Dominant Tree Species Shape Soil Microbial Community via Regulating Assembly Processes in Planted Subtropical Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Field Sampling
2.2. Soil Physicochemical Analyses
2.3. Soil DNA Isolation, PCR Amplicons Sequencing and Data Processing
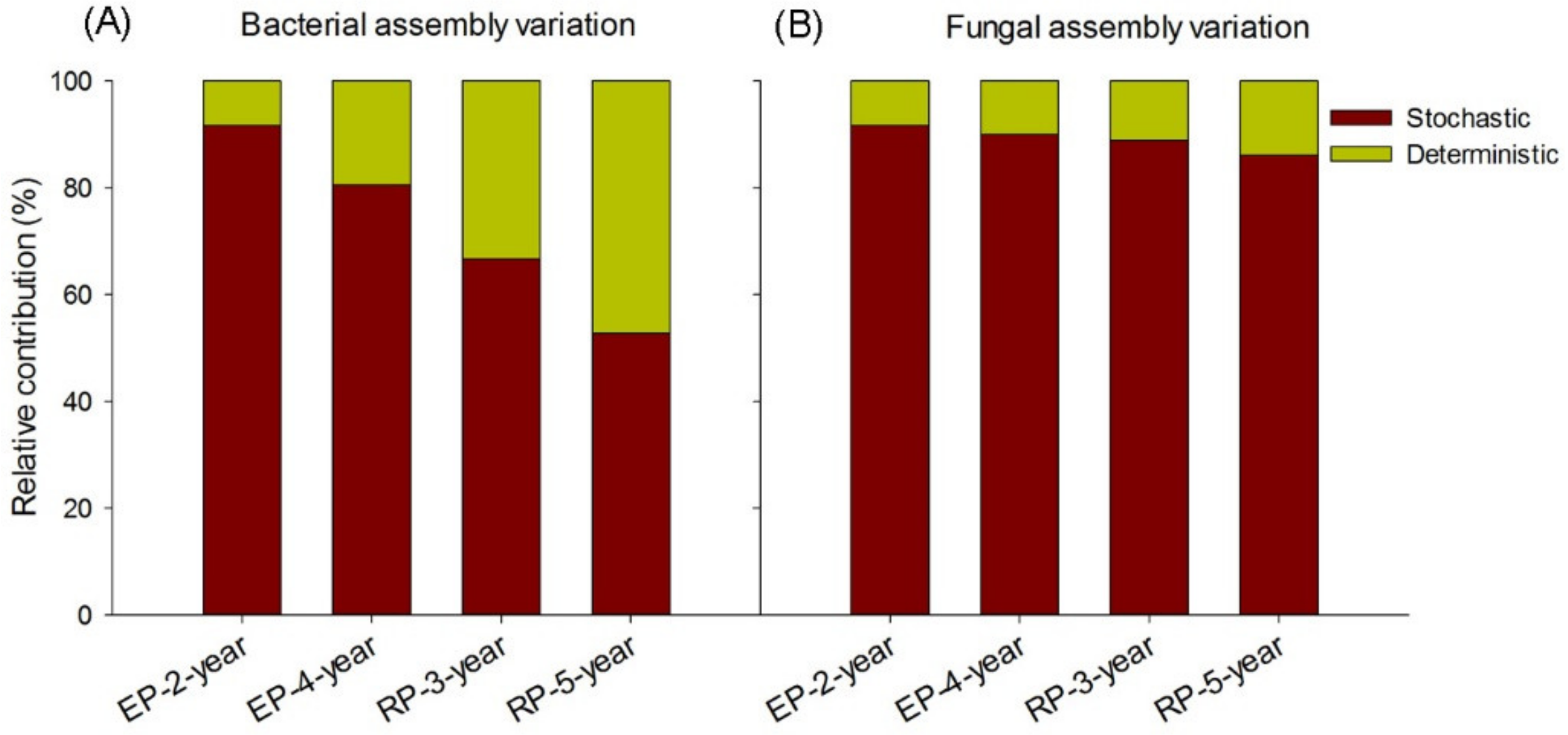
2.4. Stochastic and Deterministic Processes Determination
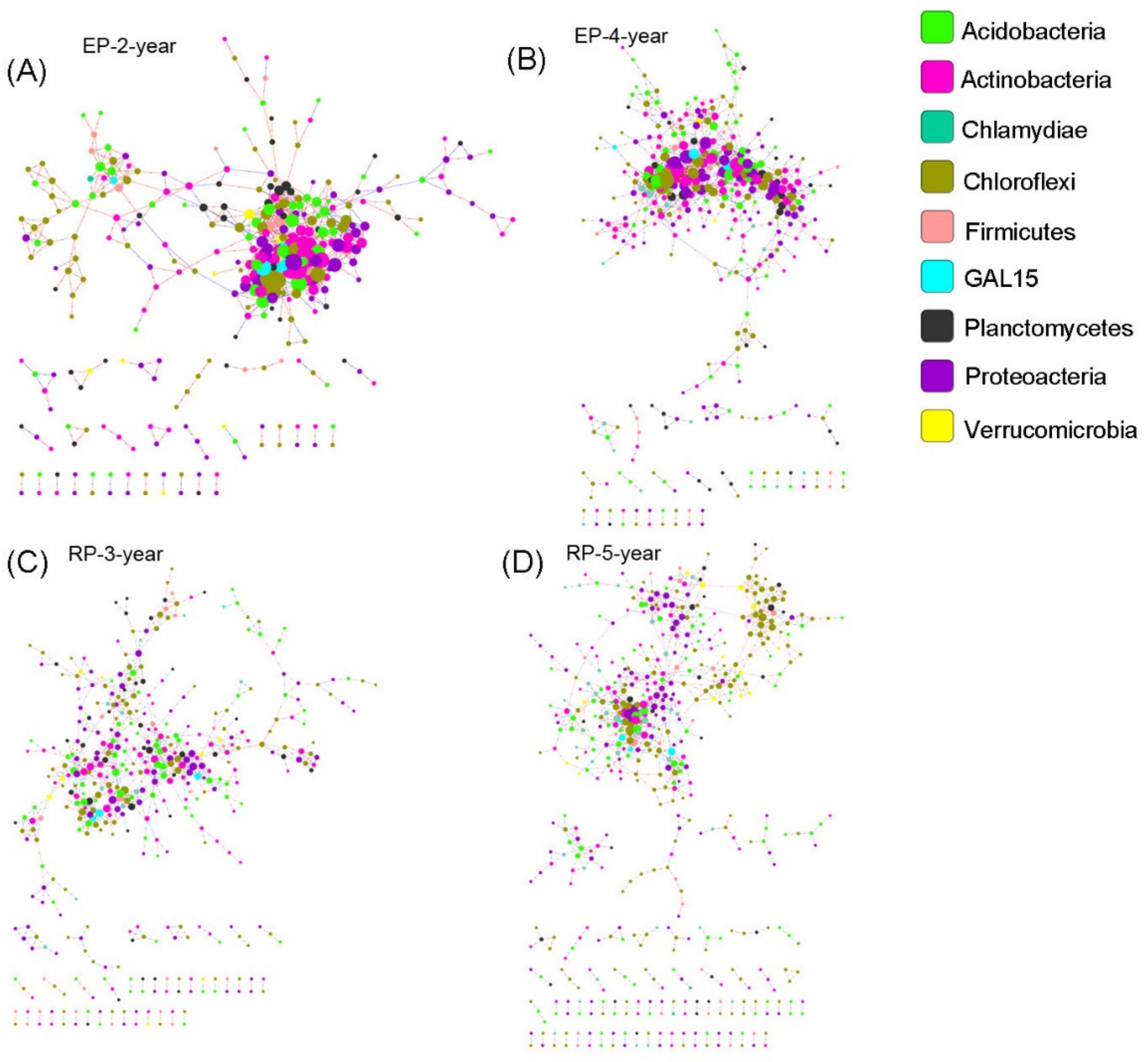
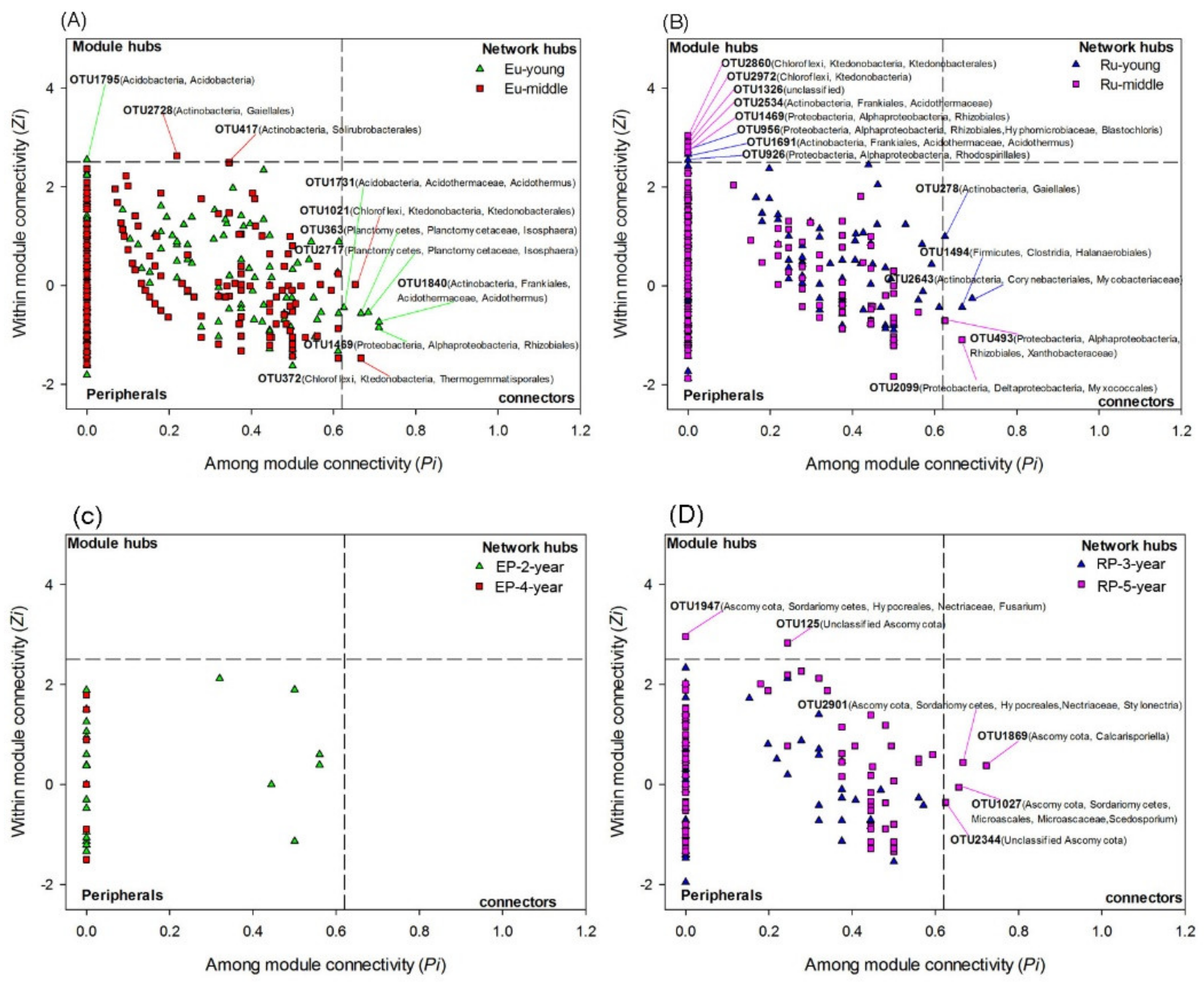
2.5. Microbial Network Analysis
2.6. Statistical Analysis
3. Results
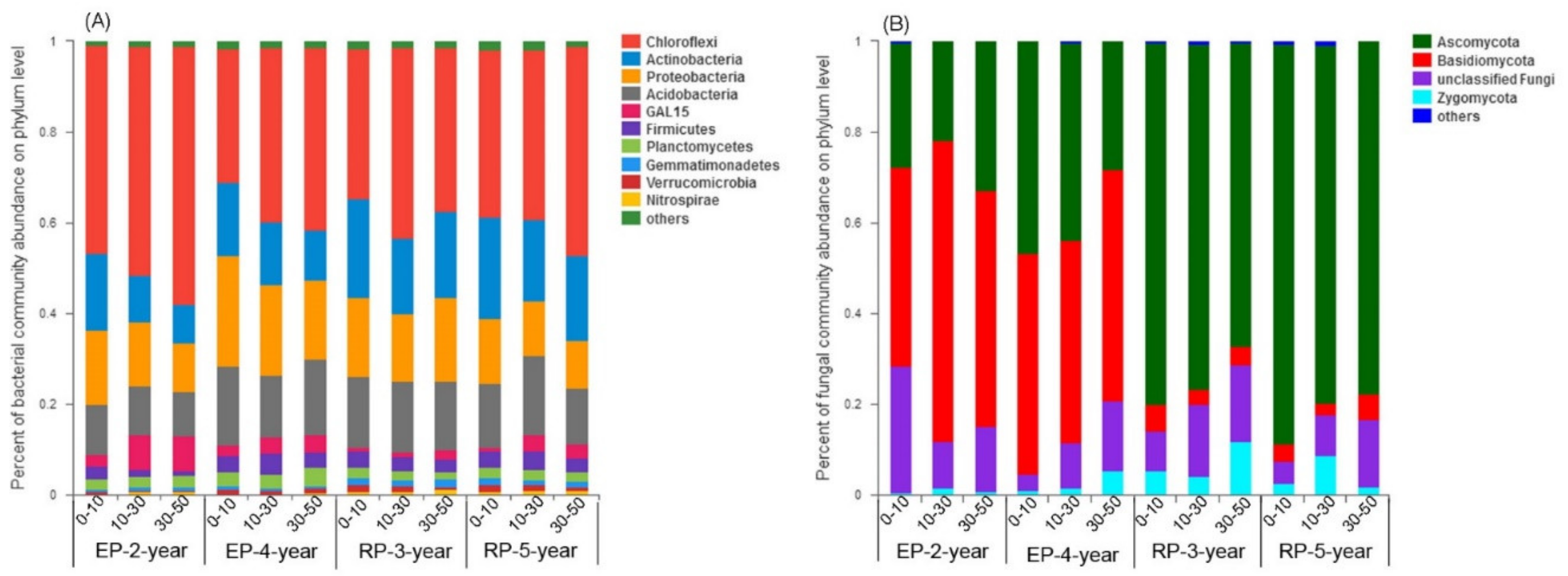
3.1. Soil Properties
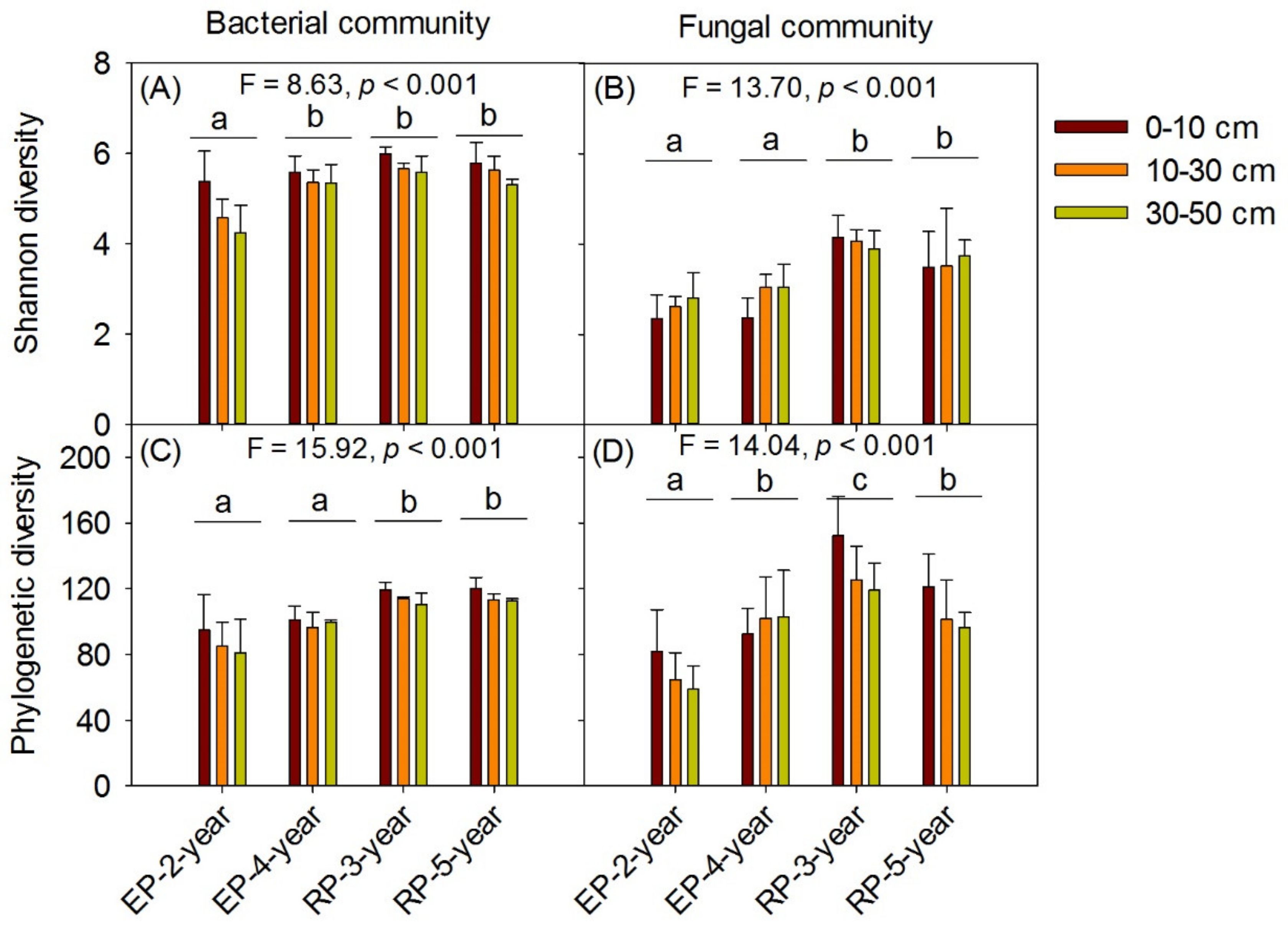
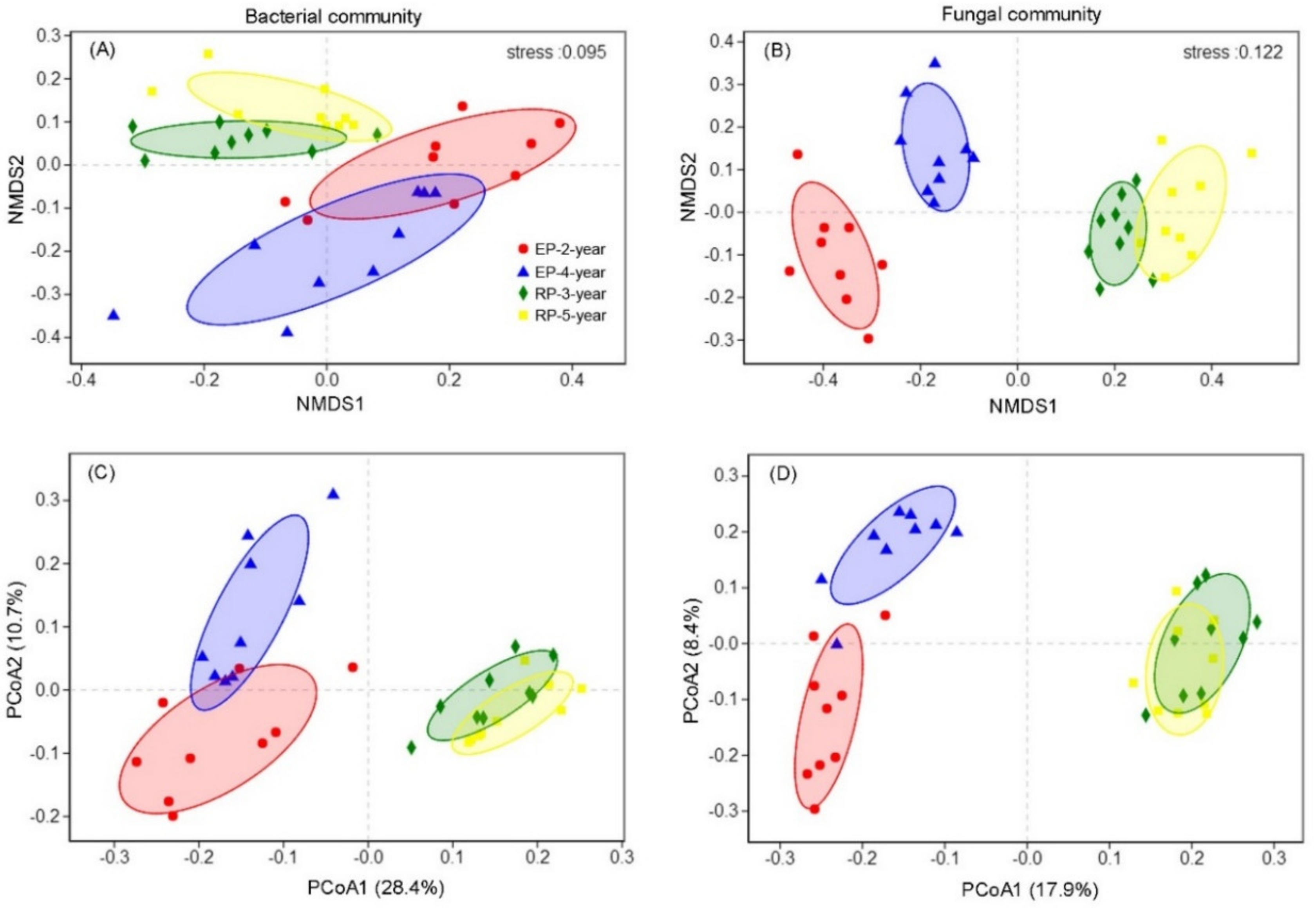
3.2. Microbial Community Diversity and Structure
3.3. Microbial Community Networks
4. Discussion
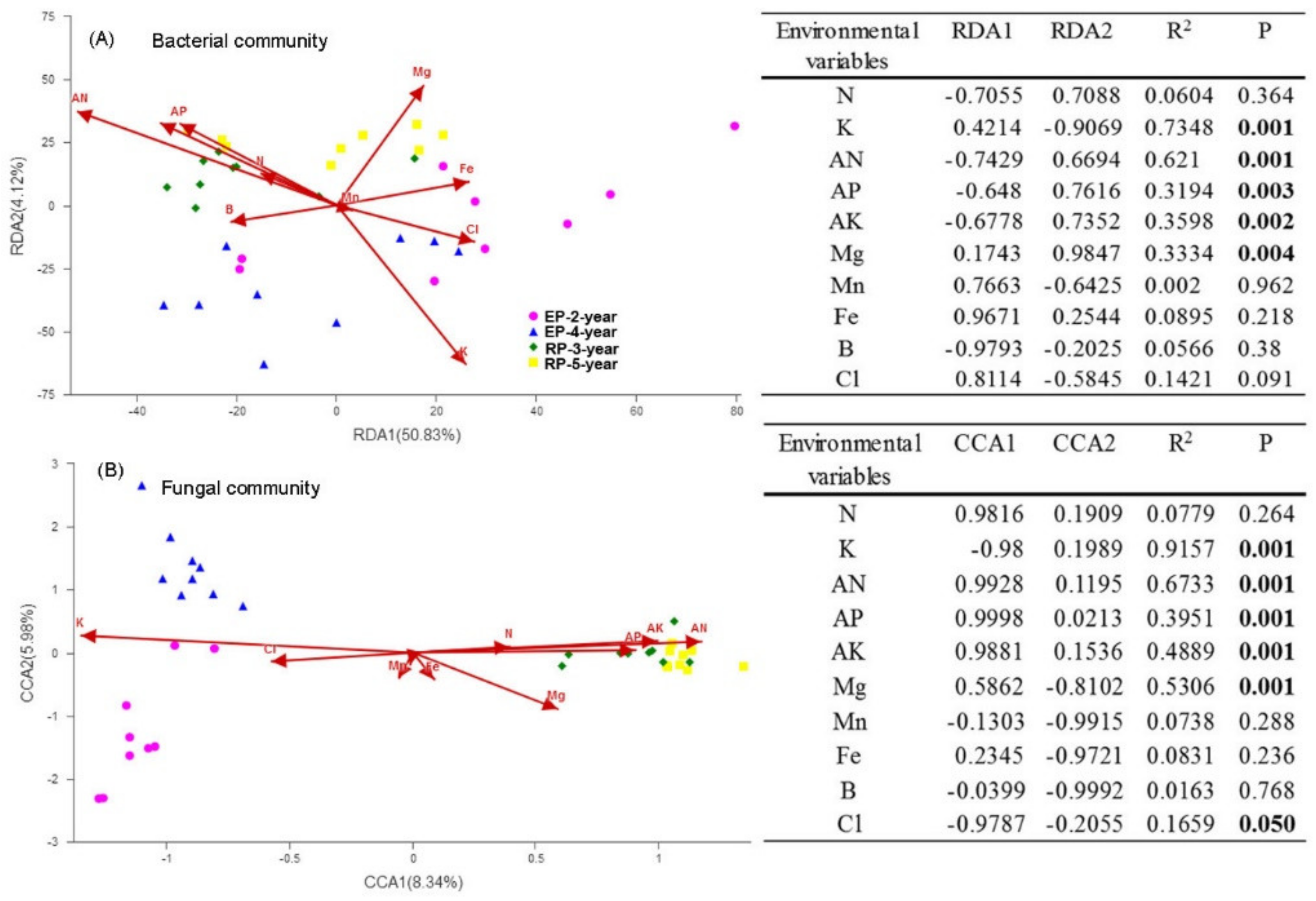
4.1. Microbial α-Diversity and Structure and Relationships to Soil Properties in Rubber and Eucalypt Plantations
4.2. Microbial Assembly Processes in Rubber and Eucalypt Plantations
4.3. Linking Microbial Species Co-Occurrence Networks to Assembly Processes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.J.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Martiny, J.B.H. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Caruso, T.; Chan, Y.K.; Lacap, D.C.; Lau, M.C.Y.; Mckay, C.P.; Pointing, S.B. Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. ISME J. 2011, 5, 1406–1413. [Google Scholar] [CrossRef]
