Abstract
This paper deals with the spatial distribution of heartwood in Scots pine stems (Pinus sylvestris L.), determined on the basis of the absence of nuclei in parenchyma cells. Samples were collected at several heights from two Scots pine stems growing in fresh coniferous stand as codominant trees. Transverse and radial sections were cut from the samples and stained with acetocarmine to detect the nuclei and with I2KI to show starch grains. Unstained sections were also observed under ultraviolet (UV) light to reveal cell wall lignification. The shapes of the nuclei in ray and axial parenchyma cells differed: the axial parenchyma cells had rounded nuclei, while the nuclei of the ray parenchyma cells were elongated. The lifespan of the parenchyma cells was found to be 16–42 years; the longest-lived were cells from the base of the stem, and the shortest-lived were from the base of the crown. The largest number of growth rings comprising heartwood was observed at a height of 1.3–3.3 m, which signifies that the distribution of heartwood within the stem is uneven. Moreover, the distance of the cells from the apical meristem and the cambium was seen to have an effect on the presence of living parenchyma cells, i.e., those with stained nuclei.
1. Introduction
The presence of living parenchyma cells in wood is, in addition to the ability to conduct sap, the generally accepted basic distinction between sapwood (SW) and heartwood (HW). According to the International Association of Wood Anatomists (IAWA) definition, heartwood consists of “inner layers of wood that have ceased to contain living cells in the growing tree, in which reserve materials (e.g., starch) have been removed or transformed into a heartwood substance” [1]. These materials provide natural durability—generally low in sapwood but much higher in heartwood—with some species being very resistant to biodeterioration [2], which is of value to the forestry and timber industry. However, heartwood formation is still not fully understood, despite its widespread presence in both gymnosperm and angiosperm trees [3] and despite its value and the opportunities it offers for financial optimisation, especially in the case of the most widely planted and distributed tree species.
In this context, Scots pine (Pinus sylvestris. L.), a long-lived coniferous tree, the most widely distributed pine found throughout Eurasia and a model tree for studying the mechanism of wood differentiation in conifers [4], would appear to be a highly promising subject for further research. The tree grows at altitudes ranging from sea level up to 2600 m in a variety of climatic conditions and ecological habitats [5]. Due to its huge genetic diversity, several different subspecies are distinguished throughout its distribution. The Scots pine thrives in poor soils, is resistant to drought and frost and, as a pioneer species, is able to colonise nutrient-poor soils in disturbed areas. However, it does not tolerate air pollution [6] or salt-laden winds and is usually out-competed by other trees on more fertile soils. The great economic importance of this species, especially in northern Europe, is due to its strong and easy-to-process wood, which makes it suitable for general construction, furniture production, and the pulp and paper industry.
In terms of anatomical structure, Scots pine wood is, in common with the majority of conifers, a relatively simple and homogeneous tissue consisting mainly of two types of cells: (i) tracheids that both conduct sap and perform support roles and (ii) parenchyma cells that are involved in the storage and radial transport of substances [7,8,9]. Tracheids die off at the end of their developmental program to undertake their role [4], whereas parenchyma cells remain alive for at least a couple of years. The formation of tracheid cells is much better studied and described and can be divided into five consecutive phases: (i) the periclinal division of cambial cells, (ii) cambial derivative enlargement, (iii) the deposition of a secondary cell wall, (iv) the impregnation of the cell walls with lignin and finally (v) programmed cell death (PCD) [10,11]. This process of the differentiation of xylem cells is under environmental and intracellular control [12]. Among the intracellular factors involved in this regulation are hormones, particularly auxin, which has some morphogen-like characteristics [13]. It is worth noting that Wolpert [14] proposed that the positional information formed by the gradient of morphogen concentration is important for the regulation of developmental events in animal systems. It follows that cells develop in relation to their position along the concentration gradient of a morphogen that forms the morphogenetic field. This theory has also been adopted for plant systems as a mechanism coordinating developmental events in plant cells. It stipulates that the gradient of auxin concentration along the axis of the tree trunk creates a morphogenetic field within which auxin acts as a positional signal for the developmental program of cambial derivatives [15,16,17].
The model of tracheid differentiation described above, in its most general outline, also applies to parenchyma cells. The differences in their differentiation mainly relate to (i) molecular factors involved in the process of parenchyma formation, (ii) the duration of individual phases of differentiation and (iii) the shift in time of wall lignification and programmed cell death that occur not earlier than during the formation of heartwood.
