Base Cation Fluxes from the Stemflow in Three Mixed Plantations in the Rainy Zone of Western China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plot Design
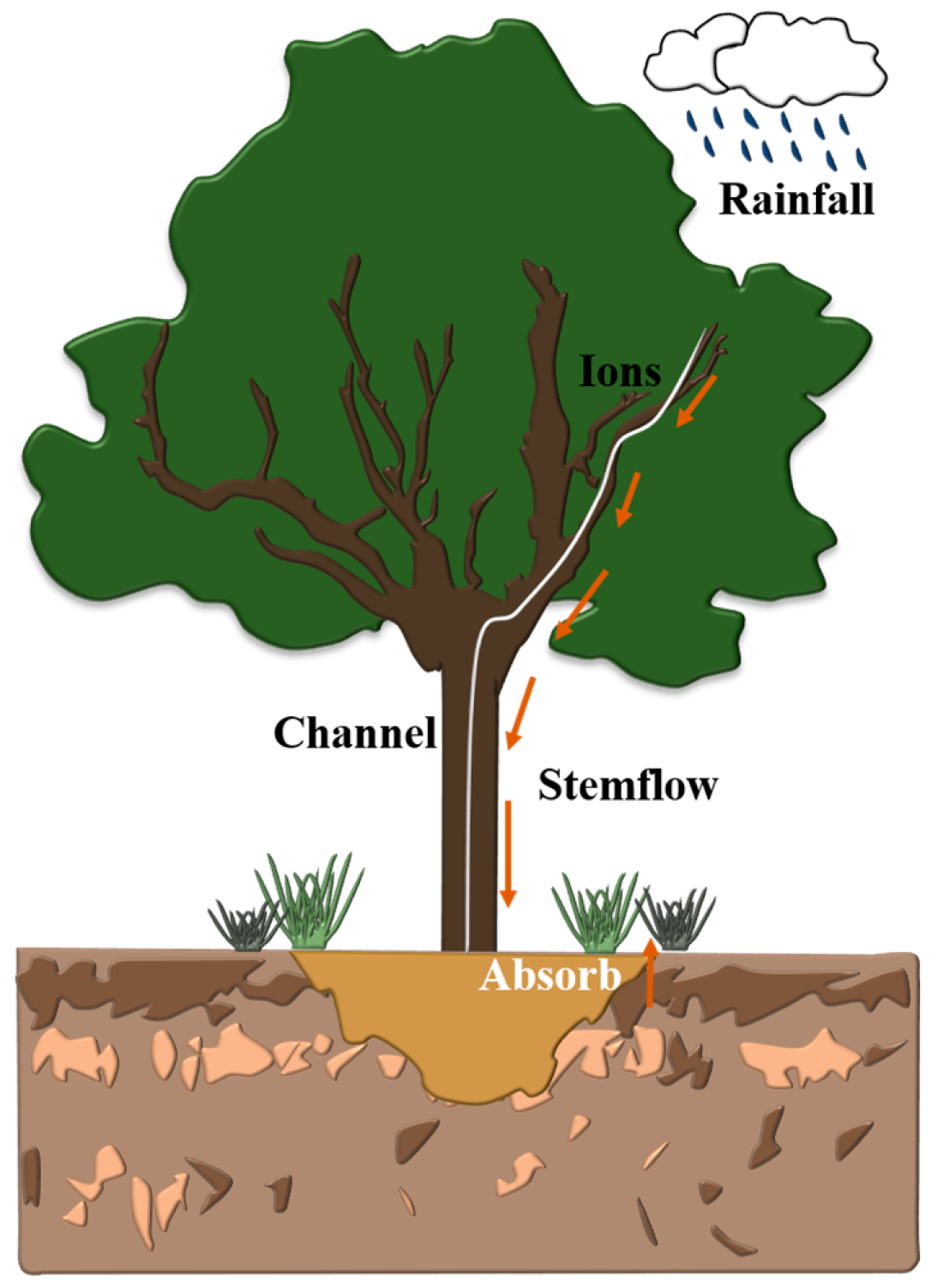
2.3. Stemflow and Rainfall Collection
2.4. Data Calculations and Statistical Analyses
3. Results
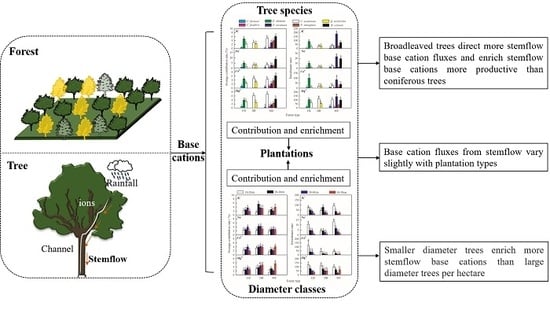
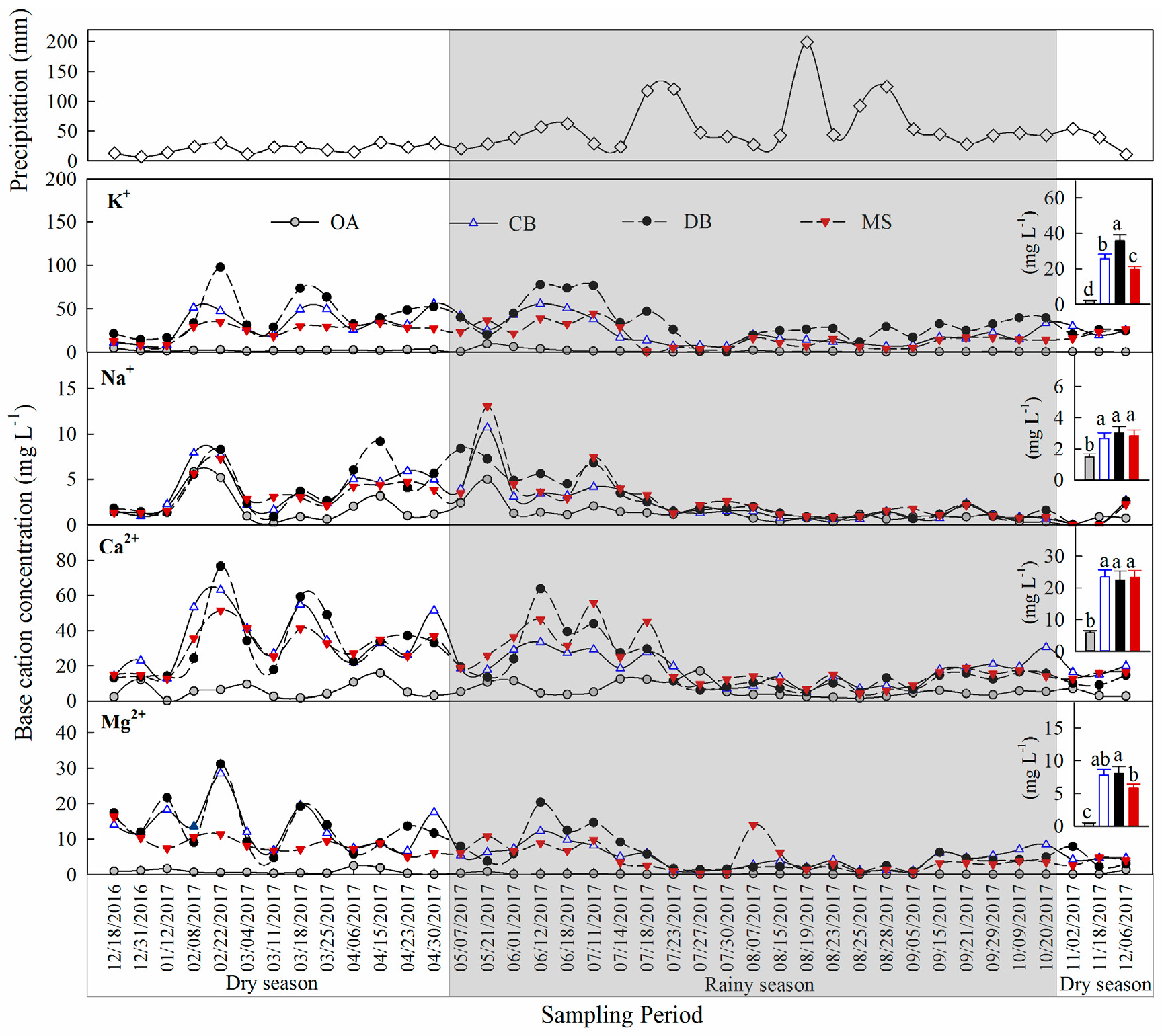
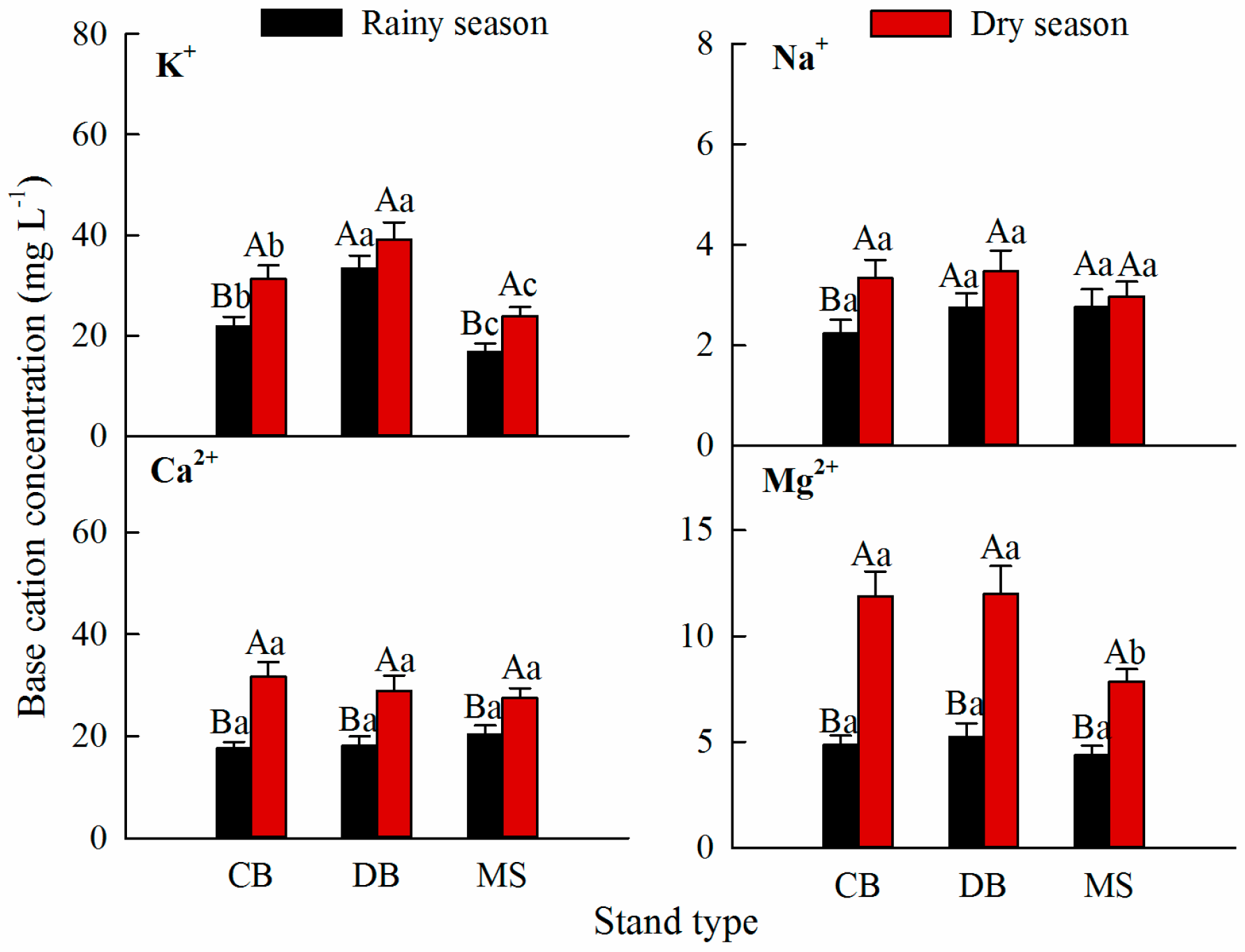
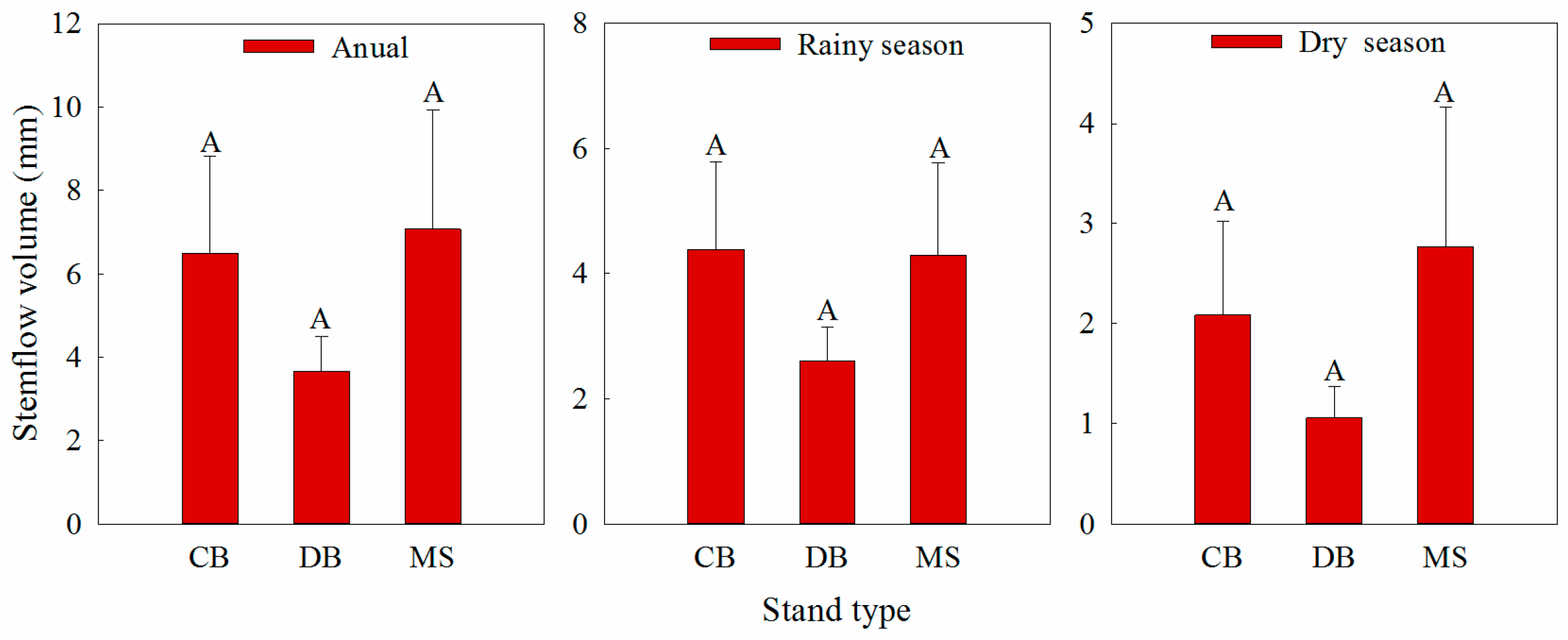
3.1. Base Cation Concentrations in Stemflow
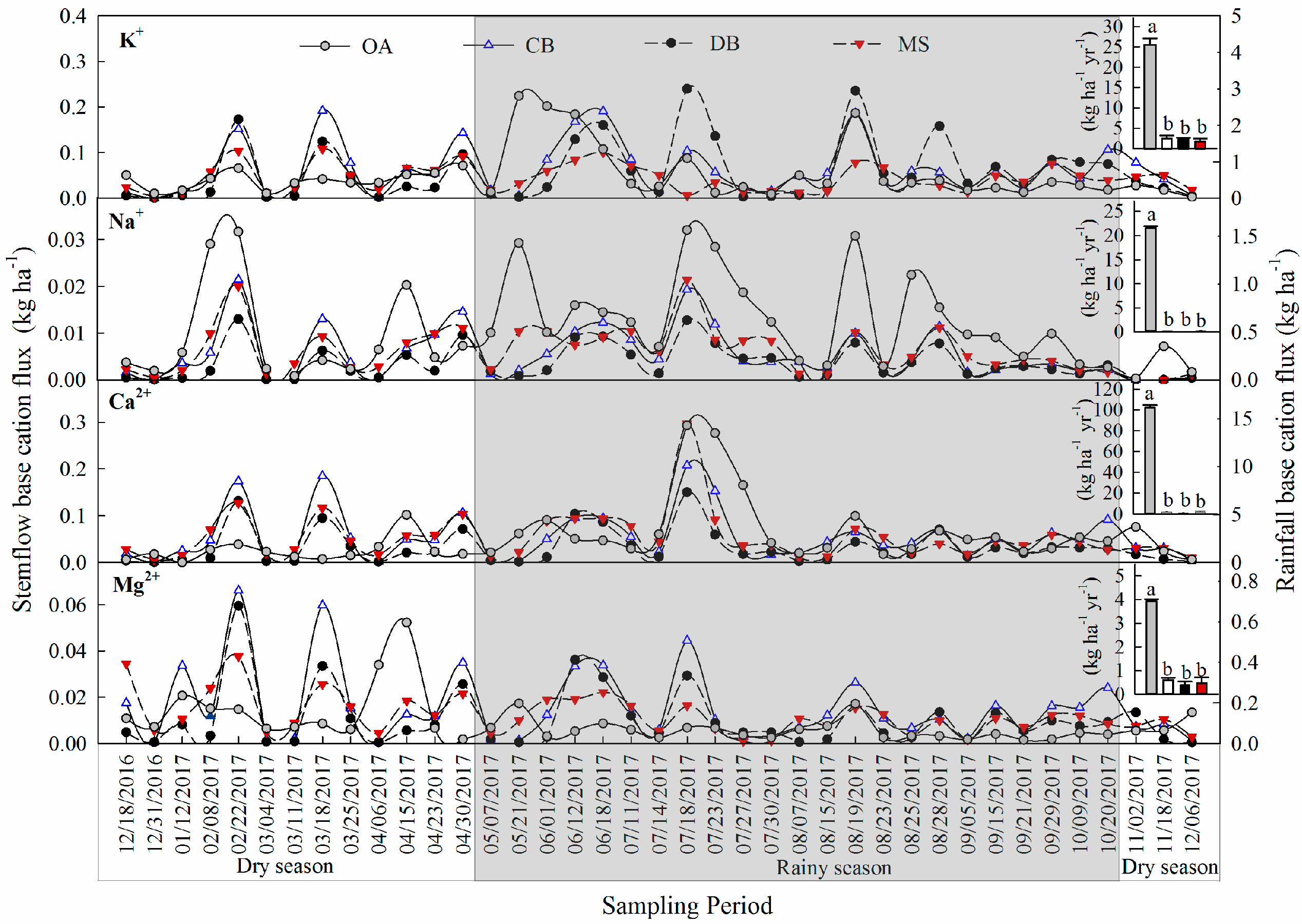
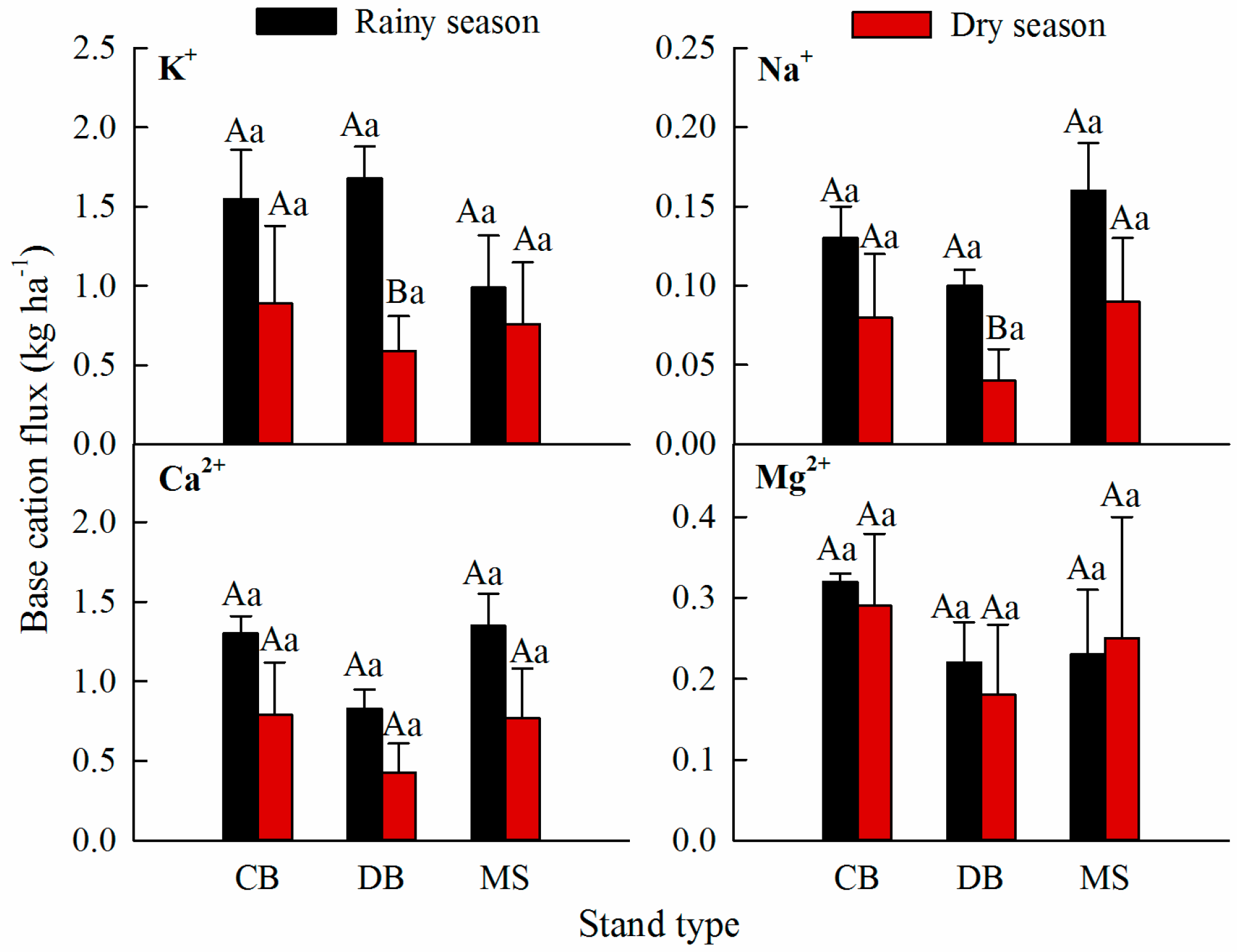
3.2. Base Cation Fluxes in the Stemflow
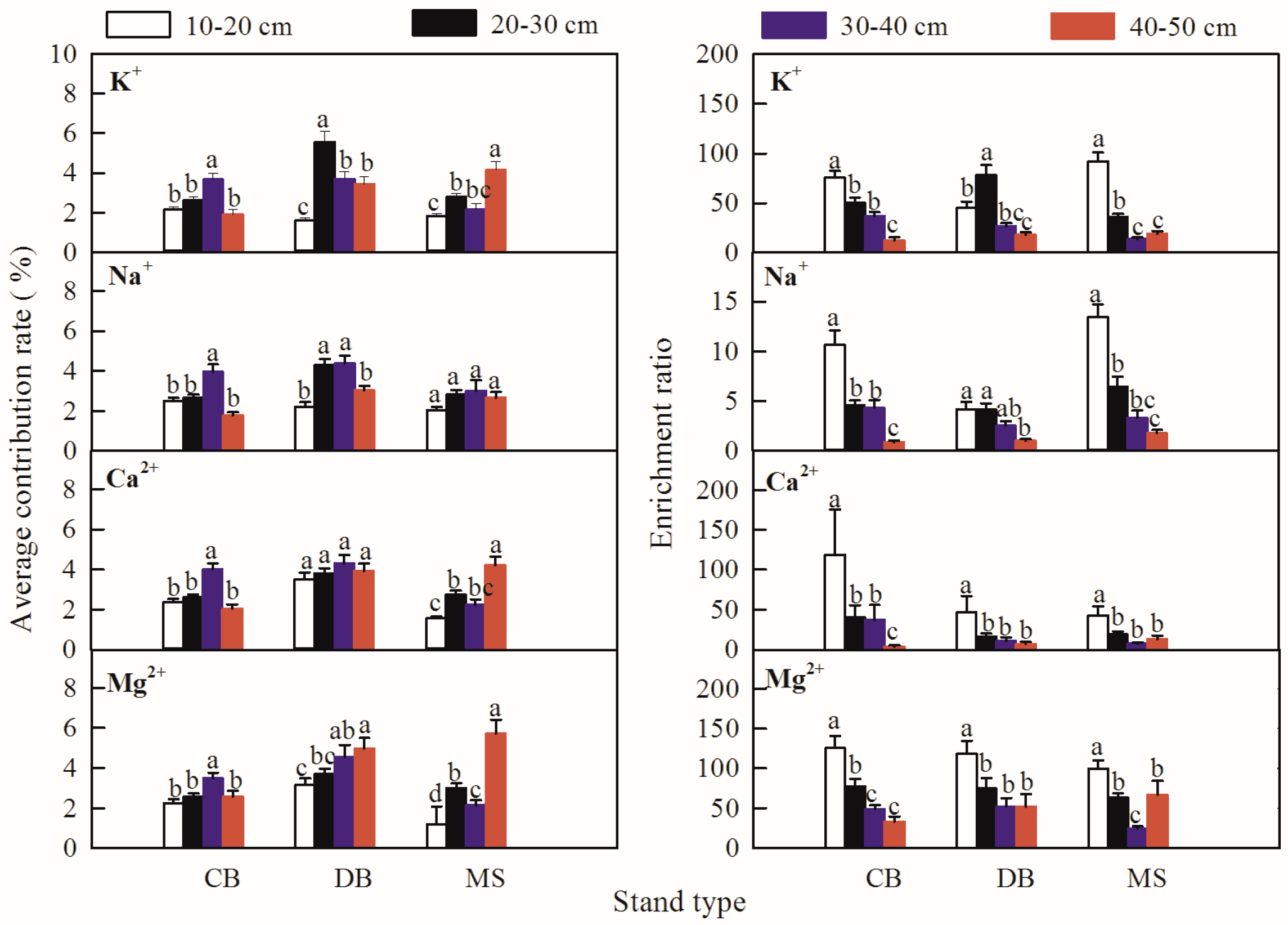
3.3. The Contribution and Enrichment Effects of the Afforestation Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahendrappa, M.K. Partitioning of rainwater and chemicals into throughfall and stemflow in different forest stands. For. Ecol. Manag. 1990, 30, 65–72. [Google Scholar] [CrossRef]
- Levia, D.F.; Frost, E.E. A review and evaluation of stemflow literature in the hydrologic and biogeochemical cycles of forested and agricultural ecosystems. J. Hydrol. 2003, 274, 1–29. [Google Scholar] [CrossRef]
- Van Stan, J.T.; Gordon, D.A. Mini-Review: Stemflow as a resource limitation to near-stem soils. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Hu, R.; Pan, Y.; Zhang, H. Stemflow in two xerophytic shrubs and its significance to soil water and nutrient enrichment. Ecol. Res. 2013, 28, 567–579. [Google Scholar] [CrossRef]
- Tan, S.; Zhao, H.; Yang, W.; Tan, B.; Ni, X.; Yue, K.; Zhang, Y.; Wu, F. The effect of canopy exchange on input of base cations in a subalpine spruce plantation during the growth season. Sci. Rep. UK 2018, 8, 9373. [Google Scholar] [CrossRef]