- Martiny, J.B.H.; Eisen, J.A.; Penn, K.; Allison, S.D.; Horner-Devine, M.C. Drivers of bacterial β-diversity depend on spatial scale. Proc. Natl. Acad. Sci. USA 2011, 108, 7850–7854. [Google Scholar] [CrossRef]
- Goss-Souza, D.; Mendes, L.W.; Rodrigues, J.L.M.; Tsai, S.M. Ecological Processes Shaping Bulk Soil and Rhizosphere Microbiome Assembly in a Long-Term Amazon Forest-to-Agriculture Conversion. Microb. Ecol. 2019. [Google Scholar] [CrossRef]
- Guo, X.; Feng, J.J.; Shi, Z.; Zhou, X.S.; Yuan, M.T.; Tao, X.Y.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Chang. 2018, 8, 813–818. [Google Scholar] [CrossRef]
- Shi, Y.; Dang, K.K.; Dong, Y.H.; Feng, M.M.; Wang, B.R.; Li, J.G.; Chu, H.Y. Soil fungal community assembly processes under long-term fertilization. Eur. J. Soil Sci. 2019. [Google Scholar] [CrossRef]
- Fuhrman, J.A. Microbial community structure and its functional implications. Nature 2009, 459, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Banning, N.; Gleeson, D.; Grigg, A.H.; Grant, C.D.; Andersen, G.L.; Brodie, E.L.; Murphy, D.V. Soil microbial community successional patterns during forest ecosystem restoration. Appl. Environ. Microb. 2011, 77, 6158. [Google Scholar] [CrossRef] [PubMed]