Heartwood occurs normally in living trees. It occupies the innermost part of the secondary xylem and contains lifeless parenchyma cells whose reserve materials have been converted into heartwood substances [1]. It can usually be distinguished from the surrounding sapwood by its darker colour [18]. It can also be identified by its lower permeability [19] and increased resistance to decay microorganisms [2,20]. The most critical change during the transition of SW into HW is the programmed death of ray and axial parenchyma cells [21] and, in gymnosperms, the aspiration of bordered pits [22,23]. The question arises whether parenchyma death is the result or the cause of heartwood formation. In 1976, Bamber [24] suggested that parenchyma death was the result of heartwood formation, an idea subsequently supported by the observations of Nobuchi et al. [25], who found that heartwood extractives were formed before parenchyma death in Robinia pseudoacacia L. Thus, the death of parenchyma cells would be the final step in heartwood formation, considered as the process of secondary wood differentiation and an active program of wood senescence [21]. Various heartwood traits are under environmental [2] and genetic control [26,27]. The transformation of sapwood into heartwood has been related to the regulation of the amount of sapwood relative to the water and nutrient balance of the tree [28,29]. Stokes and Berthier [29] postulated that drought stress is involved as the upper parts of the trunk suffer from accentuated depletion in water supply. In turn, Climent et al. [30] stipulated that resin canals and adjacent sheet parenchyma are also important in the formation of heartwood based on the observation of a higher proportion of heartwood in trees with a greater percentage of axial parenchyma. The formation of heartwood has also been described in terms of its mechanical function in the tree. However, there is a lack of direct, general evidence for the concept that heartwood has superior mechanical traits when compared to SW; the mechanical function of heartwood in tree stems is, therefore, under debate [31].
Despite the fact that several studies have shown gene expression, various enzyme activities and the involvement of other component processes in parenchyma cell death and the production of heartwood substances [32,33], HW formation is still not fully understood, and the knowledge is incomplete.
Because it is visible to the naked eye, the change in the colour of the wood is often used as an indicator of heartwood formation, even though other changes associated with heartwood formation and essential by definition, such as parenchyma death, may not have occurred [25]. Our research aims to describe, for the first time, the spatial distribution of heartwood expressed as a result of the absence of nuclei in parenchyma cells in the stem of Scots pine.
2. Results and Discussion
In SW and HW, the tracheids of both the axial and radial systems of P. sylvestris stem wood were dead as the absence of nuclei was observed in all annual growth rings stained by acetocarmine. This proves that both axial and radial tracheids, although they differ in length, lost nuclei in the final stage of their differentiation. Our results are in line with reports by Wodzicki [4] and Nakaba et al. [34], who stated that tracheids are short-lived cells; they die in the year they are formed. The entire differentiation process lasts an average of a month for earlywood tracheids and two months in the case of latewood [4]. Moreover, our findings concur with the presumed existence of a morphogenetic field in which cells are able to recognise their relative position in the tissue. It has been hypothesised that such positional information could be provided to cells in the form of a concentration gradient of a morphogen, particularly auxin [35,36], which allows cells to complete the pattern of their developmental program.
Acetocarmine-stained nuclei were detected in ray cells and in the parenchyma sheath of axial resin canals. The axial parenchyma sheath cells had rounded nuclei, while the nuclei of ray parenchyma cells were elongated (Figure 1a,b). We observed that the width of the zone containing fewer living parenchyma cells in each of the two systems (i.e., fewer cells with stained nuclei—transition zone, TZ) was equal to about one annual ring, which is in line with the report by Lim et al. [33] stating that TZ in Pinus sylvestris is usually 1–2 annual rings wide. Studies by these authors [33] on changes in the pine transcriptome associated with heartwood formation also provide further and new evidence of the involvement of auxin and PCD in this process. A group of auxin response and auxin-regulated or auxin-induced transcripts were downregulated between TZ and SW. In turn, transcripts encoding two nucleases involved in PCD were highly induced in the TZ, indicating that PCD was taking place in the TZ.