- Eaton, J.S.; Likens, G.E.; Bormann, F.H. Throughfall and stemflow chemistry in a northern hardwood forest. J. Ecol. 1973, 61, 495–508. [Google Scholar] [CrossRef]
- Crozier, C.R.; Boerner, R.E.J. Correlations of understory herb distribution patterns with microhabitats under different tree species in a mixed mesophytic forest. Oecologia 1984, 62, 337–343. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U. Effect of stemflow on beech forest soils and vegetation in Southern Sweden. J. Appl. Ecol. 1989, 26, 341–352. [Google Scholar] [CrossRef]
- Whitford, W.G.; Anderson, J.; Rice, P.M. Stemflow contribution to the “fertile island” effect in creosotebush, Larrea tridentata. J. Arid Environ. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Aboal, J.R.; Saavedra, S.; Hernández-Moreno, J.M. Edaphic heterogeneity related to below-canopy water and solute fluxes in a Canarian laurel forest. Plant Soil 2014, 387, 177–188. [Google Scholar] [CrossRef]
- Lei, S.; Xu, W.; Zhao, C.; Xie, Z.; Ju, H. Inter- and intra-specific variation in stemflow for evergreen species and deciduous tree species in a subtropical forest. J. Hydrol. 2016, 537, 1–9. [Google Scholar]
- Jian, S.; Zhang, X.; Li, D.; Wang, D.; Wu, Z.; Hu, C. The effects of stemflow on redistributing precipitation and infiltration around shrubs. J. Hydrol. Hydromech. 2018, 66, 79–86. [Google Scholar] [CrossRef]
- Ellison, D.; Morris, C.E.; Locatelli, B.; Sheil, D.; Cohen, J.; Murdiyarso, D.; Gutierrez, V.; Noordwijk, M.; Creed, I.F.; Pokorny, J.; et al. Trees, forests and water: Cool insights for a hot world. Glob. Environ. Chang. 2017, 43, 51–61. [Google Scholar] [CrossRef]
- Levia, D.F.; Germer, S. A review of stemflow generation dynamics and stemflow-environment interactions in forests and shrublands. Rev. Geophys. 2015, 53, 673–714. [Google Scholar] [CrossRef]