- Gravel, D.; Bell, T.; Barbera, C.; Combe, M.; Pommier, T.; Mouquet, N. Phylogenetic constraints on ecosystem functioning. Nat. Commun. 2012, 3, 1117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Gao, Y.; Wang, S.P.; Xu, D.P.; Yu, H.; Wu, L.W.; Lin, Q.Y.; Hu, Y.G.; Li, X.Z.; He, Z.L.; et al. The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J. 2014, 8, 430–440. [Google Scholar] [CrossRef]
- Hallam, S.J.; McCutcheon, J.P. Microbes don’t play solitaire: How cooperation trumps isolation in the microbial world. Environ. Microbiol. Rep. 2015, 7, 26–28. [Google Scholar] [CrossRef]
- Shi, S.J.; Nuccio, E.E.; Shi, Z.J.; He, Z.L.; Zhou, J.Z.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef]
- Lu, L.; Yin, S.; Liu, X.; Zhang, W.; Gu, T.; Shen, Q.; Qiu, H. Fungal networks in yield-invigorating and -debilitating soils induced by prolonged potato monoculture. Soil Biol. Biochem. 2013, 65, 186–194. [Google Scholar] [CrossRef]
- Tian, J.; He, N.; Kong, W.; Deng, Y.; Feng, K.; Green, S.M.; Wang, X.B.; Zhou, J.Z.; Kuzyakov, Y.; Yu, G.R. Deforestation decreases spatial turnover and alters the network interactions in soil bacterial communities. Soil Biol. Biochem. 2018, 123, 80–86. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.J.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Lumini, E.