Age, Growth and Death of a National Icon: The Historic Chapman Baobab of Botswana
Abstract
1. Introduction
2. Materials and Methods
2.1. The Chapman Baobab and Its Area
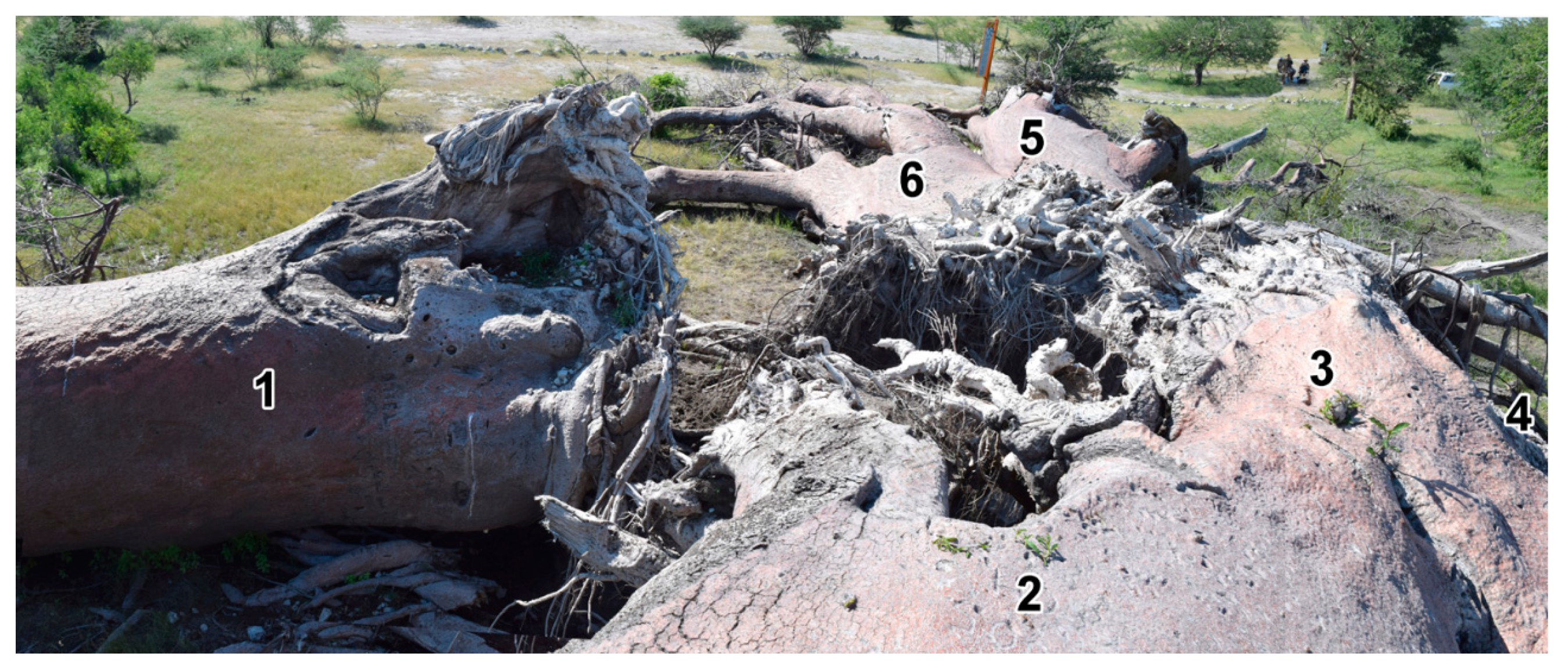
2.2. Investigation of Chapman Baobab
2.2.1. Sample Collection
2.2.2. Sample Preparation
2.2.3. AMS Measurements
2.2.4. Calibration
2.2.5. Water Content
3. Results and Discussion
3.1. Radiocarbon Dates and Calibrated Ages
3.2. Sample Ages
3.3. Tree/Stem Ages
3.4. Growth
3.5. Fall and Death
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindenmayer, D.B.; Laurence, W.F.; Franklin, J.F. Global Decline in Large Old Trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef]
- Bennett, A.C.; McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.J. Larger trees suffer most during drought in forest worldwide. Nat. Plants 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Wickens, G.E.; Lowe, P. The Baobabs: Pachycauls of Africa, Madagascar and Australia; Springer: Dordrecht, The Netherlands, 2008; pp. 245–263. ISBN 978-1-4020-6431-9. [Google Scholar]
- Baum, D.A. A systematic revision of Adansonia (Bombacaceae). Ann. Mo. Bot. Gard. 1995, 82, 440–471. [Google Scholar] [CrossRef]
- Patrut, A.; von Reden, K.F.; Mayne, D.H.; Lowy, D.A.; Patrut, R.T. AMS radiocarbon investigation of the African baobab: Searching for the oldest tree. Nucl. Instrum. Methods Phys. Res. Sect. B 2013, 294, 622–626. [Google Scholar] [CrossRef]
- Patrut, A.; Woodborne, S.; von Reden, K.F.; Hall, G.; Hofmeyr, M.; Lowy, D.A.; Patrut, R.T. African Baobabs with False Inner Cavities: The Radiocarbon Investigation of the Lebombo Eco Trail Baobab. PLoS ONE 2015, 10, e0117193. [Google Scholar] [CrossRef]
- Patrut, A.; Woodborne, S.; von Reden, K.F.; Hall, G.; Patrut, R.T.; Rakosy, L.; Danthu, P.; Leong Pock-Tsy, J.-M.; Lowy, D.A.; Margineanu, D. The growth stop phenomenon of baobabs (Adansonia spp.) indentified by radiocarbon dating. Radiocarbon 2017, 59, 435–448. [Google Scholar] [CrossRef]
- Patrut, A.; Woodborne, S.; Patrut, R.T.; Rakosy, L.; Lowy, D.A.; Hall, G.; von Reden, K.F. The demise of the largest and oldest African baobabs. Nat. Plants 2018, 4, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.L. Notes on some Historic Baobabs. Rhodesiana 1967, 16, 17–26. [Google Scholar]
- Pakenham, T. The Remarkable Baobab; Weidenfield & Nicholson: London, UK, 2004; pp. 48–53. ISBN 978-0-39-305989-2. [Google Scholar]
- The Fall of Chapman’s Baobab. Peolwane Air Botswana, April 2016; 32–37.