- Schrijver, A.D.; Geudens, G.; Augusto, L.; Staelens, J.; Mertens, J.; Wuyts, K.; Gielis, L.; Verheyen, K. The effect of forest type on throughfall deposition and seepage flux: A review. Oecologia 2007, 153, 663–674. [Google Scholar] [CrossRef]
- Robertson, S.M.C.; Hornung, M.; Kennedy, V.H. Water chemistry of throughfall and soil water under four tree species at Gisburn, northwest England, before and after felling. For. Ecol. Manag. 2000, 129, 101–117. [Google Scholar] [CrossRef]
- Levia, D.F.; Herwitz, S.R. Interspecific variation of bark water storage capacity of three deciduous tree species in relation to stemflow yield and solute flux to forest soils. Catena 2005, 64, 117–137. [Google Scholar] [CrossRef]
- André, F.; Jonard, M.; Ponette, Q. Effects of biological and meteorological factors on stemflow chemistry within a temperate mixed oak-beech stand. Sci. Total Environ. 2008, 393, 72–83. [Google Scholar] [CrossRef]
- Germer, S.; Zimmermann, A.; Neill, C.; Krusche, A.V.; Elsenbeer, H. Disproportionate single-species contribution to canopy-soil nutrient flux in an Amazonian rainforest. For. Ecol. Manag. 2012, 267, 40–49. [Google Scholar] [CrossRef]
- Tobón, C.; Sevink, J.; Verstraten, J.M. Solute fluxes in throughfall and stemflow in four forest ecosystems in northwest Amazonia. Biogeochemistry 2004, 70, 1–25. [Google Scholar] [CrossRef]
- Van Stan, J.T.; Levia, D.F.; Inamdar, S.P.; Lepori-Bui, M.; Mitchell, M.J. The effects of phenoseason and storm characteristics on throughfall solute washoff and leaching dynamics from a temperate deciduous forest canopy. Sci. Total Environ. 2012, 430, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Huang, J. Seasonal dynamics of soil N pools under Phoebe zhennan, Cunninghamia lanceolata (Lamb.) and evergreen broad-leaved forests in Dujiangyan Region, Sichuan, China. Acta Ecol. Sin. 2006, 26, 2540–2548. (In Chinese) [Google Scholar]
