Species Differences in Nitrogen Acquisition in Humid Subtropical Forest Inferred From 15N Natural Abundance and Its Response to Tracer Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Sampling and Analysis of Samples
2.4. Calculations and Statistics
3. Results
3.1. Leaf %N
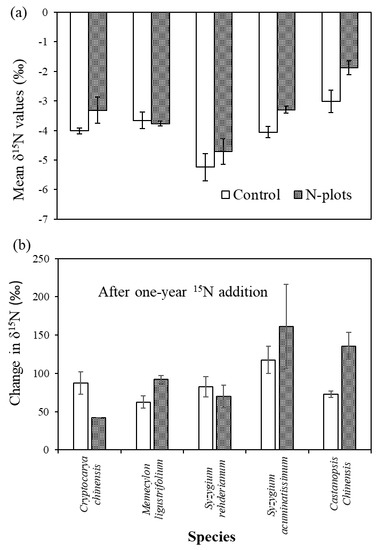
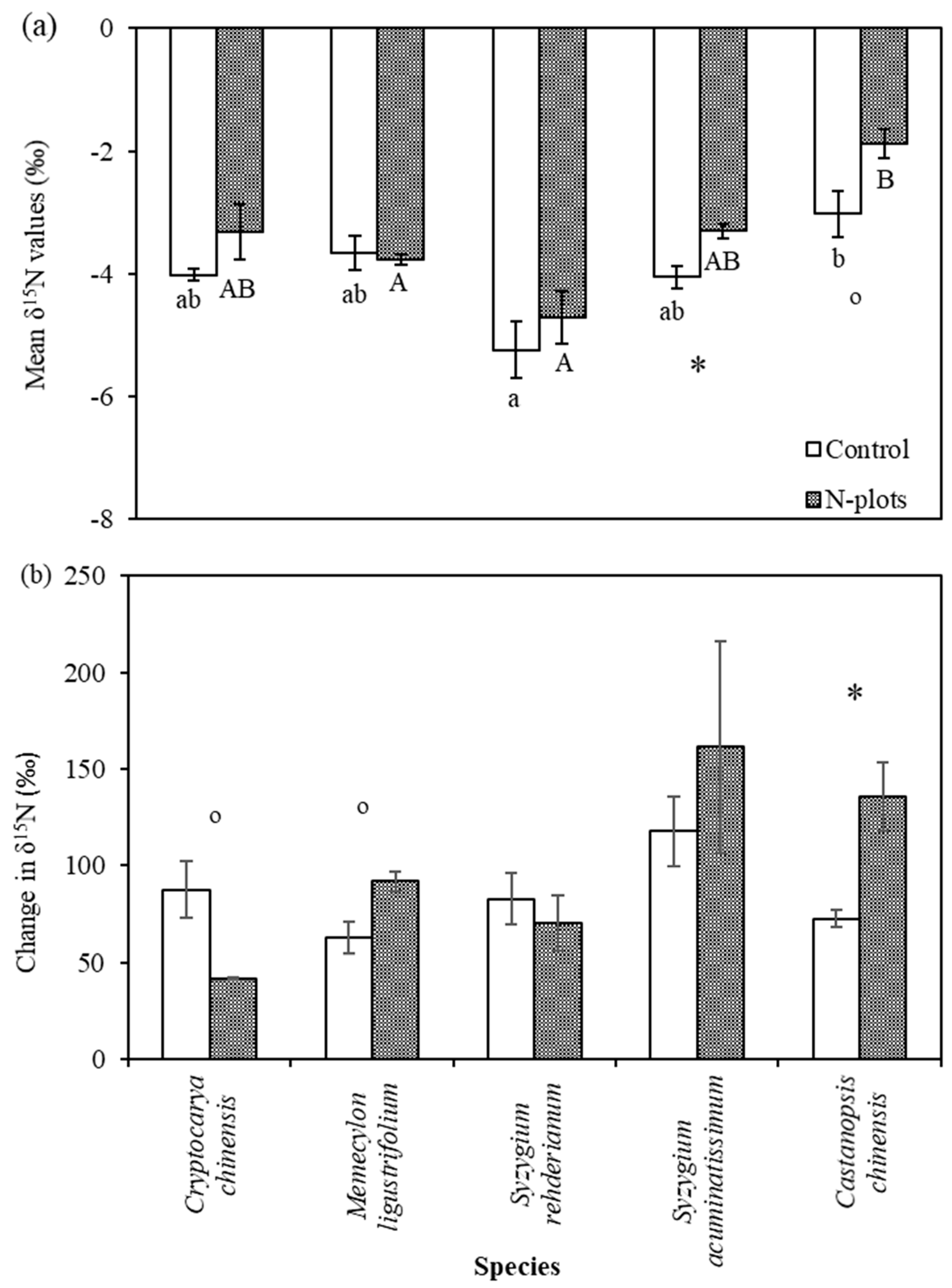
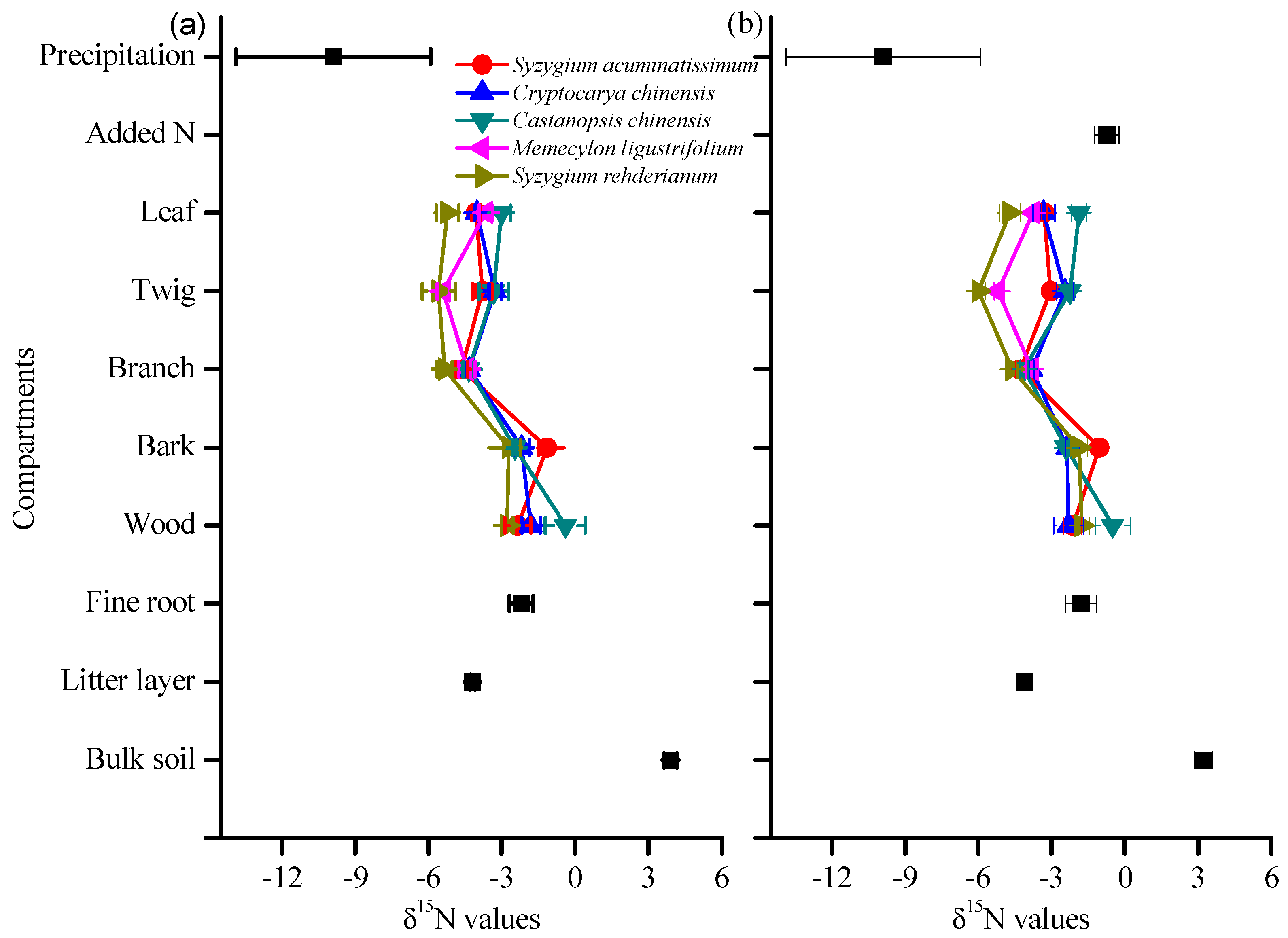
3.2. δ15N Values of Tree Compartments
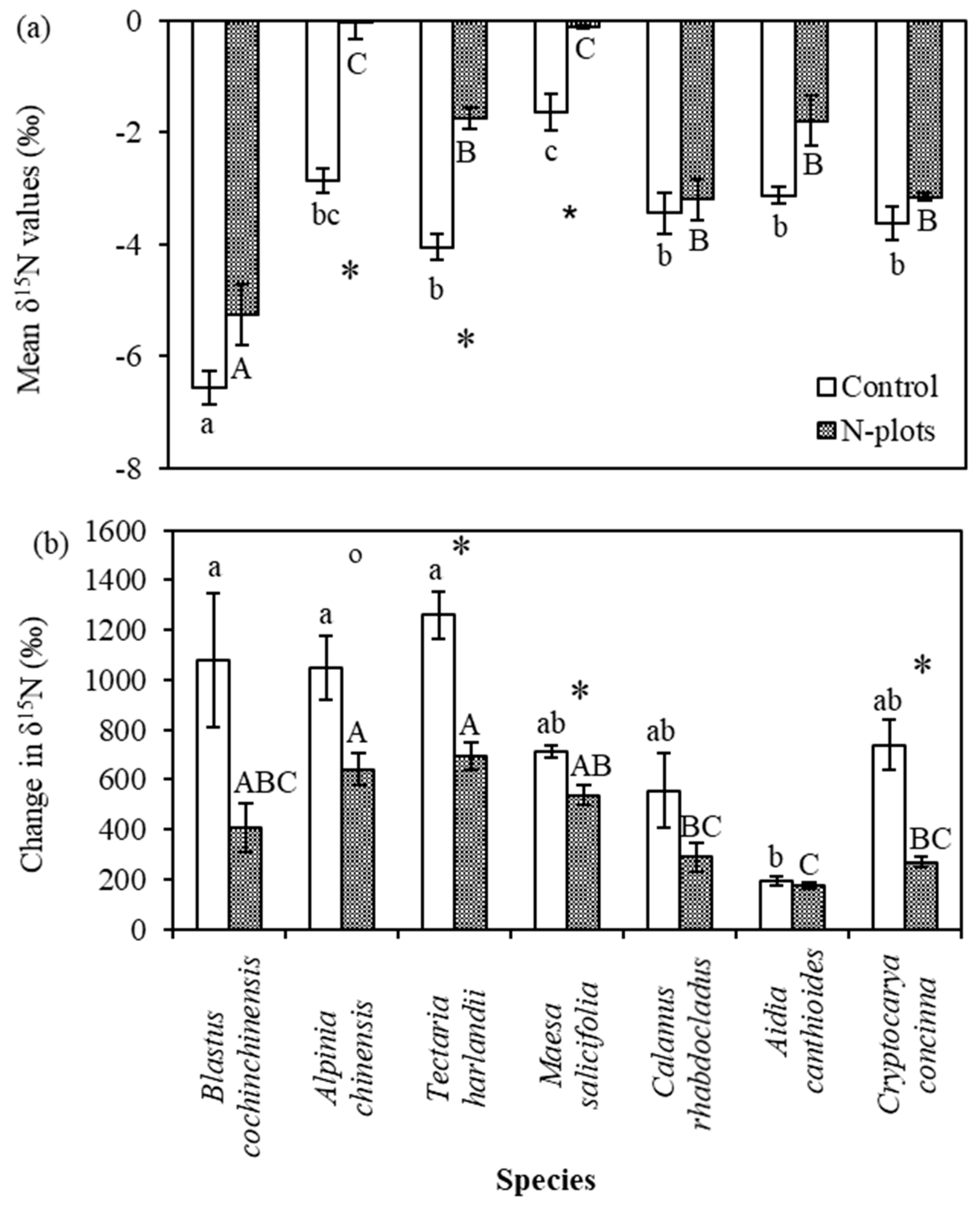
3.3. Leaf δ15N Values of Understory Plants
4. Discussion
4.1. Species Variation in %N and Effects of N Addition
4.2. 15N-Depleted Plants at DHSBR
4.3. Species Variation in δ15N and Its Response to N and 15N Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.R.; Cleveland, C.C.; Houlton, B.Z.; Alden, C.B.; White, J.W. Multi-element regulation of the tropical forest carbon cycle. Front. Ecol. Environ. 2011, 9, 9–17. [Google Scholar] [CrossRef]
- Currie, W.S.; Nadelhoffer, K.J.; Aber, J.D. Redistributions of 15N highlight turnover and replenishment of mineral soil organic N as a long-term control on forest C balance. For. Ecol. Manag. 2004, 196, 109–127. [Google Scholar] [CrossRef]
- Hobbie, S.E. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 1992, 7, 336–339. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weathers, K.C.; Arthur, M.A.; Schultz, J.C. Nitrogen cycling in a northern hardwood forest: Do species matter? Biogeochemistry 2004, 67, 289–308. [Google Scholar] [CrossRef]
- Melillo, J.M.; Aber, J.D.; Muratore, J.F. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Schmidt, I.K.; Gundersen, P.; Vesterdal, L. Soil carbon accumulation and nitrogen retention traits of four tree species grown in common gardens. For. Ecol. Manag. 2013, 309, 47–57. [Google Scholar] [CrossRef]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finér, L.; Jaroszewicz, B.; Vesterdal, L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Hietz, P.; Turner, B.L.; Wanek, W.; Richter, A.; Nock, C.A.; Wright Joseph, S.; Wright, S.J. Long-term change in the nitrogen cycle of tropical forests. Science 2011, 334, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Van der Sleen, P.; Vlam, M.; Groenendijk, P.; Anten, N.P.R.; Bongers, F.; Bunyavejchewin, S.; Hietz, P.; Pons, T.L.; Zuidema, P.A. 15N in tree rings as a bio-indicator of changing nitrogen cycling in tropical forests: An evaluation at three sites using two sampling methods. Front. Plant Sci. 2015, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Kahmen, A.; Wanek, W.; Buchmann, N. Foliar δ15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia 2008, 156, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, A.; Schmidt, I.K.; Jonasson, S.; Quarmby, C.; Sleep, D. Leaf 15N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non- and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 1996, 105, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Templer, P.H.; Mack, M.C.; Chapin, F.S.; Christenson, L.M.; Compton, J.E.; Crook, H.D.; Currie, W.S.; Curtis, C.J.; Dail, D.B.; D’Antonio, C.M.; et al. Sinks for nitrogen inputs in terrestrial ecosystems: A meta-analysis of 15N tracer field studies. Ecology 2012, 93, 1816–1829. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Schulze, E.D.; Chapin, F.S.; Gebauer, G. Nitrogen nutrition and isotope differences among life forms at the northern treeline of Alaska. Oecologia 1994, 100, 406–412. [Google Scholar] [CrossRef]
- Michelsen, A.; Quarmby, C.; Sleep, D.; Jonasson, S. Vascular plant 15N natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia 1998, 115, 406–418. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Aidar, M.P.; Bustamante, M.; Dawson, T.E.; Hobbie, E.A.; Kahmen, A.; Mack, M.C.; McLauchlan, K.K.; Michelsen, A.; et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009, 183, 980–992. [Google Scholar] [CrossRef]
- Nadelhoffer, K.; Shaver, G.; Fry, B.; Giblin, A.; Johnson, L.; McKane, R. 15N natural abundances and N use by tundra plants. Oecologia 1996, 107, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ometto, J.P.H.B.; Ehleringer, J.R.; Domingues, T.F.; Berry, J.A.; Ishida, F.Y.; Mazzi, E.; Higuchi, N.; Flanagan, L.B.; Nardoto, G.B.; Martinelli, L.A. The stable carbon and nitrogen isotopic composition of vegetation in tropical forests of the Amazon Basin, Brazil. Biogeochemistry 2006, 79, 251–274. [Google Scholar] [CrossRef]