- Loader, N.J.; Robertson, I.; Barker, A.C.; Switsur, V.R.; Waterhouse, J.S. An improved technique for the batch processing of small wholewood samples to α-cellulose. Chem. Geol. 1997, 136, 313–317. [Google Scholar] [CrossRef]
- Sofer, Z. Preparation of carbon dioxide for stable carbon isotope analysis of petroleum fractions. Anal. Chem. 1980, 52, 1389–1391. [Google Scholar] [CrossRef]
- Vogel, J.S.; Southon, J.R.; Nelson, D.E.; Brown, T.A. Performance of catalytically condensed carbon for use in accelerator mass-spectrometry. Nucl. Instrum. Methods Phys. Res. Sect. B 1984, 5, 289–293. [Google Scholar] [CrossRef]
- Povinec, P.P.; Litherland, A.E.; von Reden, K.F. Developments in Radiocarbon Technologies: From the Libby Counter to Compound-Specific AMS Analyses. Radiocarbon 2009, 51, 45–78. [Google Scholar] [CrossRef]
- Roberts, M.L.; Burton, J.R.; Elder, K.L.; Longworth, B.E.; McIntyre, C.P.; von Reden, K.F.; Han, B.X.; Rosenheim, B.E.; Jenkins, W.J.; Galutschek, E.; et al. A high-performance 14C Accelerator Mass Spectrometry system. Radiocarbon 2010, 52, 228–235. [Google Scholar] [CrossRef]
- Bronk Ramsey, C. Bayesian analysis of radiocarbon dates. Radiocarbon 2009, 51, 337–360. [Google Scholar] [CrossRef]
- Hogg, A.G.; Hua, Q.; Blackwell, P.G.; Niu, M.; Buck, C.E.; Guilderson, T.P.; Heaton, T.J.; Palmer, J.G.; Reimer, P.J.; Reimer, R.W.; et al. SHCal13 Southern Hemisphere calibration, 0-50,000 years cal BP. Radiocarbon 2013, 55, 1889–1903. [Google Scholar] [CrossRef]
- Patrut, A.; von Reden, K.F.; Lowy, D.A.; Alberts, A.H.; Pohlman, J.W.; Wittmann, R.; Gerlach, D.; Xu, L.; Mitchell, C.S. Radiocarbon dating of a very large African baobab. Tree Physiol. 2007, 27, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Patrut, A.; Mayne, D.H.; von Reden, K.F.; Lowy, D.A.; Van Pelt, R.; McNichol, A.P.; Roberts, M.L.; Margineanu, D. Fire history of a giant African baobab evinced by radiocarbon dating. Radiocarbon 2010, 52, 717–726. [Google Scholar] [CrossRef]
- Patrut, A.; von Reden, K.F.; Van Pelt, R.; Mayne, D.H.; Lowy, D.A.; Margineanu, D. Age determination of large live trees with inner cavities: Radiocarbon dating of Platland tree, a large African baobab. Ann. Forest Sci. 2011, 68, 993–1003. [Google Scholar] [CrossRef]
- Patrut, A.; Mayne, D.H.; von Reden, K.F.; Lowy, D.A.; Venter, S.; McNichol, A.P.; Roberts, M.L.; Margineanu, D. Age and Growth Rate Dynamics of an Old African Baobab determined by Radiocarbon Dating. Radiocarbon 2010, 52, 727–734. [Google Scholar] [CrossRef]