- Liu, Q.; Zhuang, L.Y.; Ni, X.Y.; You, C.M.; Yang, W.Q.; Wu, F.Z.; Tan, B.; Yue, K.; Liu, Y.; Zhang, L.; et al. Nitrogen additions stimulate litter humification in a subtropical forest, southwestern China. Sci. Rep. UK 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P. The concept of the rainy zone of west China and its significance to the biodiversity conservation in China. Chin. Biodivers. 2002, 10, 339–344. (In Chinese) [Google Scholar]
- Nakanishi, A.; Shibata, H.; Inokura, Y.; Nakao, T.; Toda, H.; Satoh, F.; Sasa, K. Chemical characteristics in stemflow of Japanese Cedar in Japan. Water Air Soil Pollut. 2001, 130, 709–714. [Google Scholar] [CrossRef]
- Levia, D.F.; Herwitz, S.R. Physical properties of water in relation to stemflow leachate dynamics: Implications for nutrient cycling. Can. J. For. Res. 2000, 30, 662–666. [Google Scholar] [CrossRef]
- Hölscher, D.; Köhler, L.; Leuschner, C.; Kappelle, M. Nutrient fluxes in stemflow and throughfall in three successional stages of an upper montane rain forest in Costa Rica. J. Trop. Ecol. 2003, 19, 557–565. [Google Scholar] [CrossRef]
- Hofhansl, F.; Wanek, W.; Drage, S.; Huber, W.; Weissenhofer, A.; Richter, A. Controls of hydrochemical fluxes via stemflow in tropical lowland rainforests: Effects of meteorology and vegetation characteristics. J. Hydrol. 2012, 452–453, 247–258. [Google Scholar] [CrossRef]
- Liu, W.; Fox, J.E.D.; Xu, Z. Nutrient fluxes in bulk precipitation, throughfall and stemflow in montane subtropical moist forest on Ailao Mountains in Yunnan, South-West China. J. Trop. Ecol. 2002, 18, 527–548. [Google Scholar] [CrossRef]