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Singh, D.; Tomlinson, K.W.; Yang, X.; Ogwu, M.C.; Slik, J.W.F.; Adams, J.M. Tropical forest conversion to rubber plantation in southwest China results in lower fungal beta diversity and reduced network complexity. FEMS Microbiol. Ecol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Slik, J.W.F.; Jeon, Y.-S.; Tomlinson, K.W.; Yang, X.; Wang, J.; Kerfashi, D.; Porazinska, D.L.; Adams, J.M. Tropical forest conversion to rubber plantation affects soil micro-& mesofaunal community & diversity. Sci. Rep. 2019, 9, 5893. [Google Scholar] [PubMed]
- Zhou, J.Z.; Deng, Y.; Luo, F.; He, Z.L.; Yang, Y.F. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. MBio 2011, 2, e00122-11. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, Y.H.; Yang, Y.F.; He, Z.L.; Luo, F.; Zhou, J.Z. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Vitali, F.; Mastromei, G.; Senatore, G.; Caroppo, C.; Casalone, E. Long lasting effects of the conversion from natural forest to poplar plantation on soil microbial communities. Microbiol. Res. 2016, 182, 89–98. [Google Scholar] [CrossRef]
- Lan, G.Y.; Li, Y.W.; Wu, Z.X.; Xie, G.S. Soil bacterial diversity impacted by conversion of secondary forest to rubber or eucalyptus plantations: A case study of hainan island, South China. Forest Sci. 2017, 63, 87–93. [Google Scholar] [CrossRef]