- Viani, R.A.G.; Rodrigues, R.R.; Dawson, T.E.; Oliveira, R.S. Functional differences between woodland savannas and seasonally dry forests from south-eastern Brazil: Evidence from 15N natural abundance studies. Austral Ecol. 2011, 36, 974–982. [Google Scholar] [CrossRef]
- Wang, A.; Fang, Y.T.; Chen, D.X.; Koba, K.; Makabe, A.; de Li, Y.; Luo, T.S.; Yoh, M. Variations in nitrogen-15 natural abundance of plant and soil systems in four remote tropical rainforests, southern China. Oecologia 2014, 174, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
- Fang, Y.; Gundersen, P.; Mo, J.; Zhu, X.W. Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 2008, 5, 339–352. [Google Scholar] [CrossRef]
- Huang, Z.L.; Kong, G.H.; Yu, Q.F.; Zhang, Z.H. The hydrological processes and nitrogen dynamics in a monsoon evergreen broad-leaved forest of Dinghu Shan. Acta Phytoecol. Sin. 2000, 24, 157–161. [Google Scholar]
- Gurmesa, G.A. Fate of Deposited Nitrogen in Tropical Forests in Southern China. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 5 February 2016. [Google Scholar]
- Lu, X.; Gilliam, F.S.; Yu, G.; Li, L.; Mao, Q.; Chen, H.; Mo, J. Long-term nitrogen addition decreases carbon leaching in a nitrogen-rich forest ecosystem. Biogeosciences 2013, 10, 3931–3941. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Peng, S.L.; Kong, G.; Mo, H.M.; Brown, S.; Peng, S.L.; Kong, G. Nitrogen availability in disturbed, rehabilitated and mature forests of tropical China. For. Ecol. Manag. 2003, 175, 573–583. [Google Scholar] [CrossRef]
- Field, C.; Merino, J.; Mooney, H.A. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 1983, 60, 384–389. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Hendricks, J.J.; Bowden, R.D.; Melillo, J.M.; Steudler, P.A. Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol. Appl. 1997, 7, 402–415. [Google Scholar] [CrossRef]