- Olson, R.K.; Reiners, W.A.; Cronan, C.S.; Lang, G.E. The chemistry and flux of throughfall and stemflow in subalpine balsam fir forests. Ecography 1981, 4, 291–300. [Google Scholar] [CrossRef]
- Corti, G.; Agnelli, A.; Cocco, S.; Cardelli, S.; Masse, J.; Courchesne, F. Soil affects throughfall and stemflow under Turkey oak (Quercus cerris L.). Geoderma 2019, 333, 43–56. [Google Scholar] [CrossRef]
- Xu, Z.; Tu, L.; Hu, T.; Schädler, M. Implications of greater than average increases in nitrogen deposition on the western edge of the Szechwan Basin, China. Environ. Pollut. 2013, 177, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Scheer, M.B. Mineral nutrient fluxes in rainfall and throughfall in a lowland Atlantic rainforest in southern Brazil. J. For. Res. 2011, 16, 76–81. [Google Scholar] [CrossRef]
- Reid, L.M.; Lewis, J. Rates, timing, and mechanisms of rainfall interception loss in a coastal redwood forest. J. Hydrol. 2009, 375, 459–470. [Google Scholar] [CrossRef]
- Crockford, R.H.; Richardson, D.P.; Sageman, R. Chemistry of rainfall, throughfall and stemflow in a eucalypt forest and a pine plantation in south-eastern Australia: 3. Stemflow and total inputs. Hydrol. Process. 1996, 10, 25–42. [Google Scholar] [CrossRef]
- De Balieiro, F.C.; Franco, A.A.; Fontes, R.L.F.; Dias, L.E.; Campello, E.F.C.; de Faria, S.M. Evaluation of the throughfall and stemflow nutrient contents in mixed and pure plantations of Acacia mangium, Pseudosamenea guachapele and Eucalyptus grandis. Rev. Árvore 2007, 31, 339–346. [Google Scholar] [CrossRef][Green Version]