- Guillaume, T.; Holtkamp, A.M.; Damris, M.; Brümmer, B.; Kuzyakov, Y. Soil degradation in oil palm and rubber plantations under land resource scarcity. Agric. Ecosyst. Environ. 2016, 232, 110–118. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Boyd, S.E.; Xu, Z.; Zhou, Q. Effects of litter quality and quantity on chemical changes during eucalyptus litter decomposition in subtropical Australia. Plant Soil 2019, 442, 65–78. [Google Scholar] [CrossRef]
- Abraham, J.; Chudek, J.A. Studies on litter characterization using 13C NMR and assessment of microbial activity in natural forest and plantation crops’ (teak and rubber) soil ecosystems of Kerala, India. Plant Soil 2008, 303, 265–273. [Google Scholar] [CrossRef]
- Wu, J.P.; Liu, Z.F.; Sun, Y.X.; Zhou, L.X.; Lin, Y.B.; Fu, S.L. Introduced eucalyptus Urophylla plantations change the composition of the soil microbial community in subtropical china. Land Degrad. Dev. 2013, 24, 400–406. [Google Scholar] [CrossRef]
- N’Dri, J.K.; Guéi, A.M.; Edoukou, E.F.; Yéo, J.G.; N’Guessan, K.K.; Lagerlöf, J. Can litter production and litter decomposition improve soil properties in the rubber plantations of different ages in côte d’ ivoire? Nutr. Cycl. Agroecosyst. 2018, 111, 203–215. [Google Scholar] [CrossRef]