- Townsend, A.; Asner, G.; Cleveland, C. The biogeochemical heterogeneity of tropical forests. Trends Ecol. Evol. 2008, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B. Photosynthesis-nitrogen relations in Amazonian tree species. Oecologia 2004, 97, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.L.; Martínez, R.V.; Mendoza, A.M.; Baker, T.R.; Vargas, P.N. Large lianas as hyperdynamic elements of the tropical forest canopy. Ecology 2005, 86, 1250–1258. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gilliam, F.S.; Zhou, G.; Fang, Y. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob. Chang. Biol. 2010, 16, 2688–2700. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Zhou, G.; Zou, X.; Bai, E.; Scanlon, T.M.; Hou, E.; et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef]
- Fang, Y.; Yoh, M.; Mo, J.; Gundersen, P.; Zhou, G. Response of Nitrogen Leaching to Nitrogen Deposition in Disturbed and Mature Forests of Southern China. Pedosphere 2009, 19, 111–120. [Google Scholar] [CrossRef]
- Fang, Y.; Gundersen, P.; Vogt, R.D.; Koba, K.; Chen, F.; Chen, X.Y.; Yoh, M. Atmospheric deposition and leaching of nitrogen in Chinese forest ecosystems. J. For. Res. 2011, 16, 341–350. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Lu, X.; Gundersen, P.; Mao, Q.; Zhou, K.; Fang, Y.; Mo, J. High retention of 15N-labeled nitrogen deposition in a nitrogen saturated old-growth tropical forest. Glob. Chang. Biol. 2016, 22, 3608–3620. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; McGroddy, M.E.; Wen, D. Nutrient Limitation on Ecosystem Productivity and Processes of Mature and Old-Growth Subtropical Forests in China. PLoS ONE 2012, 22, 3608–3620. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Piccolo, M.C.; Townsend, A.R.; Vitousek, P.M.; Cuevas, E.; McDowell, W.; Robertson, G.P.; Santos, O.C.; Treseder, K. Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry 1999, 46, 45–65. [Google Scholar] [CrossRef]
- Mayor, J.R.; Wright Joseph, S.; Schuur, E.A.; Brooks, M.E.; Turner, B.L. Stable nitrogen isotope patterns of trees and soils altered by long-term nitrogen and phosphorus addition to a lowland tropical rainforest. Biogeochemistry 2014, 119, 293–306. [Google Scholar] [CrossRef]
