophyll conductance to CO2 and photosynthesis in two related Mediterranean Abies species. Plant. Cell Environ. 2012, 35, 2121–2129. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Peguero-Pina, J.J.; Flexas, J.; Herbette, S.; Cochard, H.; Niinemets, U.; Gil-Pelegrín, E. Coping with low light under high atmospheric dryness: Shade acclimation in a Mediterranean conifer (Abies pinsapo Boiss.). Tree Physiol. 2014, 34, 1321–1333. [Google Scholar] [CrossRef]
- Lechuga, V.; Carraro, V.; Viñegla, B.; Carreira, J.A.; Linares, J.C. Managing drought-sensitive forests under global change. Low competition enhances long-term growth and water uptake in Abies pinsapo. For. Ecol. Manag. 2017, 406, 72–82. [Google Scholar] [CrossRef]
- Sperry, J.S.; Donnelly, J.R.; Tyree, M.T. A method for measuring hydraulic conductivity and embolism in xylem. Plant. Cell Environ. 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer: Berlin, Germany, 1983; p. 144. [Google Scholar]
- Cochard, H.; Cruiziat, P.; Tyree, M.T. Use of positive pressures to establish vulnerability curves. Plant. Physiol. 1992, 100, 205–209. [Google Scholar] [CrossRef]
- Sperry, J.S.; Saliendra, N.Z. Intra- and inter-plant variation in xylem cavitation in Betula occidentalis. Plant. Cell Environ. 1994, 17, 1233–1241. [Google Scholar] [CrossRef]
- Sperry, J.S.; Ikeda, T. Xylem cavitation in roots and stems of Douglas-fir and white-fir. Tree Physiol. 1997, 17, 275–280. [Google Scholar]
- Pammenter, N.W.; Willigen, C.V. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef]
- Granier, A. Une nouvelle methode pour la measure du flux de seve brute dans le tronc des arbres. Ann. Sci. Forest. 1985, 42, 193–200. [Google Scholar] [CrossRef]
- Moore, G.W.; Bond, B.J.; Jones, J.A.; Meinzer, F.C. Thermal-dissipation sap flow sensors may not yield consistent sap-flux estimates over multiple years. Trees 2010, 24, 165–174. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Meinzer, F.C.; Andrade, J.L.; Goldstein, G.; Holbrook, N.M. Potential errors in measurement of non-uniform sap flow using heat dissipation probes. Tree Physiol. 1999, 19, 681–687. [Google Scholar] [CrossRef]
- Poyatos, R.; Cěrmák, J.; Llorens, P. Variation in the radial patterns of sap flux density in pubescent oak (Quercus pubescens Willd.) and its implications for tree and stand transpiration measurements. Tree Physiol. 2007, 27, 537–548. [Google Scholar] [CrossRef]
- Ford, C.R.; McGuire, M.A.; Mitchell, R.J.; Teskey, R.O. Assessing variation in the radial profile of sap flux density in Pinus species and its effect on daily water use. Tree Physiol. 2004, 24, 241–249. [Google Scholar] [CrossRef]
- Popp, M.; Lied, W.; Meyer, A.J.; Richter, A.; Schiller, P.; Schwitte, H. Sample preservation for determination of organic compounds: Microwave versus freeze-drying. J. Exp. Bot. 1996, 47, 1469–1473. [Google Scholar] [CrossRef]
- Hansen, J.; Moller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Sutton, B.G.; Ting, I.P.; Sutton, R. Carbohydrate metabolism of cactus in a desert environment. Plant. Physiol. 1981, 68, 784–787. [Google Scholar] [CrossRef]
- Rose, R.; Rose, C.L.; Omi, S.K.; Forry, K.R.; Durall, D.M.; Bigg, W.L. Starch determination by perchloric acid vs. enzymes: Evaluating the accuracy and precision of six colorimetric methods. J. Agric. Food Chem. 1991, 39, 2–11. [Google Scholar] [CrossRef]
- Friedrich, S.; Brunner, E.; Pauly, M. Permuting longitudinal data in spite of the dependencies. J. Multivar. Anal. 2017, 153, 255–265. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R. Development Core Team: Vienna, Austria, 2019. [Google Scholar]