- Holmgren, K.; Karlén, W.; Lauritzen, S.E.; Lee-Thorp, J.A.; Partridge, T.C.; Piketh, S.; Repinski, P.; Stevenson, C.; Svanered, O.; Tyson, P.D. A 3000-year high-resolution stalagmite based record of paleoclimate for northeastern South Africa. Holocene 1999, 9, 295–309. [Google Scholar] [CrossRef]
- Woodborne, S.; Hall, G.; Robertson, I.; Patrut, A.; Rouault, M.; Loader, N.J.; Hofmeyr, M. A 1000-Year Carbon Isotope Rainfall Proxy Record from South African Baobab Trees (Adansonia digitata L.). PLoS ONE 2015, 10, e0124202. [Google Scholar] [CrossRef] [PubMed]
- Woodborne, S.; Gandiwa, P.; Hall, G.; Patrut, A.; Finch, J. A regional stable carbon isotope dendro-climatology from South African summer rainfall area. PLOS ONE 2016, 11, e0159361. [Google Scholar] [CrossRef] [PubMed]
- Tyson, P.D.; Karlen, W.; Holmgren, K.; Heiss, G.A. The little ice and medieval warming in South Africa. S. Afr. J. Sci. 2000, 96, 121–126. [Google Scholar]
- Sundqvist, H.S.; Holmgren, K.; Fohlmeister, J.; Zhang, Q.; Bar Matthews, M.; Spötl, C.; Körnich, H. Evidence of a large cooling between 1690 and 1740 AD in southern Africa. Sci. Rep. 2013, 3, 1767. [Google Scholar] [CrossRef]
- Chapotin, S.M.; Razanameharizaka, J.H.; Holbrook, N.M. A biomechanical perspective on the role of large stem volume and high water content in baobab trees (Adansonia spp.; Bombacaceae). Am. J. Bot. 2006, 93, 1251–1264. [Google Scholar] [CrossRef]
- Petignat, A.; Jasper, L. Baobabs of the World: The upside-down Trees of Madagascar, Africa and Australia; Struik Publishers: Cape Town, South Africa, 2016; p. 19. ISBN 978-1-77-584370-2. [Google Scholar]
- Engelbrecht, F.; Adegoke, J.; Bopape, M.-J.; Naidoo, M.; Garland, R.; Thatcher, M.; McGregor, J.; Katzfey, J.; Werner, M.; Ichoku, C.; et al. Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 2015, 10, 085004. [Google Scholar] [CrossRef]





| Sample/Segment Code (Stem) | Depth * (Height) (m) | Radiocarbon Date (Error) (14C Year BP) | Cal AD Range 1σ (Confidence Interval) | Assigned Year (Error) (cal AD) | Sample/Segment Age (Error) (cal AD) | Accession # |
|---|---|---|---|---|---|---|
| CH-1x (1) | 0.75 (1.47) | 618 (±18) | 1326–1341 (28.9%) 1390–1404 (39.3%) | 1397 (±7) | 620 (±5) | OS-125224 |
| CH-2x (2) | 0.70 (1.60) | 353 (±25) | 1508–1584 (61.4%) 1620–1628 (6.8%) | 1551 (+33, −43) | 465 (+35, −45) | OS-109251 |
| CH-3x (3) | 1.90 (1.20) | 1381 (±22) | 654–681 (63.0%) 749–752 (5.2%) | 669 (+12, −15) | 1345 (+10, −15) | OS-95069 |
| CH-4x (4) | 0.78 (1.38) | 340 (±24) | 1510–1576 (56.0%) 1622–1636 (12.2%) | 1546 (+30, −36) | 470 (+30, −35) | OS-95070 |
