Future Climate Change Will Have a Positive Effect on Populus davidiana in China
Abstract
:1. Introduction
2. Materials and Methods
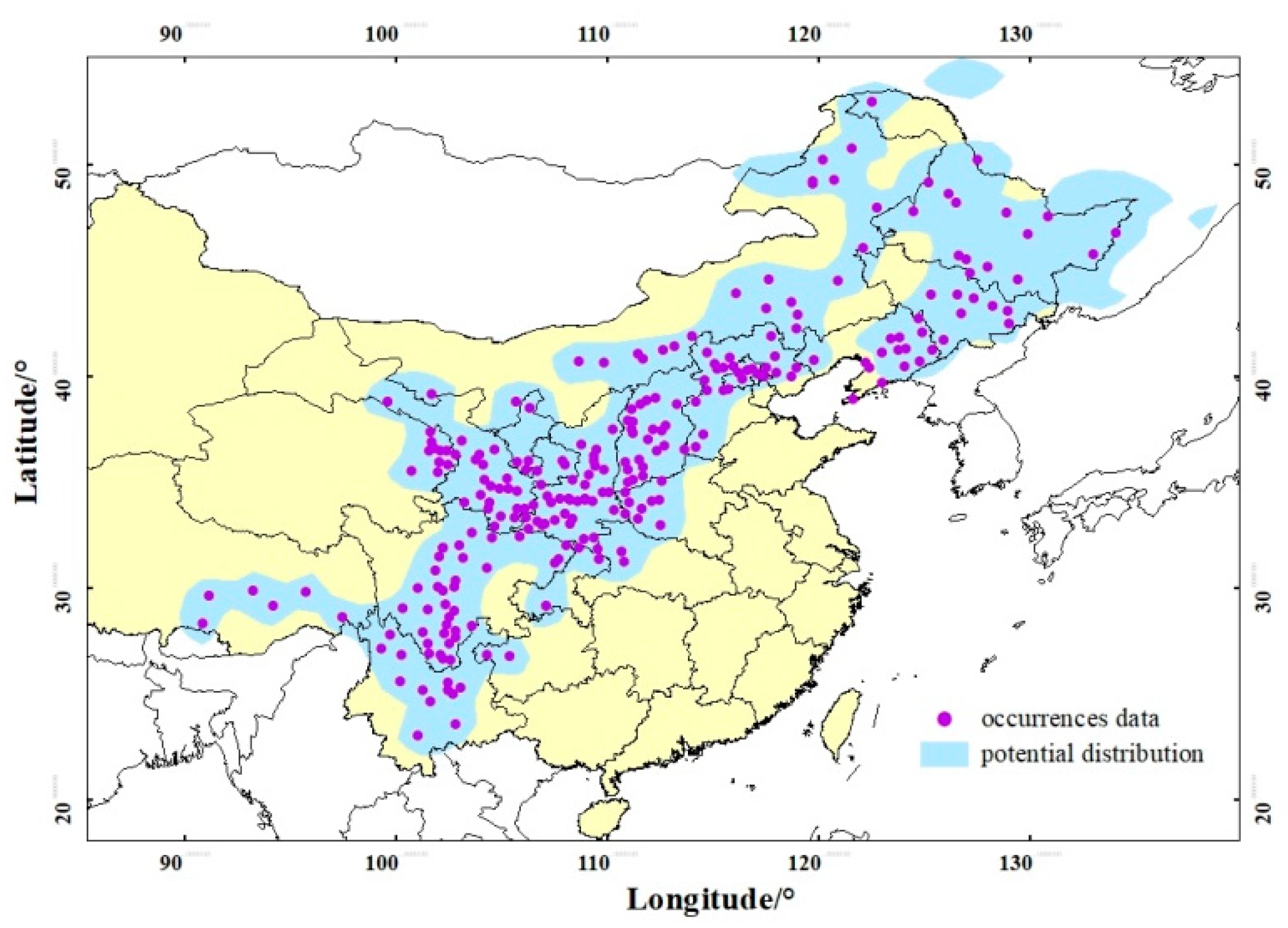
2.1. Species Occurrence Data
2.2. Climatic Variables and Their Layers under Current and Future Conditions
2.3. Species Distribution Simulation under Current and Future Climate
2.4. Measures of Species Vulnerability to Climate Change in Different Components
2.4.1. Vulnerability in the Component of Range Change (VRC)
2.4.2. Vulnerability in the Component of Protected Area (VPA)
2.4.3. Vulnerability in the Component of Human Footprint (VHF)
2.4.4. Vulnerability in the Component of Fragmentation (VFr)
2.4.5. Overall Vulnerability Index (V)
3. Results
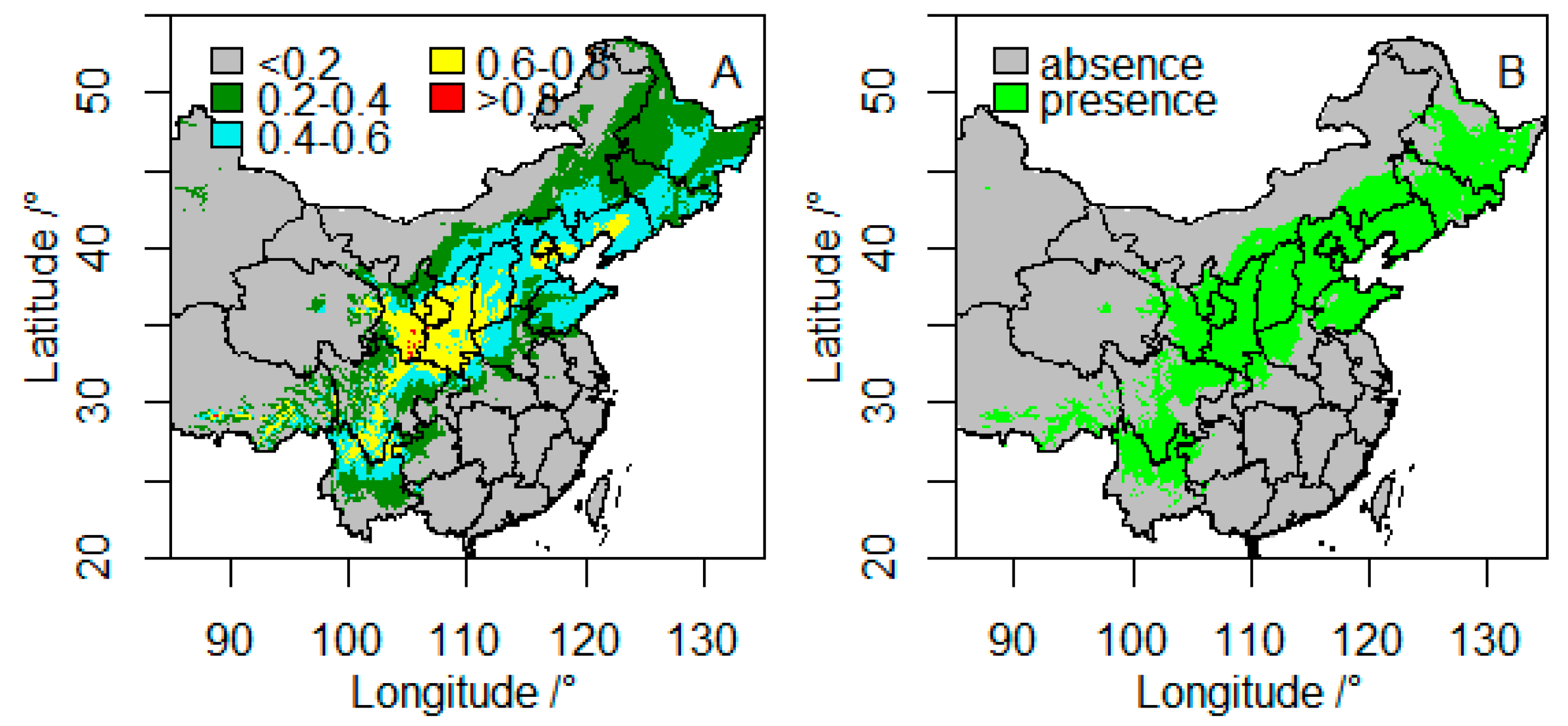
3.1. Model Accuracy and Current Suitable Climatic Habitats
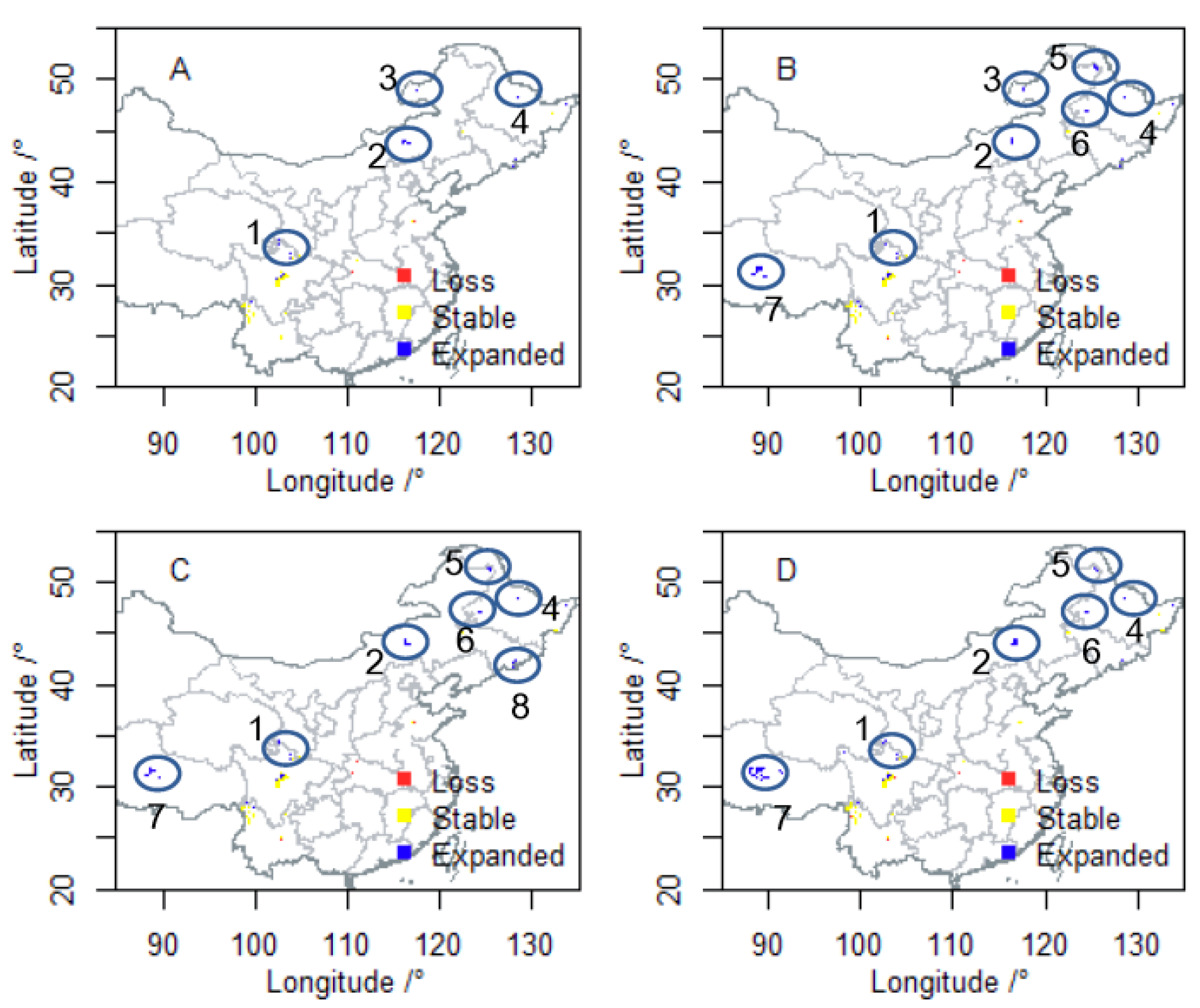
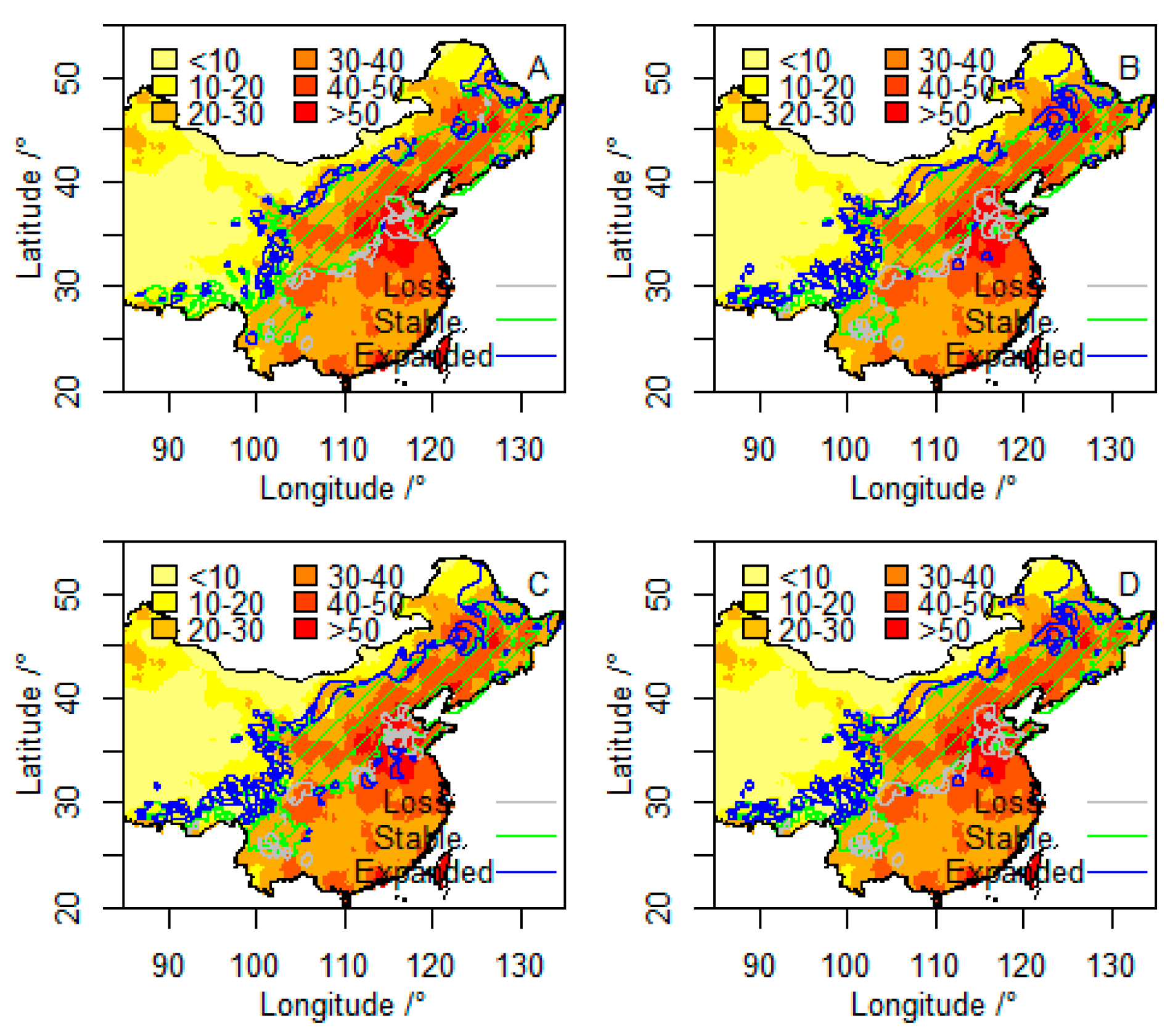
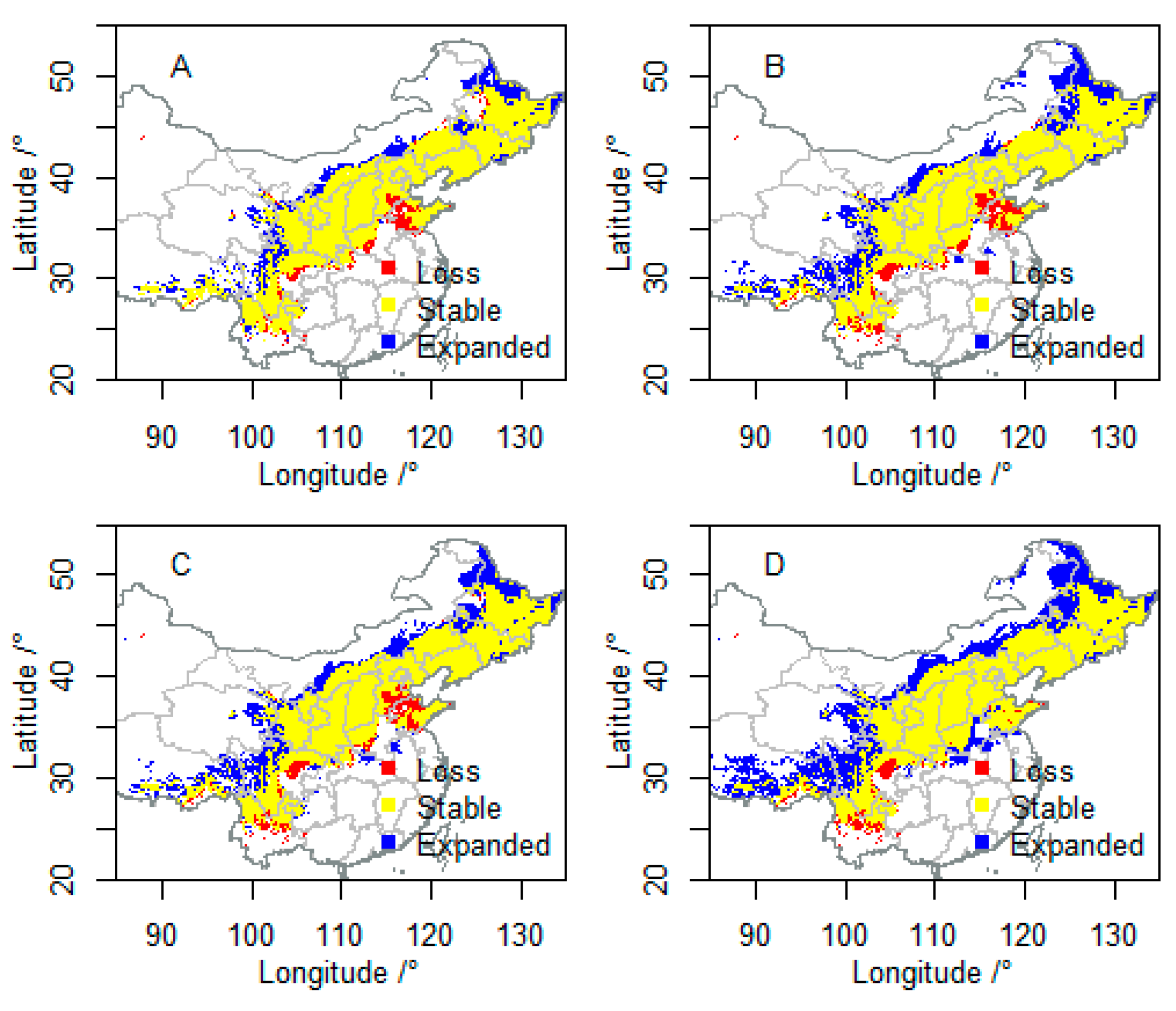
3.2. Future Suitable Climatic Habitats
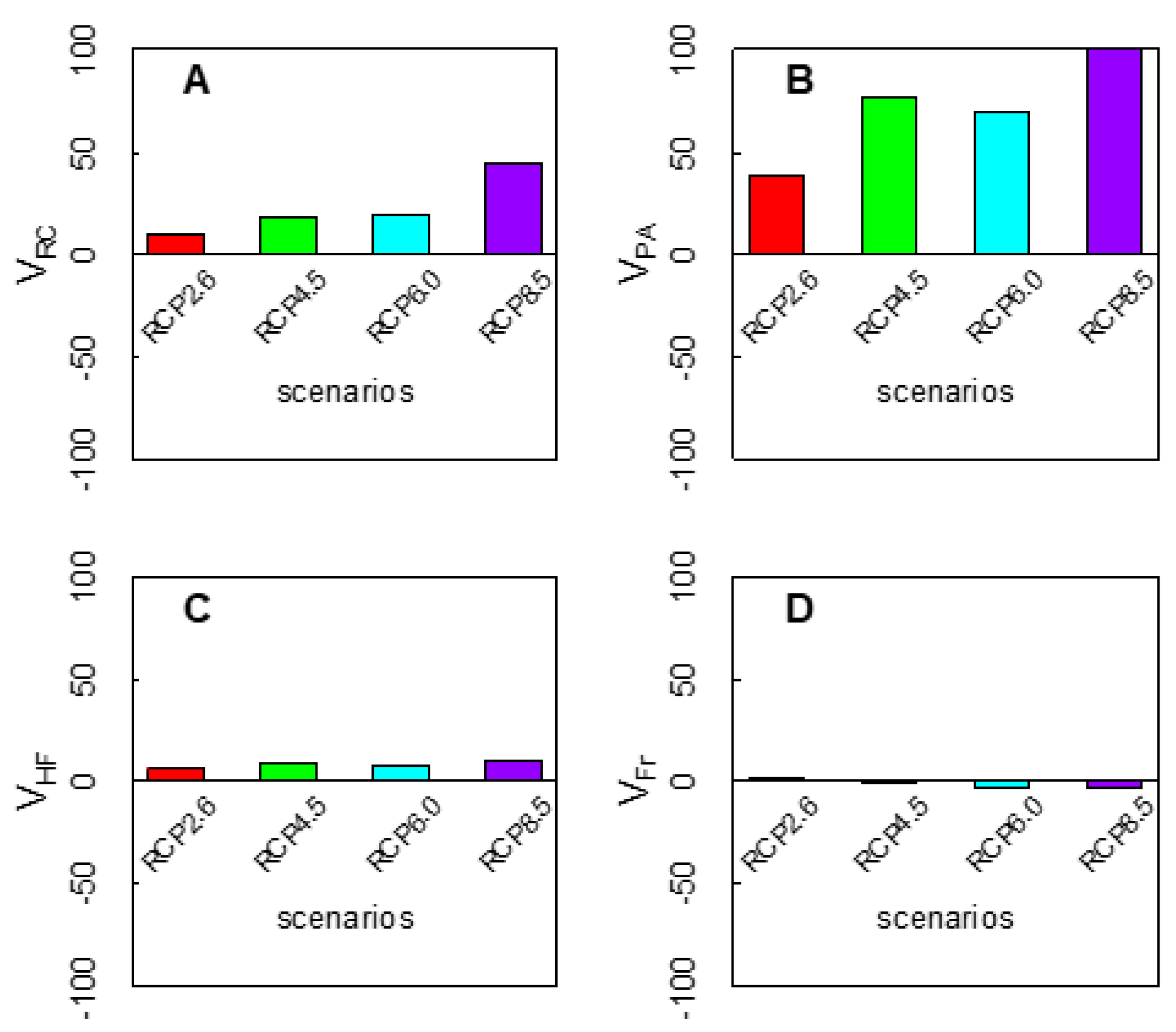
3.3. Vulnerability to Climatic Change of P. davidiana in Four Components
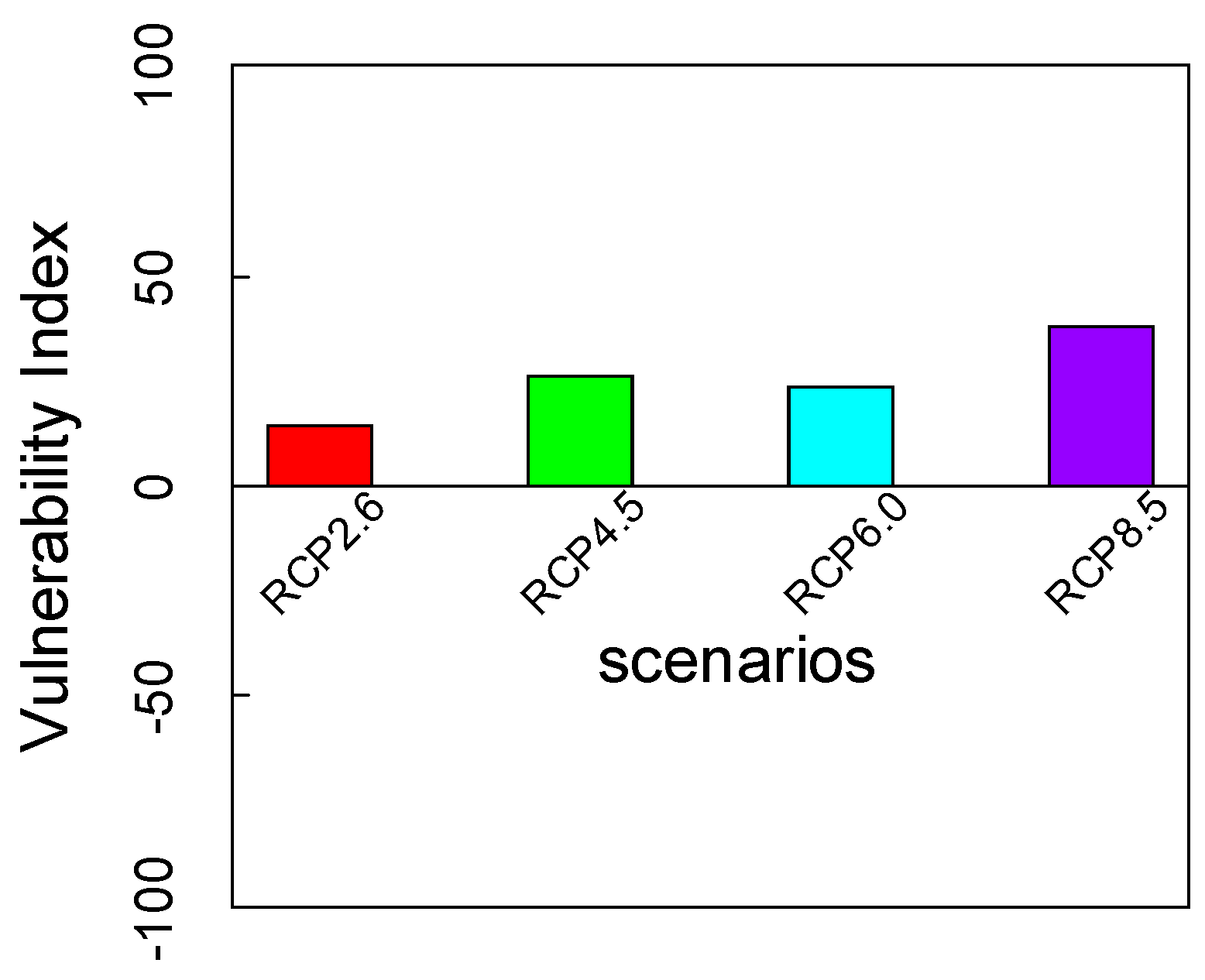
3.4. Integration of Exposure Components into an Overall Vulnerability Index
4. Discussion