- Giorgi, F.; Lionello, P. Climate change projects for the Mediterranean region. Gl. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Lagergren, F.; Lindroth, A. Variation in sap flow and stem growth in relation to tree size, competition and thinning in a mixed forest of pine and spruce in Sweden. For. Ecol. Manag. 2004, 188, 51–63. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Hauck, M.; Bader, M.; Oyungerel, S.; Dalaikhuu, O.; Nyambayar, S.; Leuschner, C. The different strategies of Pinus sylvestris and Larix sibirica to deal with summer drought in a northern Mongolian forest-steppe ecotone suggest a future superiority of pine in a warming climate. Can. J. For. Res. 2009, 39, 2520–2528. [Google Scholar] [CrossRef]
- Anfodillo, T.; Rento, S.; Carraro, V.; Furlanetto, L.; Urbinati, C.; Carrer, M. Tree water relations and climatic variations at the alpine timberline: Sesonal changes of sap flux and xylem water potential in Larix decidua, Picea abies and Pinus cembra. Ann. For. Sci. 1998, 55, 159–172. [Google Scholar] [CrossRef]
- Linares, J.C.; Covelo, F.; Carreira, J.A.; Merino, J. Phenological and water-use patterns underlying maximum growing season length at the highest elevations: Implications under climate change. Tree Physiol. 2012, 32, 161–170. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Piñol, J.; Beven, K. A hydraulic model to predict drought-induced mortality in woody plants: An application to climate change in the Mediterranean. Ecol. Model. 2002, 155, 127–147. [Google Scholar] [CrossRef]
- Piñol, J.; Sala, A. Ecological implications of xylem cavitation for several Pinaceae in the Pacific Northern USA. Funct. Ecol. 2000, 14, 538–545. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Cochard, H.; Barredo, G.; Villarroya, D.; Gil-Pelegrín, E. Hydraulic traits are associated with the distribution range of two closely related Mediterranean firs, Abies alba Mill. and Abies pinsapo Boiss. Tree Physiol. 2011, 31, 1067–1075. [Google Scholar] [CrossRef]
- Oliveras, I.; Martínez-Vilalta, J.; Jiménez-Ortiz, T.; Lledó, M.J.; Escarré, A.; Piñol, J. Hydraulic properties of Pinus halepensis, Pinus pinea and Tetraclinis articulata in a dune ecosystem of Eastern Spain. Plant. Ecol. 2003, 169, 131–141. [Google Scholar] [CrossRef]
- Irvine, J.; Perks, M.P.; Magnani, F.; Grace, J. The response of Pinus sylvestris to drought: Stomatal control of transpiration and hydraulic conductance. Tree Physiol. 1998, 18, 393–402. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Aldea, J.; Bravo, F.; Bravo-Oviedo, A.; Ruiz-Peinado, R.; Rodriguez, F.; del Rio, M. Thinning enhances the species-specific radial increment response to drought in Mediterranean pine-oak stands. Agr. Forest. Meteorol. 2017, 237, 371–383. [Google Scholar] [CrossRef]
- Elkin, C.; Giuggiola, A.; Rigling, A.; Bugmann, H. Short- and long-term efficacy of forest thinning to mitigate drought impacts in mountain forests in the European Alps. Ecol. Appl. 2015, 25, 108–1098. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.W.; Bradford, J.B.; Fraver, S.; Palik, B.J. Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol. Appl. 2013, 23, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.; Tobin, B.; Black, K.; Gioria, M.; Nieuwenhuis, M.; Osborne, B.A. Thinning effects on the net ecosystem carbon exchange of a sitka spruce forest are temperature-dependent. Agr. For. Meteorol. 2012, 157, 1–10. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Flexas, J.; Galmés, J.; Niinemets, Ü.; Gil-Pelegrín, E. Light acclimation of photosynthesis in two closely related firs (Abies pinsapo Boiss. and Abies alba Mill.): The role of leaf anatomy and mesophyll conductance to CO2. Tree Physiol. 2016, 36, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Non-structural carbon in woody plants. Annu. Rev. Plant. Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; Helliker, B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol. 2012, 195, 285–289. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Oberhuber, W.; Swidrak, I.; Pirkebner, D.; Gruber, A. Temporal dynamics of nonstructural carbohydrates and xylem growth in Pinus sylvestris exposed to drought. Can. J. For. Res. 2011, 1597, 1590–1597. [Google Scholar] [CrossRef]