| CH-6x (6) | 1.25 (2.57) | 745 (±18) | 1278–1300 (68.2%) | 1288 (+12, −10) | 730 (±10) | OS-126138 |
| CH-3’a (3) | 0.25 (2.80) | 211 (±22) | 1670–1678 (8.9%) 1732–1785 (53.0%) 1794–1800 (6.3%) | 1758 (+17, −16) | 260 (±15) | OS-126074 |
| CH-3’b (3) | 0.35 (2.80) | 342 (±22) | 1510–1576 (57.6%) 1622–1634 (10.6%) | 1546 (+30, −36) | 470 (+30, −35) | OS-125229 |
| CH-3’c (3) | 0.45 (2.80) | 630 (±16) | 1324–1342 (45.4%) 1390–1398 (22.8%) | 1394 (±4) | 620 (±5) | OS-127129 |
| CH-3’d (3) | 0.65 (2.80) | 687 (±21) | 1300–1320 (26.5%) 1350–1386 (41.7%) | 1311 (+9, −11) | 705 (±10) | OS-126075 |
| CH-3’e (3) | 0.85 (2.80) | 841 (±22) | 1222–1266 (68.2%) | 1243 (+23, −21) | 775 (+25, −20) | OS-126076 |
| CH-3’f (3) | 1.06 (2.80) | 985 (±18) | 1044–1054 (7.5%) 1060–1068 (6.1%) 1078–1147 (54.5%) | 1110 (+37, −32) | 905 (+35, −30) | OS-125231 |
| Stem | Height (m) | Circumference */Radius * (m) | Age (Year) |
|---|---|---|---|
| 1 | 21.4 | 9.25/1.47 | 800–1000 |
| 2 | 14.5 (broken) | 6.20/0.99 | 500–600 |
| 3 | 19.0 | 12.39/1.97 | 1350–1400 |
| 4 | 22.2 | 5.64/0.90 | 500–600 |
| 5 | 19.7 | 9.64/1.53 | 800–1000 |
| 6 | 22.6 | 9.23/1.47 | 800–1000 |
| Age Range (AD) | Increase in Radius * (m) | Growth Rate * (10−2 m Year−1) |
|---|---|---|
| 669–1110 | 0.84 | 0.190 |
| 1110–1243 | 0.21 | 0.158 |
| 1243–1311 | 0.20 | 0.294 |
| 1311–1394 | 0.20 | 0.241 |
| 1394–1546 | 0.10 | 0.066 |
| 1546–1758 | 0.10 | 0.047 |
| 1758–2016 | 0.25 | 0.096 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrut, A.; Woodborne, S.; Patrut, R.T.; Hall, G.; Rakosy, L.; Winterbach, C.; von Reden, K.F. Age, Growth and Death of a National Icon: The Historic Chapman Baobab of Botswana. Forests 2019, 10, 983. https://doi.org/10.3390/f10110983
Patrut A, Woodborne S, Patrut RT, Hall G, Rakosy L, Winterbach C, von Reden KF. Age, Growth and Death of a National Icon: The Historic Chapman Baobab of Botswana. Forests. 2019; 10(11):983. https://doi.org/10.3390/f10110983
Chicago/Turabian StylePatrut, Adrian, Stephan Woodborne, Roxana T. Patrut, Grant Hall, Laszlo Rakosy, Christiaan Winterbach, and Karl F. von Reden. 2019. "Age, Growth and Death of a National Icon: The Historic Chapman Baobab of Botswana" Forests 10, no. 11: 983. https://doi.org/10.3390/f10110983
APA StylePatrut, A., Woodborne, S., Patrut, R. T., Hall, G., Rakosy, L., Winterbach, C., & von Reden, K. F. (2019). Age, Growth and Death of a National Icon: The Historic Chapman Baobab of Botswana. Forests, 10(11), 983. https://doi.org/10.3390/f10110983