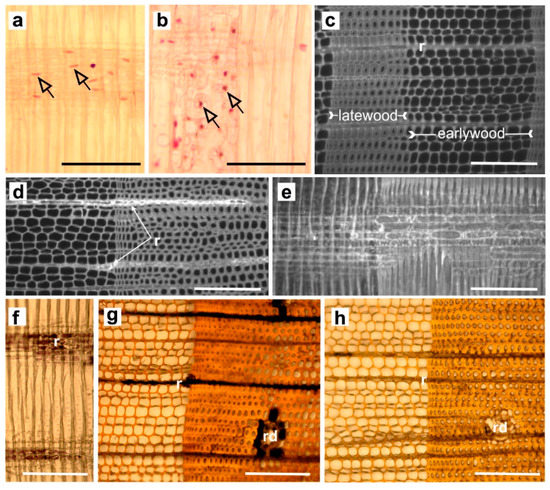
Figure 1.
Details of the wood parenchyma in a Pinus sylvestris stem. (a) The ray parenchyma cells with elongated nuclei in sapwood (SW). (b) The parenchyma sheath cells of axial resin canals with rounded nuclei in SW. (c) Transverse section of SW and (d) heartwood (HW). (e) Radial view of the ray parenchyma cells under ultraviolet (UV) light in HW. (f–g) Starch grain distribution shown with I2KI staining in SW on radial and transverse sections, respectively. (h) HW stained with I2KI. All pictures are for breast height (BH) and for the 18th SW and the 41st HW annual ring counting from the cambium. The cambial side is the left border of the photographs. Abbreviations: r—parenchyma cells; rd—resin duct. Black arrowheads indicate the nuclei. Scale bars = 200 µm.
Wood parenchyma cells were found to remain alive for 16–42 years depending on their location in the stem; the longest-lived parenchyma cells were at the base of the stem, and those with the shortest life span were at the base of the crown. This means that the occurrence of parenchyma cells with stained nuclei depends not only on the distance from the cambium [9,37,38,39] but also on the distance from the apical meristem (Table 1). Spicer and Holbrook [40] stated that wood parenchyma cells may have long lives, and their age varies from 2 to 200 years with different life spans specific to a given species, although this can also be influenced by the environment. For example, in Pinus strobus L., living parenchyma cells were found in roughly 20 annual growth rings of wood, compared with 48 in Fraxinus americana L. [40]. The observation made by Climent et al. [41] suggests that the lifespan of the parenchyma cell in Pinus canariensis C. Smith. stems measured at breast height exceeds 34.4 years. According to research made on Cunninghamia lanceolata (Lamb.) Hook., ray parenchyma could survive for at least 13 years [42].

Table 1.
Spatial distribution of SW and HW rings in a Scots pine stem. Each column represents average numbers of SW and HW starting from the base of the stem and proceeding towards breast height (1.3 m above ground level) and then every 1 m to the base of the crown.
The largest number of annual growth rings without nuclei in parenchyma cells (i.e., constituting heartwood) was observed in wood located at a height of 1.3–3.33 m, and this number decreased towards the crown and the base of the tree (Figure 2) unlike SW, where the number of SW rings decreased acropetally. Our results therefore confirm that HW is not uniformly distributed along the trunk [29,43]. It seems reasonable to assume, after Berthier et al. [44], that HW formation is a developmental process, regulated internally within the tree as a consequence of changing biomechanical and hydraulic requirements for the efficient and safe functioning of the tree. Climent et al. [43] presented a mechanical–physiological hypothesis to explain the irregular pattern of HW variation along the stem, a feature particularly prominent in trees with the crown located in the upper half (i.e., with higher ratio of crown height to total tree height) and proposed a model based on the heartwood radius at BH and tree height explaining 90% of the HW radius variability along the stem. They postulated that a higher proportion of heartwood between 4 and 8 m of the stem resulting from earlier or faster HW formation is rather a nonadaptive response to mechanical stress due to an increase in ethylene levels caused by tree sway as heartwood confers little advantage in facing mechanical demands in comparison to sapwood [44,45].
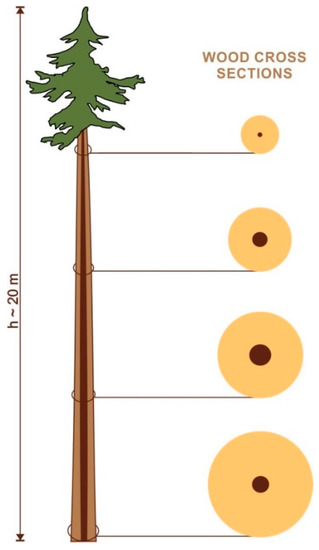
Figure 2.