4.1. Future Climatic Conditions Will Benefit the Survival of P. davidiana
4.2. Uncertainty and Potential Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Fragmentation | RCP2.6 | RCP4.5 | RCP6.0 | RCP8.5 |
|---|---|---|---|---|
| Ed | −12.64 | −1.50 | 0.15 | 2.88 |
| P_A | −9.09 | 0.00 | 4.55 | 4.55 |
| Shape | −9.52 | 1.96 | 3.63 | 6.48 |
| CA | 1.22 | 0.00 | −1.22 | −1.22 |
| Vulnerability | 1.80 | −0.87 | −2.31 | −2.34 |
References
- Booth, T.H. Why understanding the pioneering and continuing contributions of BIOCLIM to species distribution modeling is important. Austral. Ecol. 2018, 43, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Booth, T.H.; Broadhurst, L.M.; Pinkard, E.; Prober, S.M.; Dillon, S.K. Native forests and climate change: Lessons from Eucalypts. For. Ecol. Manag. 2015, 347, 18–29. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunez, S.; Arets, E.; Alkemade, R.; Verwer, C.; Leemans, R. Assessing the impacts of climate change on biodiversity: Is below 2 °C enough? Clim. Chang. 2019, 154, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; De Siqueira, M.F.; Grainger, A.; Hannah, L. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Levinsky, I.; Skov, F.; Svenning, J.C.; Rahbek, C. Potential impacts of climate change on the distributions and diversity patterns of European mammals. Biodivers. Conserv. 2007, 16, 3803–3816. [Google Scholar] [CrossRef]