- König, S.; Worrich, A.; Centler, F.; Wick, L.Y.; Miltner, A.; Kästner, M.; Thullner, M.; Frank, K.; Banitz, T. Modelling functional resilience of microbial ecosystems: Analysis of governing processes. Environ. Model. Softw. 2017, 89, 31–39. [Google Scholar] [CrossRef]
- Lan, G.; Li, Y.; Wu, Z.; Xie, G. Impact of tropical forest conversion on soil bacterial diversity in tropical region of China. Eur. J. Soil Biol. 2017, 83, 91–97. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Pei, C.; Lai, K.; Jeffries, T.C.; Tang, G. Variation of soil bacterial communities along a chronosequence of Eucalyptus plantation. Peer J. 2018, 6, e5648. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Chen, F.; Li, C.; Wu, L. Effects of the successive planting of Eucalyptus urophylla on the soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land Degrad. Dev. 2019. [Google Scholar] [CrossRef]
- Lan, G.; Li, Y.; Lesueur, D.; Wu, Z.; Xie, G. Seasonal changes impact soil bacterial communities in a rubber plantation on Hainan Island, China. Sci. Total Environ. 2018, 626, 826–834. [Google Scholar] [CrossRef]
- Lan, G.; Wu, Z.; Li, Y.; Chen, B. The drivers of soil bacterial communities in rubber plantation at local and geographic scales. Arch. Agron. Soil Sci. 2019. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Lin, C.; Liu, X.; Chen, G.; Yang, Y. Conversion of a natural evergreen broadleaved forest into coniferous plantations in a subtropical area: Effects on composition of soil microbial communities and soil respiration. Biol. Fert. Soils 2016, 52, 799–809. [Google Scholar] [CrossRef]
- He, R.; Yang, K.; Li, Z.; Schadler, M.; Yang, W.Q.; Wu, F.Z.; Tan, B.; Zhang, L.; Xu, Z.F. Effects of forest conversion on soil microbial communities depend on soil layer on the eastern Tibetan Plateau of china. PLoS ONE 2017, 12, e0186053. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, K.; Wang, Y.; Wu, A.; Zheng, H. Impacts of converting natural secondary forests to exotic eucalyptus plantations on structure and function of soil microbial communities. Acta Ecol. Sin. 2018, 38, 8070–8079. [Google Scholar]
- Ihrmark, K.; Bodeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Clemmensen, K.E.; Lindahl, B.D.; et al. New primers to amplify the fungal ITS2 region-evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.; Ley, R. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.V.A.; Kembel, S.W. Phylogenetic community structure and phylogenetic turnover across space and edaphic gradients in western Amazonian tree communities. Ecography 2011, 34, 552–565. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2005; Available online: www.r-project.org.