A schematic diagram showing the proportion of SW and HW along the stem axis of a 20 m high (h) Pinus sylvestris tree. The proportion of SW and HW is based on the number of annual rings. The axial distance between the cross sections shown is ca. 4 m. SW—light brown, HW—dark brown.
The location of the ray parenchyma cells also influenced their life expectancy, i.e., whether they were in contact with short-lived ray tracheids (usually located at the edges of the wood ray) [34,40,46]). The ray parenchyma cells that were in contact with ray tracheids died first. Prior to death, the secondary layers were deposited on their thin primary walls, which underwent lignification, and the starch disappeared (Figure 1c–h). The absence of nuclei is an essential indicator of the presence of heartwood. This development process of parenchyma cells was observed in Pinus densiflora S. et Z. or Pinus rigida Mill. by Nakaba et al. [34]. Murakami et al. [47] studied PCD of parenchyma cells in Populus maximowiczii Henry wood. In the wood of this species, ray parenchyma cells appear as isolation and contact cells. The contact cells form contiguity with vessels, whereas isolation cells do not. The aforementioned authors found that the lifespan and death of contact cells was determined by the proximity of vessels as the contact cells formed secondary walls at approximately the same time as the adjoining vessel elements. The lignification of the cell walls of contact cells and vessel elements began earlier than that of the fibres and isolation cells. Thus, the formation of the secondary wall of the contact cells, including lignification, might occur at the same time as that of the vessel elements to which they are directly connected.
As mentioned above, many papers have described the histochemical changes in parenchyma cells during heartwood formation, which is usually identified by colour [48] and the literature cited there. However, the colour of the wood may change, not because of the presence of heartwood extractives but due, for example, to fungal pathogen activity. Therefore, the criterion for identifying heartwood based on dead parenchyma cells would appear to be more precise and more dependable. Moreover, understanding the spatial distribution of heartwood in trees is pertinent for foresters as it may allow them to guide this process by applying various silvicultural practices.
3. Materials and Methods
Two healthy Scots pines (Pinus sylvestris L.) growing in a fresh coniferous stand and belonging to codominant trees in the canopy layer according to Kraft’s biosocial classification [49] were used in this study. The stand was located in Kraśnik Forest District (51°05′55.1″N, 21°54′35.2″E). Samples containing bark, cambium and wood were taken from various heights of the pine stems, i.e., from the base, breast height (1.3 m above ground level) and then in 1 m increments up to the base of the crown. The total height measured was 20.7 and 20.5 m for pines 1 and 2, respectively. The height of the crown base was 14.9 m for pine 1 and 14 m for pine 2. The diameter of the trunks of both pines at breast height (DBH) was 14.8 cm. These samples were taken during the dormant cambium season, i.e., during lasts days of April 2019.
For light microscopy observation, blocks with a width of approximately 1 cm were cut from the collected samples along one radial direction from the pith to the bark, avoiding compression wood. All block samples were fixed in an equal-part solution of formaldehyde (Alchem, Toruń, Poland), glacial acetic acid (Alchem, Toruń, Poland) and 70% ethanol (Alchem, Toruń, Poland) for a few days at room temperature [50]. Then, the samples were rinsed in 70% ethanol twice, and slices of transverse and radial sections of approximately 25 µm thickness were cut on a Microm HM 440E sliding microtome (GMI Imc, Ramseyy, MN, USA). The transverse sections were useful in counting the number of annual growth rings formed by cambium at each stem height. The radial sections were stained with acetocarmine to detect the nuclei and with I2KI to show the presence of starch grains [50]. Additionally, fluorescence microscopy with UV excitation (Olympus, Tokyo, Japan) (λext. = 365 nm) was used to observe sections to detect cell wall lignification [51,52,53]. The stained radial and unstained transverse sections were observed under an OLYMPUS BX61 light microscope (Olympus, Tokyo, Japan) equipped with a motorised table and Color View OLYMPUS digital microscope camera (Olympus Soft Imaging System GmbH, Műnster, Germany) as well as OLYMPUS Cell P software (ver. 3.4, Olympus Soft Imaging System GmbH, Műnster, Germany) for image acquisition and archiving.