- Arribas, P.; Abellan, P.; Velasco, J.; Bilton, D.T.; Millan, A.; Sanchez-Fernandez, D. Evaluating drivers of vulnerability to climate change: A guide for insect conservation strategies. Glob. Chang. Biol. 2012, 18, 2135–2146. [Google Scholar] [CrossRef]
- Foden, W.B.; Butchart, S.H.M.; Stuart, S.N.; Vie, J.C.; Akcakaya, H.R.; Angulo, A.; DeVantier, L.M.; Gutsche, A.; Turak, E.; Cao, L. Identifying the world’s most climate change vulnerable species: A systematic trait-based assessment of all birds, amphibians and corals. PloS ONE 2013, 8, e65427. [Google Scholar] [CrossRef] [Green Version]
- Booth, T.H. Estimating potential range and hence climatic adaptability in selected tree species. For. Ecol. Manag. 2016, 366, 175–183. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Hughes, L. Potential changes in the distributions of latitudinally restricted Australian butterfly species in response to climate change. Glob. Chang. Biol. 2002, 8, 954–971. [Google Scholar] [CrossRef]
- Harrison, P.A.; Berry, P.M.; Butt, N.; New, M. Modelling climate change impacts on species’ distributions at the European scale: Implications for conservation policy. Environ. Sci. Policy 2006, 9, 116–128. [Google Scholar] [CrossRef]
- Huntley, B.; Collingham, Y.C.; Willis, S.G.; Green, R.E. Potential impacts of climatic change on European breeding birds. PloS ONE 2008, 3, e1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, R.M.; Didham, R.K. The effect of fragment shape and species’ sensitivity to habitat edges on animal population size. Conserv. Biol. 2007, 21, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Liu, C.C.; Liu, Y.G.; Yang, J.; Zhang, X.S.; Guo, K. Effects of climate, disturbance and soil factors on the potential distribution of Liaotung oak (Quercus wutaishanica Mayr) in China. Ecol. Res. 2012, 27, 427–436. [Google Scholar] [CrossRef]
- Cianfrani, C.; Broennimann, O.; Loy, A.; Guisan, A. More than range exposure: Global otter vulnerability to climate change. Biol. Conserv. 2018, 221, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Rogers, P.C.; Pinno, B.D.; Šebesta, J.; Albrectsen, B.R.; Li, G.Q.; Ivanova, N.; Kusbach, A.; Kuuluvainen, T.; Landhäusser, S.M.; Liu, H.Y.; et al. A global view of aspen: Conservation science for widespread keystone systems. Glob. Ecol. Conserv. 2019, 21, e00828. [Google Scholar] [CrossRef]