- Nardoto, G.B.; Quesada, C.A.; Patino, S.; Saiz, G.; Baker, T.R.; Schwarz, M.; Schrodt, F.; Feldpausch, T.R.; Domingues, T.F.; Marimon, B.S.; et al. Basin-wide variations in Amazon forest nitrogen-cycling characteristics as inferred from plant and soil N-15:N-14 measurements. Plant Ecol. Divers. 2014, 7, 173–187. [Google Scholar] [CrossRef]
- Domingues, T.F.; Martinelli, L.A.; Ehleringer, J.R. Ecophysiological traits of plant functional groups in forest and pasture ecosystems from eastern Amazonia, Brazil. Plant Ecol. 2007, 193, 101–112. [Google Scholar] [CrossRef]
- Koba, K.; Isobe, K.; Takebayashi, Y.; Fang, Y.; Sasaki, Y.; Saito, W.; Yoh, M.; Mo, J.; Liu, L.; Lu, X.; et al. Delta N-15 of soil N and plants in a N-saturated, subtropical forest of southern China. Rapid Commun. Mass Spectrom. 2010, 24, 2499–2506. [Google Scholar] [CrossRef]
- Fang, Y.; Yoh, M.; Koba, K.; Zhu, W.; Takebayashi, Y.; Xiao, Y.; Lei, C.; Mo, J.; Zhang, W.; Lu, X. Nitrogen deposition and forest nitrogen cycling along an urban-rural transect in southern China. Glob. Chang. Biol. 2011, 17, 872–885. [Google Scholar] [CrossRef]
- Kitayama, K.; Iwamoto, K. Patterns of natural 15N abundance in the leaf-to-soil continuum of tropical rain forests differing in N availability on Mount Kinabalu, Borneo. Plant Soil 2001, 229, 203–212. [Google Scholar] [CrossRef]
- Liu, C.P.; Yeh, H.W.; Sheu, B.H. N isotopes and N cycle in a 35-year-old plantation of the Guandaushi subtropical forest ecosystem, central Taiwan. For. Ecol. Manag. 2006, 235, 84–87. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Lu, X.; Gundersen, P.; Fang, Y.; Mao, Q.; Hao, C.; Mo, J. Nitrogen input 15N signatures are reflected in plant 15N natural abundances in subtropical forests in China. Biogeosciences 2017, 14, 2359–2370. [Google Scholar] [CrossRef]
- Evans, R.D. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001, 6, 121–126. [Google Scholar] [CrossRef]
- Kolb, K.J.; Evans, R.D. Implications of leaf nitrogen recycling on the nitrogen isotope composition of deciduous plant tissues. New Phytol. 2002, 156, 57–64. [Google Scholar] [CrossRef]