- Piper, F. Drought induces opposite changes in the concentration of non-structural carbohydrates of two evergreen Nothofagus species of differential drought resistance. Ann. For. Sci. 2011, 68, 415–424. [Google Scholar] [CrossRef]
- DeSoto, L.; Olano, J.M.; Rozas, V. Secondary Growth and Carbohydrate Storage Patterns Differ between Sexes in Juniperus thurifera. Front. Plant. Sci. 2016, 7, 723. [Google Scholar] [CrossRef]
- Carbone, M.S.; Czimczik, C.I.; Keenan, T.F.; Murakami, P.F.; Pederson, N.; Schaberg, P.G.; Xu, X.; Richardson, A.D. Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phytol. 2013, 200, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Forner, N.; Biel, C.; Savé, R.; Martinez-Vilalta, J. 2016. Isohydric species are not necessarily more carbon limited than anisohydric species during drought. Tree Physiol. 2016, 37, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.S.; Zhang, K.R.; Zhang, Q.F.; Xu, Y.M. Temporal variations of mobile carbohydrates in Abies fargesii at the upper tree limits. Plant. Biol. 2015, 17, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Piper, F.; Pfund, L.; Körner, C.; Hoch, G. Variation of mobile carbon reserves in trees at the alpine treeline ecotone is under environmental control. New Phytol. 2012, 195, 794–802. [Google Scholar] [CrossRef]
| Treatment | CL | TL | CH | ||||
|---|---|---|---|---|---|---|---|
| Basal area | (m2 ha−1) | 31.45 | (3.8) | 13.40 | (1.41) | 9.15 | (2.76) |
| Density | (trees ha−1) | 1616 | (664) | 571 | (15) | 502 | (62) |
| Tree diameter | (DBH, cm) | 13.00 | (1.70) | 14.15 | (2.05) | 7.90 | (0.57) |
| Tree age | (years at DBH) | 45 | (2) | 46 | (2) | 29 | (1) |
| Treatment | CL | TL | CH | ||||
|---|---|---|---|---|---|---|---|
| Tree diameter | (DBH, cm) | 8.16 | (1.10) | 7.63 | (0.85) | 5.25 | (2.13) |
| Branch diameter | (mm) | 9.70 | (0.30) | 9.60 | (0.30) | 8.30 | (0.30) |
| Branch age | (years) | 13 | (2) a | 11 | (2) a | 5 | (2) b |
| Kh | (kg m4 MPa−1 s−1) | 2.51 × 10−5 | (8.4 × 10−6) | 2.3 × 10−5 | (3.8 × 10−6) | 1.05 × 10−5 | (4.7 × 10−6) |
| Ks | (kg m2 MPa−1 s−1) | 0.33 | (0.09) | 0.32 | (0.05) | 0.19 | (0.08) |
| Kl | (kg m2 MPa−1 s−1) | 1.16 × 10−4 | (2.6 × 10−5) | 0.91 × 10−4 | (1.6 × 10−5) | 0.68 × 10−4 | (2.8 × 10−5) |
| Al | (m2) | 0.21 | (0.02) ab | 0.25 | (0.02) a | 0.14 | (0.01) b |
| a | 0.64 | (0.12) | 0.76 | (0.12) | 0.93 | (0.11) | |
| b (PLC50) | (MPa) | −6.94 | (0.7) | −6.55 | (0.11) | −5.73 | (0.72) |
| PLC12 | (MPa) | −3.83 | (0.7) | −3.82 | (0.74) | −3.59 | (0.72) |
| PLC88 | (MPa) | −10.05 | (0.7) | −9.29 | (0.74) | −7.89 | (0.72) |
| R2 | 0.82 | (0.07) | 0.84 | (0.05) | 0.91 | (0.01) | |
| N | 7 | 7 | 4 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechuga, V.; Carraro, V.; Viñegla, B.; Carreira, J.A.; Linares, J.C. Carbon Limitation and Drought Sensitivity at Contrasting Elevation and Competition of Abies pinsapo Forests. Does Experimental Thinning Enhance Water Supply and Carbohydrates? Forests 2019, 10, 1132. https://doi.org/10.3390/f10121132
Lechuga V, Carraro V, Viñegla B, Carreira JA, Linares JC. Carbon Limitation and Drought Sensitivity at Contrasting Elevation and Competition of Abies pinsapo Forests. Does Experimental Thinning Enhance Water Supply and Carbohydrates? Forests. 2019; 10(12):1132. https://doi.org/10.3390/f10121132
Chicago/Turabian StyleLechuga, Victor, Vinicio Carraro, Benjamín Viñegla, José Antonio Carreira, and Juan Carlos Linares. 2019. "Carbon Limitation and Drought Sensitivity at Contrasting Elevation and Competition of Abies pinsapo Forests. Does Experimental Thinning Enhance Water Supply and Carbohydrates?" Forests 10, no. 12: 1132. https://doi.org/10.3390/f10121132
APA StyleLechuga, V., Carraro, V., Viñegla, B., Carreira, J. A., & Linares, J. C. (2019). Carbon Limitation and Drought Sensitivity at Contrasting Elevation and Competition of Abies pinsapo Forests. Does Experimental Thinning Enhance Water Supply and Carbohydrates? Forests, 10(12), 1132. https://doi.org/10.3390/f10121132