4. Conclusions
In this study, conducted on two sample Scots pine trees, we found that assessment of the lifespan of parenchyma cells based on the number of rings containing cells with nuclei amounted to 16–42 years; the longest-lived were cells from the base of the stem, and the shortest-lived were from the base of the crown. The largest number of annual growth rings without nuclei in parenchyma cells (i.e., constituting heartwood) was observed in wood sampled at a height of 1.3–3.33 m. This was unlike sapwood, where the number of rings steadily decreased acropetally. Therefore, the distance from apical meristem and cambium, the two main sources of auxin, was recognised to have an impact on the presence of living parenchyma cells, i.e., with stained nuclei. The death of parenchyma cells occurred simultaneously in the rays and the sheath of the axial resin ducts and the width of the zone, with a decreasing number of cells with stained nuclei, i.e., the transition zone was about one annual ring. To our knowledge, this report is the first to describe the distribution of heartwood and sapwood along the tree stem based on the absence or presence of nuclei in parenchyma cells, respectively. Determining whether the revealed pattern of distribution is universal or it can be modified under the influence of conditions and rate of tree growth and whether this shows species variability requires further research.
Author Contributions
Conceptualization, M.T.; investigation and methodology, M.T. and J.J.-M.; writing—review and editing, M.T., A.B., J.J.-M. and K.M.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Marcin Kobuc from the Kraśnik Forest District for his help in collecting wood samples for the investigations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Committee on Nomenclature International Association of Wood Anatomists (IAWA). Multilingual Glossary of Terms Used in Wood Anatomy; Verlagsanstalt Buchdruckerei Konkordia: Winterthur, Switzerland, 1964. [Google Scholar]
- Taylor, A.M.; Gartner, B.; Morrell, J. Heartwood formation and natural durability—A review. Wood Fiber Sci. 2002, 4, 587–611. [Google Scholar]
- Panshin, A.J.; de Zeeuw, C. Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada, 4th ed.; McGraw-Hill Series in Forest Resources: New York, NY, USA, 1980; ISBN 9780070484412. [Google Scholar]
- Wodzicki, T.J. Mechanism of xylem differentiation in Pinus silvestris L. J. Exp. Bot. 1971, 22, 670–687. [Google Scholar] [CrossRef]
- Białobok, S.; Boratyński, A.; Bugała, W. Biology of Scots Pine Sorus; Institute of Dendrology: Poznań-Kórnik, Poland, 1993; ISBN 83-85599-21-5. [Google Scholar]
- Leyton, L.; Juniper, B.E. Cuticle structure and water relations of pine needles. Nature 1963, 198, 770–771. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, K. Symplasmic transport in wood: The importance of living xylem cells. In Symplasmic Transport in Vascular Plants; Sokołowska, K., Sowiński, P., Eds.; Springer Science-Business Media: New York, NY, USA, 2013; pp. 101–132. [Google Scholar]
- Spicer, R. Symplasmic networks in secondary vascular tissues: Parenchyma distribution and activity supporting long-distance transport. J. Exp. Bot. 2014, 65, 1829–1848. [Google Scholar] [CrossRef]
- Wodzicki, T.J.; Brown, C.L. Cellular differentiation of the cambium in the Pinaceae. Bot. Gaz. 1973, 134, 139–146. [Google Scholar] [CrossRef]
- Rathgeber, C.B.K.; Cuny, H.E.; Fonti, P. Biological basis of tree-ring formation: A crash course. Front. Plant Sci. 2016, 7, 734. [Google Scholar] [CrossRef]
- Wodzicki, T.J. Natural factors affecting wood structure. Wood Sci. Technol. 2001, 35, 5–26. [Google Scholar] [CrossRef]
- Zajączkowski, S.; Wodzicki, T.J. Auxin and plant morphogenesis—A model of regulation. Acta Soc. Bot. Pol. 1978, 47, 233–243. [Google Scholar] [CrossRef]
- Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969, 25, 1–47. [Google Scholar] [CrossRef]