- Emmett, B.A.; Kjonaas, O.J.; Gundersen, P.; Koopmans, C.; Tietema, A.; Sleep, D. Natural abundance of N-15 in forests across a nitrogen deposition gradient. For. Ecol. Manag. 1998, 101, 9–18. [Google Scholar] [CrossRef]
- Pardo, L.H.; Semaoune, P.; Schaberg, P.G.; Eagar, C.; Sebilo, M. Patterns in δ15N in roots, stems, and leaves of sugar maple and American beech seedlings, saplings, and mature trees. Biogeochemistry 2013, 112, 275–291. [Google Scholar] [CrossRef][Green Version]
- Dijkstra, P.; Williamson, C.; Menyailo, O.; Doucett, R.; Koch, G.; Hungate, B.A. Nitrogen stable isotope composition of leaves and roots of plants growing in a forest and a meadow. Isot. Environ. Health Stud. 2003, 112, 275–291. [Google Scholar] [CrossRef]
- Handley, L.L.; Raven, J.A. The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ. 1992, 15, 965–985. [Google Scholar] [CrossRef]
- Sheng, W.; Yu, G.; Fang, H.; Liu, Y.; Wang, Q.; Chen, Z.; Zhang, L. Regional patterns of 15N natural abundance in forest ecosystems along a large transect in eastern China. Sci. Rep. 2014, 4, 4249. [Google Scholar] [CrossRef]
- Craine, J.M.; Towne, E.G.; Ocheltree, T.W.; Nippert, J.B. Community traitscape of foliar nitrogen isotopes reveals N availability patterns in a tallgrass prairie. Plant Soil 2012, 356, 395–403. [Google Scholar] [CrossRef]
- Xu, Y.; He, J.; Cheng, W.; Xing, X.; Li, L. Natural 15N abundance in soils and plants in relation to N cycling in a rangeland in Inner Mongolia. J. Plant Ecol. 2010, 3, 201–207. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Michelsen, A.; Olsson, M.O.; Quarmby, C.; Sleep, D. Plant nitrate use in deciduous woodland: The relationship between leaf N, 15N natural abundance of forbs and soil N mineralisation. Soil Biol. Biochem. 2004, 36, 1885–1891. [Google Scholar] [CrossRef]
- Wania, R.; Hietz, P.; Wanek, W. Natural 15N abundance of epiphytes depends on the position within the forest canopy: Source signals and isotope fractionation. Plant Cell Environ. 2002, 25, 581–589. [Google Scholar] [CrossRef]