- Carnol, M.; Bazgir, M. Nutrient return to the forest floor through litter and throughfall under 7 forest species after conversion from Norway spruce. For. Ecol. Manag. 2013, 309, 66–75. [Google Scholar] [CrossRef]
- Schooling, J.T.; Levia, D.F.; Carlyle-Moses, D.E.; Dowtin, A.L.; Brewer, S.E.; Donkor, S.A.; Borden, S.A.; Grzybowski, A.A. Stemflow chemistry in relation to tree size: A preliminary investigation of eleven urban park trees in British Columbia, Canada. Urban For. Urban Green. 2017, 21, 129–133. [Google Scholar] [CrossRef]
- Neary, A.J.; Gizyn, W.I. Throughfall and stemflow chemistry under deciduous and coniferous forest canopies in south-central Ontario. Can. J. For. Res. 1994, 24, 1089–1100. [Google Scholar] [CrossRef]
- Levia, D.F. Winter stemflow leaching of nutrient-ions from deciduous canopy trees in relation to meteorological conditions. Agric. For. Meteorol. 2003, 117, 39–51. [Google Scholar] [CrossRef]
- Levia, D.F.; Frost, E.E. Variability of throughfall volume and solute inputs in wooded ecosystems. Prog. Phys. Geogr. 2006, 30, 605–632. [Google Scholar] [CrossRef]
- Macinnis-Ng, C.M.O.; Flores, E.E.; Müller, H.; Schwendenmann, L. Rainfall partitioning into throughfall and stemflow and associated nutrient fluxes: Land use impacts in a lower montane tropical region of Panama. Biogeochemistry 2012, 111, 661–676. [Google Scholar] [CrossRef]
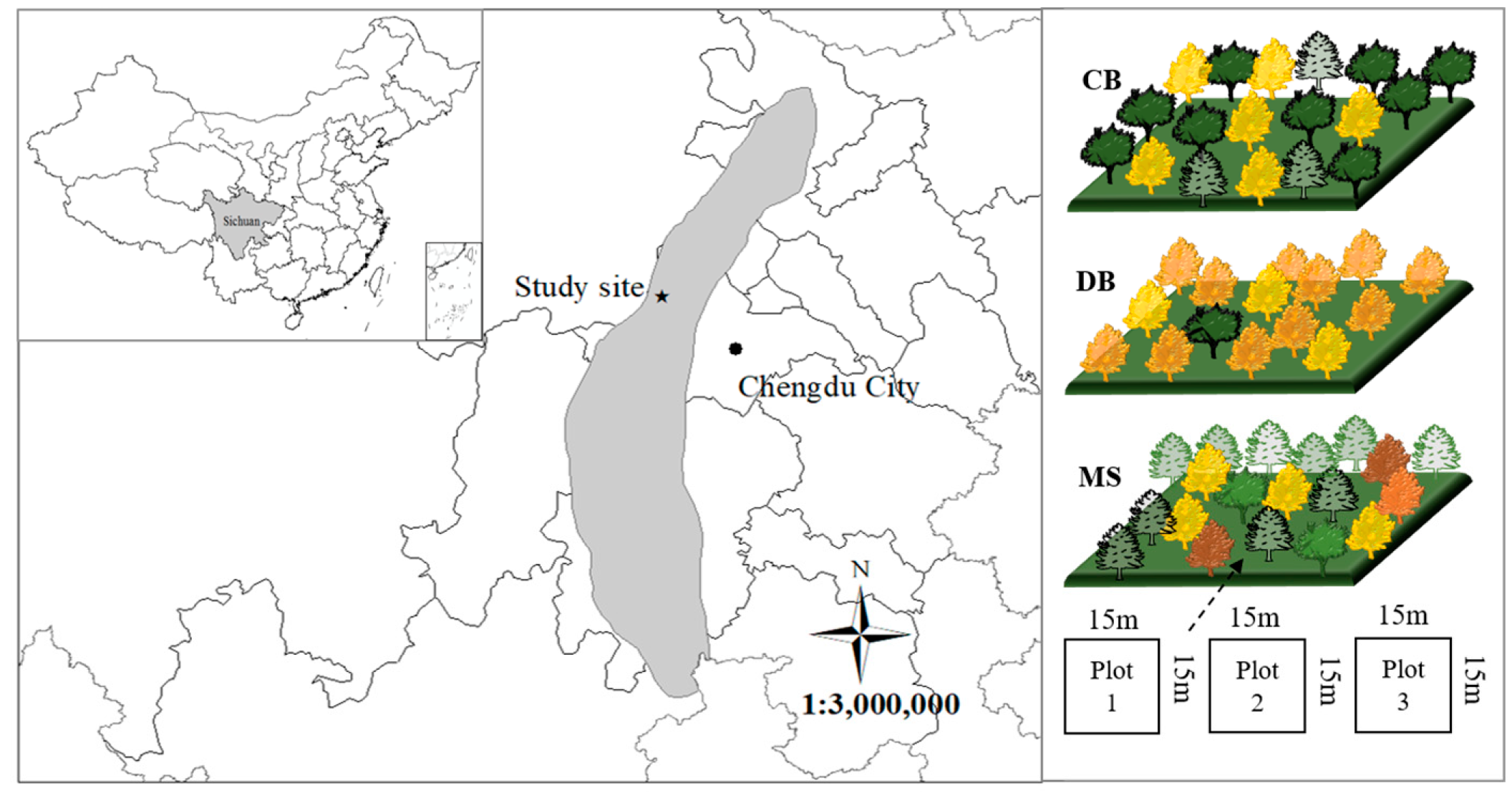

| Stand Type | Altitude (m) | Canopy Density | Stand Density (Trees ha−1) | Smallest DBH (cm) | Largest DBH (cm) | Total Basal Area (m2 ha−1) | Tree Species Composition (Proportion) | Proportion of Coniferous Tree Species | Diameter Classes Composition (Proportion) |
|---|---|---|---|---|---|---|---|---|---|
| CB | 1009 | 0.75 | 504 ± 30 | 14.0 | 49.0 | 40.92 ± 6.34 | Cryptomeria fortune (18%), Phoebe zhennan (44%), Camptotheca acuminate (38%) | 18% | 10–20 cm (12%), 20–30 cm (38%), 30–40 cm (26%), 40–50 cm (24%) |