- Zajączkowski, S.; Wodzicki, T.J.; Bruinsma, J. A possibile mechanizm for whole-plant morphogenesis. Physiol. Plant. 1983, 57, 306–310. [Google Scholar] [CrossRef]
- Zajączkowski, S.; Wodzicki, T.J.; Romberger, J.A. Auxin waves and plant morphogenesis. In Encyclopedia of Plant Physiology; Scott, T.K., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 10, pp. 244–262. [Google Scholar]
- Uggla, C.; Melerowicz, E.J.; Sundberg, B. Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol. 1998, 117, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Hillis, W.E. Heartwood and Tree Exudates; Springer: Berlin/Heidelberg, Germany, 1987; ISBN 978-3-642-72536-4. [Google Scholar]
- Kuroda, K.; Yamashita, K.; Fujiwara, T. Cellular level observation of water loss and the refilling of tracheids in the xylem of Cryptomeria japonica during heartwood formation. Trees 2009, 23, 1163–1172. [Google Scholar] [CrossRef]
- Nakada, R.; Fukatsu, E. Seasonal variation of heartwood formation in Larix kaempferi. Tree Physiol. 2012, 32, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Spicer, R. Senescence in secondary xylem: Heartwood formation as an active developmental program. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 457–475. [Google Scholar]
- Fujii, T.; Harada, H.; Saiki, H. The layered structure of ray parenchyma secondary wall in the wood of 49 Japanese angiosperm species. Mokuzai Gakkaishi 1979, 25, 251–257. [Google Scholar]
- Krahmer, R.L.; Côté, W.A., Jr. Changes in coniferous wood cells associated with heartwood formation. TAPPI 1963, 46, 42–49. [Google Scholar]
- Bamber, R.K. Heartwood, its function and formation. Wood Sci. Technol. 1976, 10, 1–8. [Google Scholar] [CrossRef]
- Nobuchi, T.; Sato, T.; Iwata, R.; Harada, H. Season of heartwood formation and the related cytological structure of ray parenchyma cells in Robinia pseudoacaccia. Mokuzai Gakkaishi 1984, 30, 636–638. [Google Scholar]
- Bito, N.; Nakada, R.; Fukatsu, E.; Matsushita, Y.; Fukushima, K.; Imai, T. Clonal variation in heartwood norlignans of Cryptomeria japonica: Evidence for independent control of agatharesinol and sequirin C biosynthesis. Ann. For. Sci. 2011, 68, 1049–1056. [Google Scholar] [CrossRef]
- Bush, D.; McCarthy, K.; Meder, R. Genetic variation of natural durability traits in Eucalyptus cladocalyx (sugar gum). Ann. For. Sci. 2011, 68, 1057–1066. [Google Scholar] [CrossRef]
- Reyes-Garcia, C.; Andrade, J.J. Sapwood to heartwood ratio affects whole-tree water use in dry forest legume and non-legume trees. Trees 2012, 26, 1317–1330. [Google Scholar] [CrossRef]
- Stokes, A.; Berthier, S. Irregular heartwood formation in Pinus pinaster Ait. is related to eccentric, radial, stem growth. For. Ecol. Manag. 2000, 135, 115–121. [Google Scholar] [CrossRef]
- Climent, J.M.; Gil, L.; Pardos, J.A. Xylem anatomical traits related to resinous heartwood formation in Pinus canariensis Sm. Trees 1998, 12, 139–145. [Google Scholar] [CrossRef]
- Gartner, B.L. Patterns of xylem variation within a tree and their hydraulic and mechanical consequences. In Tree Stems; Gartner, B.L., Ed.; Academic Press: London, UK, 1995; pp. 125–149. [Google Scholar]
- Huang, Z.; Meilan, R.; Woeste, K.A. Knat3-like homeobox gene from Juglans nigra L., JnKNAT3-like, highly expressed during heartwood formation. Plant Cell Rep. 2009, 28, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Paasela, T.; Harju, A.; Venäläinen, M.; Paulin, L.; Auvinen, P.; Kärkkäinen, K.; Teeri, T.H. Developmental changes in Scots pine transcriptome during heartwood formation. Plant Physiol. 2016, 172, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Nakaba, S.; Kubo, T.; Funada, R. Differences in patterns of cell death between ray parenchyma cells and ray tracheids in the conifers Pinus densiflora and Pinus rigida. Trees 2008, 22, 623–630. [Google Scholar] [CrossRef]