- Hao, Y.-R.; Peng, S.-L.; Mo, J.-M.; Liu, X.-W.; Chen, Z.-Q.; Zhou, K.; Wu, J.-R. Roots of pioneer trees in the lower sub-tropical area of Dinghushan, Guangdong, China. J. Zhejiang Univ. Sci. B 2006, 7, 377–385. [Google Scholar] [CrossRef]
- Vallano, D.M.; Sparks, J.P. Foliar δ15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 2013, 172, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, E.A.; Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Mayor, J.R.; Mack, M.C.; Schuur, E.A.G. Decoupled stoichiometric, isotopic, and fungal responses of an ectomycorrhizal black spruce forest to nitrogen and phosphorus additions. Soil Biol. Biochem. 2015, 88, 247–256. [Google Scholar] [CrossRef]
- Tedersoo, L.; Naadel, T.; Bahram, M.; Pritsch, K.; Buegger, F.; Leal, M.; Kõljalg, U.; Põldmaa, K. Enzymatic activities and stable isotope patterns of ectomycorrhizal fungi in relation to phylogeny and exploration types in an afrotropical rain forest. New Phytol. 2012, 195, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.; Templer, P.; Goodale, C.; Duke, S.; Groffman, P.; Adams, M.; Boeckx, P.; Boggs, J.; Campbell, J.; Colman, B.; et al. Regional assessment of N saturation using foliar and root delta N-15. Biogeochemistry 2006, 80, 143–171. [Google Scholar] [CrossRef]
- Mayor, J.R.; Schuur, E.A.G.; Henkel, T.W. Elucidating the nutritional dynamics of fungi using stable isotopes. Ecol. Lett. 2009, 12, 171–183. [Google Scholar] [CrossRef]
- Goodale, C.L. Multiyear fate of a 15 N tracer in a mixed deciduous forest: Retention, redistribution, and differences by mycorrhizal association. Glob. Chang. Biol. 2017, 23, 867–880. [Google Scholar] [CrossRef]
- Lin, G.; McCormack, M.L.; Ma, C.; Guo, D. Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol. 2017, 213, 1440–1451. [Google Scholar] [CrossRef]
| Species Name | Growth Form | %N | C:N Ratio | ||
|---|---|---|---|---|---|
| Control | N-plots | Control | N-plots | ||
| Trees | |||||
| Syzygium acuminatissimum | Tree | 1.95 (0.09)a | 2.06 (0.04)a | 25.5 (1.0)b | 23.7 (0.4)c |
| Castanopsis chinensis | Tree | 1.94 (0.08)a | 1.76 (0.02)b | 24.9 (1.1)b | 27.2 (0.2)bc |