- Hou, G.K.; Duan, S.G.; Zhao, S. Main Tree Species of Conversion Farmland to Forest in China (Volume of the North); China Agriculture Press: Beijing, China, 2004. [Google Scholar]
- ECVAC-Editorial Committee for Vegetation Atlas of China. 1:100 Million Vegetation Atlas of China; Science Press: Beijing, China, 2001. [Google Scholar]
- Chen, B.Q.; Zhang, J.J.; Zhang, Y.T.; Tian, N.N. Whole-tree sap flow of Quercus liaotungensis and Populus davidiana in response to environmental factors in the loess plateau area of western Shanxi Province, northern China. Appl. Ecol. J. 2016, 27, 746–754. [Google Scholar]
- Sun, Y.; Chen, S.; Huang, H.; Jiang, J.; Bai, S.; Liu, G. Improved salt tolerance of Populus davidiana x P. bolleana overexpressed LEA from Tamarix androssowii. J. For. Res. 2014, 25, 813–818. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, N.; Li, C. Physiological and growth responses of Populus davidiana ecotypes to different soil water contents. J. Arid Environ. 2005, 60, 567–579. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, Y.Y.; Hyun, J.O. Genetic variation in populations of Populus davidiana Dode based on microsatellite marker analysis. Genes Genomics 2011, 33, 163–171. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Kwon, S.H.; Jo, A.; Kim, Y.G.; Lee, J.W. Single nucleotide polymorphism (SNP) characterization of drought-responsive genes to estimate genetic variation of Populus tremula var. davidiana and eight other Populus species. Can. J. For. Res. 2018, 48, 689–696. [Google Scholar]
- Li, S.H.; Miao, R.; Chang, Y.; Li, J.N.; Yan, X.J.; Liu, Z.Y.; Zhang, R.S. Differential expression of PodaPIN9 gene in tissues of Populus davidiana x P-alba var. pyramidlis induced by Trichoderma. Bull. Bot. Res. 2019, 39, 267–275. [Google Scholar]
- Mun, B.G.; Hussain, A.; Park, E.J.; Lee, S.U.; Sharma, A.; Imran, Q.M.; Jung, K.H.; Yun, B.W. Profile and time-scale dynamics of differentially expressed genes in transcriptome of Populus davidiana under drought stress. Plant Mol. Biol. Rep. 2017, 35, 647–660. [Google Scholar] [CrossRef]
- Wang, N.; Dou, K.; Wang, Z.Y.; Zhang, R.S.; Wang, Y.C.; Liu, Z.H. Rooting and transplanting method of Populus davidiana x P-bolleana tissue culture seedlings. Bull. Bot. Res. 2014, 34, 380–385. [Google Scholar]