| DB | 1027 | 0.85 | 444 ± 26 | 16.8 | 47.2 | 29.73 ± 4.87 | Quercus acutissima (70%), Phoebe zhennan (7%), Camptotheca acuminate (23%) | 0% | 10–20 cm (13%), 20–30 cm (54%), 30–40 cm (20%), 40–50 cm (13%) |
| MS | 911 | 0.85 | 622 ± 44 | 10.1 | 61.8 | 47.23 ± 6.21 | Cupressus funebris (29%), Padus racemosa (10%), Pterocarya stenoptera (7%), Michelia wilsonii (10%), Cryptomeria fortune (21%), Alnus cremastogyne (4%), Camptotheca acuminate (19%) | 50% | 10–20 cm (21%), 20–30 cm (41%), 30–40 cm (24%), 40–50 cm (7%), 50–60 cm (5%), 60–70 cm (2%) |
| Stand Type | Total Trees in the Plots | Total Trees Sampled in the Plots | ||
|---|---|---|---|---|
| Tree Species and Quantities (Brackets) in Each Diameter Class | Total Quantity | Tree Species And Quantities (Brackets) in Each Diameter Class | Total Quantity | |
| CB | P. zhennan: 10–20 cm (4), 20–30 cm (8), 30–40 cm (3); C. acuminate: 20–30 cm (2), 30–40 cm (4), 40–50 cm (7); C. fortune: 20–30 cm (3), 30–40cm (2), 40–50 cm (1). | 34 | P. zhennan: 10–20 cm (3), 20–30 cm (3), 30–40 cm (3); C. acuminate: 20–30 cm (2), 30–40 cm (3), 40–50 cm (3), C. fortune: 20–30 cm (3), 30–40cm (2), 40–50 cm (1) | 23 |
| DB | C. acuminate: 10–20 cm (1), 20–30 cm (3), 30–40 cm (1), 40–50 cm (2); Q. acutissima: 10–20 cm (3), 20–30 cm (11), 30–40 cm (5), 40–50 cm (2); P. zhennan: 20–30 cm (2). | 30 | C. acuminate: 10–20 cm (1), 20–30 cm (3), 30–40 cm (1), 40–50 cm (2); Q. acutissima: 10–20 cm (3), 20–30 cm (3), 30–40 cm (3), 40–50 cm (2); P. zhennan: 20–30 cm (2). | 20 |