- Uggla, C.; Magel, E.; Moritz, T.; Sundberg, B. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiol. 2001, 125, 2029–2039. [Google Scholar] [CrossRef]
- Bhalerao, R.; Bennett, M.J. The case for morphogens in plants. Nat. Cell Biol. 2003, 5, 939–943. [Google Scholar]
- Shain, L.; Mackay, J.F.G. Seasonal fluctuations in respiration of aging xylem in relation to heartwood formation in Pinus radiata. Can. J. Bot. 1973, 51, 737–741. [Google Scholar] [CrossRef]
- Nakaba, S.; Sano, Y.; Kubo, T.; Funada, R. The positional distribution of cell death of ray parenchyma in a conifer, Abies sachalinensis. Plant Cell Rep. 2006, 25, 1143–1148. [Google Scholar] [CrossRef]
- Nakaba, S.; Yamagishi, Y.; Sano, Y.; Funada, R. Temporally and spatially controlled death of parenchyma cells is involved in heartwood formation in pith regions of branches of Robinia pseudoaccaia var. inermis. J. Wood Sci. 2012, 58, 69–76. [Google Scholar] [CrossRef]
- Spicer, R.; Holbrook, N.M. Perenchyma cell respiration and survival in secondary xylem: Does metabolic activity decline with cell age? Plant Cell Environ. 2007, 30, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Climent, J.; Gil, L.; Pardos, J. Heartwood and sapwood development and its relationship to growth and environment in Pinus canariensis Chr.Sm. ex DC. For. Ecol. Manag. 1993, 59, 165–174. [Google Scholar] [CrossRef]
- Song, K.; Liu, B.; Jiang, X.; Yin, Y. Cellular changes of tracheids and ray parenchyma cells from cambium to heartwood in Cunninghamia lanceolata. J. Trop. For. Sci. 2011, 23, 478–487. [Google Scholar]
- Climent, J.; Chambel, M.R.; Gil, L.; Pardos, J.A. Vertical heartwood variation patterns and prediction of heardwood volume in Pinus canariensis Sm. For. Ecol. Manag. 2003, 174, 203–211. [Google Scholar] [CrossRef]
- Berthier, S.; Kokutse, D.A.; Stokes, A.; Fourcaud, T. Irregular heartwood formation in Maritime pine (Pinus pinaster Ait.): Consequences for biomechanical and hydraulic tree functioning. Ann. Bot. 2001, 87, 19–25. [Google Scholar] [CrossRef]
- Mencuccini, M.; Grace, J.; Fioravanti, M. Biomechanical and hydraulic determinants of tree structure in Scots pine: Anatomical characteristics. Tree Physiol. 1997, 17, 105–113. [Google Scholar] [CrossRef]
- Nakaba, S.; Begum, S.; Yamagishi, Y.; Jin, H.O.; Kubo, T.; Funada, R. Differences in the timing of cell death, differentiation and function among three different types of ray parenchyma cells in the hardwood Populus sieboldii×P. grandidentata. Trees 2012, 26, 743–750. [Google Scholar] [CrossRef]
- Murakami, Y.; Funada, R.; Sano, Y.; Ohtani, J. The differentiation of contact cells and isolation cells in the xylem ray parenchyma of Populus maximowiczii. Ann. Bot. 1999, 84, 429–435. [Google Scholar] [CrossRef][Green Version]
- Hillis, W.E. Wood quality and utilization. In Eucalypts for Wood Production; Hillis, W.E., Brown, A.G., Eds.; CSIRO: East Melbourne, Australia, 1978; pp. 259–289. [Google Scholar]
- Kraft, G. Zur Lehre von den Durch Forstungen; Schlagstellungen und Lichtungshieben: Hanover, Germany, 1884. [Google Scholar]
- Broda, B. Metody Histochemii Roślinnej; Państwowy Zakład Wydawnictw Lekarskich: Warszawa, Poland, 1971. [Google Scholar]
- Albinsson, B.; Li, S.; Lundquist, K.; Stomberg, R. The origin of lignin fluorescence. J. Mol. Struct. 1999, 508, 19–27. [Google Scholar] [CrossRef]
- Zheng, P.; Aoki, D.; Yoshida, M.; Matsushita, Y.; Imai, T.; Fukushima, K. Lignification of ray parenchyma cells in the xylem of Pinus densiflora. Part I: Microscopic investigation by POM, UV microscopy, and TOF-SIMS. Holzforschung 2014, 68, 897–906. [Google Scholar] [CrossRef]
- Mishra, G.; Collings, D.A.; Altaner, M.A. Cell organelles and fluorescence of parenchyma cells in Eucalyptus bosistoana sapwood and heartwood investigated by microscopy. N. Z. J. For. Sci. 2018, 48, 13. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).