- Lu, M.M.; Liu, Z.H.; Wang, H.; Zhu, G.D.; Yang, X.T.; Zhang, R.S. Effects of Trichoderma asperellum on the physical and chemical properties and nutrient components of the pot coil culturing tissue cultured Populus davidiana x P. bolleana seedlings. Bull. Bot. Res. 2015, 35, 289–296. [Google Scholar]
- Shao, F.L.; Yu, X.X.; Song, S.M.; Zhao, Y. Spatial structural characteristics of natural Populus davidiana-Betula platyphylla secondary forest. Appl. Ecol. J. 2011, 22, 2792–2798. [Google Scholar]
- Zhao, P.W.; Xu, C.Y.; Zhoul, M.; Zhang, B.; Ge, P.; Zeng, N.; Liu, H.Y. Rapid regeneration offsets losses from warming-induced tree mortality in an aspen dominated broad-leaved forest in northern China. PloS ONE 2018, 13, e0195630. [Google Scholar] [CrossRef]
- Li, G.; Xu, G.H.; Guo, K.; Du, S. Geographical distribution and climatic analysis of Pinus tabuliformis in China: Insight on its afforestation. Ecol. Eng. 2016, 86, 75–84. [Google Scholar] [CrossRef]
- Huang, J.H.; Li, G.Q.; Li, J.; Zhang, X.Q.; Yan, M.J.; Du, S. Projecting the range shifts in climatically suitable habitat for Chinese Sea Buckthorn under climate change scenarios. Forests 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Chinese Virtual Herbarium. Available online: http://www.cvh.ac.cn (accessed on 21 March 2018).