| MS | A. cremastogyne: 10–20 cm (1), 40–50 cm (1); C. acuminate: 20–30 cm (4), 30–40 cm (1), 40–50 cm (2), 50–60 cm (1); C. fortune: 10–20 cm (3), 20–30 cm (2), 30–40 cm (4); C. funebris: 20–30 cm (8), 30–40 cm (4); M. wilsonii: 10–20 cm (1), 20–30 cm (2), 30–40 cm (1); P. racemose: 10–20 cm (4); P. stenoptera: 20–30 cm (1), 50–60 cm (1), 60–70 cm (1). | 42 | A. cremastogyne: 10–20 cm (1), 40–50 cm (1); C. acuminate: 20–30 cm (3), 30–40 cm (1), 40–50 cm (2), 50–60 cm (1); C. fortune: 10–20 cm (3), 20–30 cm (2), 30–40 cm (3); C. funebris: 20–30 cm (3), 30–40 cm (3); M. wilsonii: 10–20 cm (1), 20–30 cm (2), 30–40 cm (1); P. racemose: 10–20 cm (3); P. stenoptera: 20–30 cm (1), 50–60 cm (1), 60–70 cm (1). | 33 |
| Stand Type | Curve Equation | R2 | p Value |
|---|---|---|---|
| CB | Y = 0.0066x − 0.021 | 0.598 | 0.000 |
| DB | Y = 0.0047x − 0.0423 | 0.623 | 0.000 |
| MS | Y = 0.0053x + 0.0361 | 0.480 | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, L.; You, C.; Li, H.; Tan, S.; Tan, B.; Yang, W. Base Cation Fluxes from the Stemflow in Three Mixed Plantations in the Rainy Zone of Western China. Forests 2019, 10, 1101. https://doi.org/10.3390/f10121101
Liu Y, Jiang L, You C, Li H, Tan S, Tan B, Yang W. Base Cation Fluxes from the Stemflow in Three Mixed Plantations in the Rainy Zone of Western China. Forests. 2019; 10(12):1101. https://doi.org/10.3390/f10121101
Chicago/Turabian StyleLiu, Yilin, Long Jiang, Chengming You, Han Li, Siyi Tan, Bo Tan, and Wanqin Yang. 2019. "Base Cation Fluxes from the Stemflow in Three Mixed Plantations in the Rainy Zone of Western China" Forests 10, no. 12: 1101. https://doi.org/10.3390/f10121101
APA StyleLiu, Y., Jiang, L., You, C., Li, H., Tan, S., Tan, B., & Yang, W. (2019). Base Cation Fluxes from the Stemflow in Three Mixed Plantations in the Rainy Zone of Western China. Forests, 10(12), 1101. https://doi.org/10.3390/f10121101