| Cryptocarya chinensis | Tree | 1.97 (0.00)a | 1.95 (0.12)ab | 25.9 (0.2)b | 26.5 (1.7)bc |
| Memecylon ligustrifolium | Tree | 1.34 (0.04)b | 1.35 (0.08)c | 32.2 (0.9)a | 32.4 (1.9)ab |
| Syzygium rehderianum | Tree | 1.37 (0.02)b | 1.33 (0.04)c | 34.3 (0.6)a | 36.0 (1.0)a |
| Understory plants | |||||
| Alpinia chinensis | Herb | 1.40 (0.10)c | 1.53 (0.02)c | 32.5 (3.3)a | 29.2 (0.3)a |
| Blastus cochinchinensis | Woody shrub | 2.09 (0.08)ab | 2.15 (0.03)ab | 21.3 (0.7)b | 20.5 (0.2)c |
| Calamus rhabdocladus | Liana | 1.86 (0.06)b | 1.58 (0.03)c | 24.3 (0.9)b | 28.2 (0.5)a |
| Cryptocarya concinna | Understory tree | 2.50 (0.10)a | 2.34 (0.05)a | 20.2 (0.8)b | 21.5 (0.5)c |
| Tectaria harlandii | Fern | 2.16 (0.04)ab | 2.13 (0.00)ab | 20.0 (0.3)b | 20.7 (0.1)c |
| Maesa salicifolia | Shrub | 2.01(0.02)b | 1.96 (0.04)b | 23.6 (0.2)b | 24.3 (0.6)b |
| Aidia canthioides | Understory tree | 2.23 (0.06)ab | 2.18 (0.07)ab | 20.6 (0.6)b | 21.0 (0.7)c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurmesa, G.A.; Lu, X.; Gundersen, P.; Mao, Q.; Fang, Y.; Mo, J. Species Differences in Nitrogen Acquisition in Humid Subtropical Forest Inferred From 15N Natural Abundance and Its Response to Tracer Addition. Forests 2019, 10, 991. https://doi.org/10.3390/f10110991
Gurmesa GA, Lu X, Gundersen P, Mao Q, Fang Y, Mo J. Species Differences in Nitrogen Acquisition in Humid Subtropical Forest Inferred From 15N Natural Abundance and Its Response to Tracer Addition. Forests. 2019; 10(11):991. https://doi.org/10.3390/f10110991
Chicago/Turabian StyleGurmesa, Geshere Abdisa, Xiankai Lu, Per Gundersen, Qinggong Mao, Yunting Fang, and Jiangming Mo. 2019. "Species Differences in Nitrogen Acquisition in Humid Subtropical Forest Inferred From 15N Natural Abundance and Its Response to Tracer Addition" Forests 10, no. 11: 991. https://doi.org/10.3390/f10110991
APA StyleGurmesa, G. A., Lu, X., Gundersen, P., Mao, Q., Fang, Y., & Mo, J. (2019). Species Differences in Nitrogen Acquisition in Humid Subtropical Forest Inferred From 15N Natural Abundance and Its Response to Tracer Addition. Forests, 10(11), 991. https://doi.org/10.3390/f10110991