- Roger, G.; Amaral, L.A.N. Functional cartography of complex metabolic networks. Nature 2005, 433, 895. [Google Scholar]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef]
- Martius, C.; HöFer, H.; Garcia, M.V.B.; RöMbke, J.; Hanagarth, W. Litter fall, litter stocks and decomposition rates in rainforest and agroforestry sites in central amazonia. Nutr. Cycl. Agroecosyst. 2004, 68, 137–154. [Google Scholar] [CrossRef]
- Kotowska, M.M.; Leuschner, C.; Triadiati, T.; Hertel, D. Conversion of tropical lowland forest reduces nutrient return through litterfall, and alters nutrient use efficiency and seasonality of net primary production. Oecologia 2016, 180, 601–618. [Google Scholar] [CrossRef]
- Cizungu, L.; Staelens, J.; Huygens, D.; Walangululu, J.; Muhindo, D.; Van Cleemput, O.; Boeckx, P. Litterfall and leaf litter decomposition in a central African tropical mountain forest and eucalyptus plantation. Forest Ecol. Manag. 2014, 326, 109–116. [Google Scholar] [CrossRef]
- Valadao, M.B.X.; Carneiro, K.M.S.; Inkotte, J.; Ribeiro, F.P.; Miguel, E.P.; Gatto, A. Litterfall, litter layer and leaf decomposition in Eucalyptus stands on Cerrado soils. Sci. For. 2019, 47, 256–264. [Google Scholar] [CrossRef]
- Jatoi, M.T.; Lan, G.; Wu, Z.; Sun, R.; Yang, C.; Tan, Z. Comparison of soil microbial composition and diversity between mixed and monoculture rubber plantations in Hainan province, China. Trop. Conserv. Sci. 2019, 12. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture–status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B. Physiology of cereals for mineral nutrient uptake, use, and efficiency. In Crops as Enhancers of Nutrient Use; Baligar, V.C., Duncan, R.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 131–209. [Google Scholar]
- Santolamazza-Carbone, S.; Durán-Otero, M.; Calviño-Cancela, M. Context dependency, co-introductions, novel mutualisms, and host shifts shaped the ectomycorrhizal fungal communities of the alien tree Eucalyptus globulus. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Malajczuk, N.; Brundrett, M. Fruiting of putative ectomycorrhizal fungi under blue gum (Eucalyptus globulus) plantations of different ages in Western Australia. Mycorrhiza 1999, 8, 255–261. [Google Scholar] [CrossRef]
- Ducousso, M.; Duponnois, R.; Thoen, D.; Prin, Y. Diversity of ectomycorrhizal fungi associated with Eucalyptus in Africa and Madagascar. Int. J. For. Res. 2012, 2012, 450715. [Google Scholar]
- Wang, Z.H. Advances in researches of arbuscular mycorrhizae in Hevea brasiliensis. For. Res. 2001, 14, 345–348. [Google Scholar]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Matthias, K. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Liu, C.; Guénard, B.; Blanchard, B.; Peng, Y.Q.; Economo, E.P. Reorganization of taxonomic, functional, and phylogenetic ant biodiversity after conversion to rubber plantation. Ecol. Monogr. 2016, 86, 215–227. [Google Scholar] [CrossRef]
- Kageyama, S.A.; Posavatz, N.R.; Waterstripe, K.E.; Jones, S.J.; Bottomley, P.J.; Cromack, K.; Myrold, D.D. Fungal and bacterial communities across meadow-forest ecotones in the western Cascades of Oregon. Can. J. For. Res. 2008, 38, 1053–1060. [Google Scholar] [CrossRef]
- Nie, M.; Meng, H.; Li, K.; Wan, J.R.; Quan, Z.X.; Fang, C.M.; Chen, J.K.; Li, B. Comparison of bacterial and fungal communities between natural and planted pine forests in subtropical china. Curr. Microbiol. 2012, 64, 34–42. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Power, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones. BioScience 1996, 46, 609–620. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Zou, W.; Yang, J.; Hogan, J.A.; Xu, H.; Chen, J. Dominant Tree Species Shape Soil Microbial Community via Regulating Assembly Processes in Planted Subtropical Forests. Forests 2019, 10, 978. https://doi.org/10.3390/f10110978
Ma H, Zou W, Yang J, Hogan JA, Xu H, Chen J. Dominant Tree Species Shape Soil Microbial Community via Regulating Assembly Processes in Planted Subtropical Forests. Forests. 2019; 10(11):978. https://doi.org/10.3390/f10110978
Chicago/Turabian StyleMa, Haibin, Wentao Zou, Jinchang Yang, J. Aaron Hogan, Han Xu, and Jie Chen. 2019. "Dominant Tree Species Shape Soil Microbial Community via Regulating Assembly Processes in Planted Subtropical Forests" Forests 10, no. 11: 978. https://doi.org/10.3390/f10110978
APA StyleMa, H., Zou, W., Yang, J., Hogan, J. A., Xu, H., & Chen, J. (2019). Dominant Tree Species Shape Soil Microbial Community via Regulating Assembly Processes in Planted Subtropical Forests. Forests, 10(11), 978. https://doi.org/10.3390/f10110978