- Li, G.; Du, S.; Guo, K. Evaluation of limiting climatic factors and simulation of a climatically suitable habitat for Chinese Sea Buckthorn. PloS ONE 2015, 10, e0131659. [Google Scholar]
- Holdridge, L.R. Determination of world plant formations from simple climatic data. Science 1947, 105, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Kira, T. A New Classification of Climate in Eastern Asia as the Basis for Agricultural Geography; Horticultural Institute Kyoto University: Kyoto, Japan, 1945. [Google Scholar]
- WorldClim. Global Climata Data—Free Climate Data for Ecological Modeling and GIS. Available online: http://www.worldclim.org/ (accessed on 2 May 2017).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Hughes, L.; Pitman, A.J. Why is the choice of future climate scenarios for species distribution modeling important. Ecol. Lett. 2008, 11, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, C.H. Preliminary assessment of simulations of climate changes over China by CMIP5 multi-models. Atmos. Ocean. Sci. Lett. 2012, 5, 489–494. [Google Scholar]
- Giorgi, F.; Mearns, L. Calculation of average, uncertainty range, and reliability of regional climate changes from AOGCM simulations via the reliability ensemble averaging (REA) method. J. Clim. 2002, 15, 1141–1158. [Google Scholar] [CrossRef]
- McMahon, S.M.; Harrison, S.P.; Armbruster, W.S.; Bartlein, P.J.; Beale, C.M.; Edwards, M.E.; Kattge, J.; Midgly, G.; Morin, X.; Prentice, I.C. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends Ecol. Evol. 2011, 26, 249–259. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Richardson, A.J.; Molinos, J.G.; Hoffmann, A.; Buckley, L.B.; Moore, P.J.; Brown, C.J.; Bruno, J.F.; Duarte, C.M.; et al. Geographical limits to species-range shifts are suggested by climate velocity. Nature 2014, 507, 492–495. [Google Scholar] [CrossRef]
- Soberon, J.; Nakamura, M. Niches and distributional areas: Concepts, methods, and assumptions. Proc. Natl. Acad. Sci. USA 2009, 106, 19644–19650. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, S.J.; White, M.D.; Newell, G.R. How useful are species distribution models for managing biodiversity under future climates? Ecol. Soc. 2010, 15, 8. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettman, F.; Lehmann, A. Novel methods improve prediction of species’ distribution from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Tsoar, A.; Allouche, O.; Steinitz, O.; Rotem, D.; Kadmon, R. A comparative evaluation of presence-only methods for modeling species distribution. Divers. Distrib. 2007, 13, 397–405. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Monserud, R.A.; Leemans, R. Comparing global vegetation maps with the Kappa-Statistic. Ecol. Model. 1992, 62, 275–293. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, Q.; He, S.A.; Liu, Y.; Wang, M.T.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PloS ONE 2018, 13, e0192153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarnevich, C.S.; Reynolds, L.V. Challenges of predicting the potential distribution of a slow-spreading invader: A habitat suitability map for an invasive riparian tree. Biol. Invasions 2011, 13, 153–163. [Google Scholar] [CrossRef]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The human footprint and the last of the wild. Bioscience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Vanderwal, J.; Falconi, L.; Januchowski, S.; Shoo, L.; Storlie, C. Sdmtools: Species Distribution Modelling Tools: Tools for Processing Data Associated with Species Distribution Modelling Exercises. R Package Version 1.1-221. 2014. Available online: https://cran.R-project.Org/package=sdmtools (accessed on 20 May 2016).
- Wu, Q.F.; Wang, J.S.; Lu, Z.F. Research progress of poplar plantation degradation and recovery. Chin. Agric. Sci. Bull. 2015, 31, 1–6. [Google Scholar]
- Li, H.F.; Zhang, B.Z.; Wang, J.M. Investigation on the causes of death of natural Populus davidiana forest in Ziwuling. Gansu Sci. Technol. 2008, 24, 166–167. [Google Scholar]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef] [Green Version]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, B.A. Ecology’s oldest pattern? Endeavour 2001, 25, 133–134. [Google Scholar] [CrossRef]
- Sakai, A.; Malla, M.S. Winter hardiness of tree species at high altitudes in the east Himalaya, Nepal. Ecology 1981, 62, 1288–1298. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T. Global imprint of climate change on marine life. Nat. Clim. Chang. 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, H.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Lomolino, M.V. Ecology’s most general, yet protean pattern: The species-area relationship. J. Biogeogr. 2000, 27, 17–26. [Google Scholar] [CrossRef]
| No. | Variable | Abbr. |
|---|---|---|
| 1 | Annual mean temperature | AMT |
| 2 | Maximum temperature of the warmest month | MTWM |
| 3 | Minimum temperature of the coldest month | MTCM |
| 4 | Annual temperature range | ART |
| 5 | Annual precipitation | AP |
| 6 | Precipitation of the wettest month | PWM |
| 7 | Precipitation of the driest month | PDM |
| 8 | Precipitation of seasonality (monthly coefficient of variation of precipitation) | PSD |
| 9 | Annual biotemperature 1 | ABT |
| 10 | Warmth index (WI = ∑(T − 5), where T > 5 °C) | WI |
| 11 | Coldness index (CI = −∑(5−T), where T < 5 °C) | CI |
| 12 | Potential evapotranspiration rate (PER = 58.93 × ABT/AP) | PER |
| 13 | Humidity index (HI = AP/WI) | HI |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, G.; Lu, Q.; Zhang, Y.; Li, G.; Du, S. Future Climate Change Will Have a Positive Effect on Populus davidiana in China. Forests 2019, 10, 1120. https://doi.org/10.3390/f10121120
Li J, Liu G, Lu Q, Zhang Y, Li G, Du S. Future Climate Change Will Have a Positive Effect on Populus davidiana in China. Forests. 2019; 10(12):1120. https://doi.org/10.3390/f10121120
Chicago/Turabian StyleLi, Jie, Guan Liu, Qi Lu, Yanru Zhang, Guoqing Li, and Sheng Du. 2019. "Future Climate Change Will Have a Positive Effect on Populus davidiana in China" Forests 10, no. 12: 1120. https://doi.org/10.3390/f10121120
APA StyleLi, J., Liu, G., Lu, Q., Zhang, Y., Li, G., & Du, S. (2019). Future Climate Change Will Have a Positive Effect on Populus davidiana in China. Forests, 10(12), 1120. https://doi.org/10.3390/f10121120





