Historical Development of the Portuguese Forest: The Introduction of Invasive Species
Abstract
1. Introduction
2. Characterization and Evolution of the Forest in Portugal
2.1. From the Early Beginning to the Arrival of Humans
2.2. Influence of Humans on Forest Development
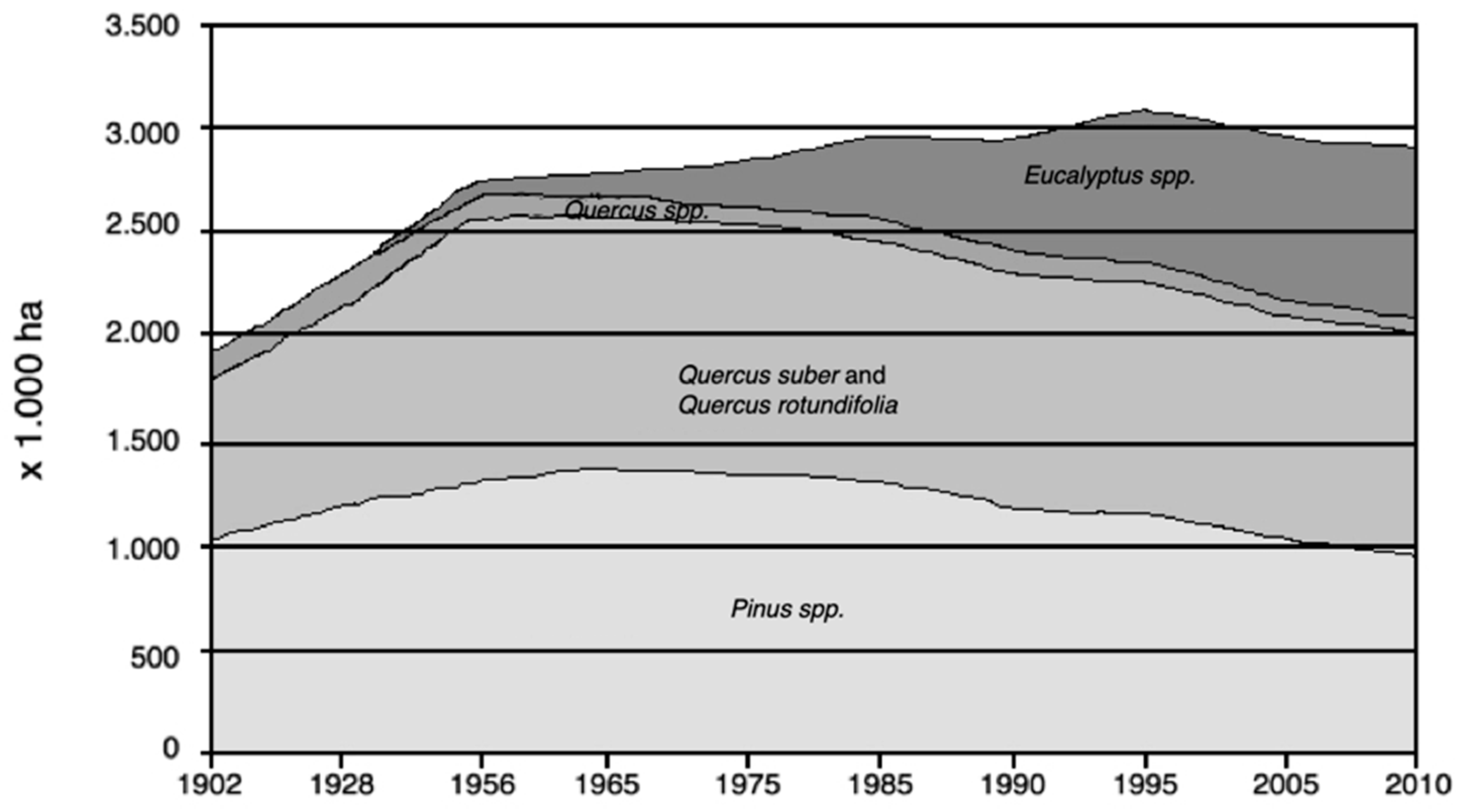
2.3. Current Forest Development
3. Historical Evolution of Invasive Species in Portugal
3.1. Exotic and Invasive Species
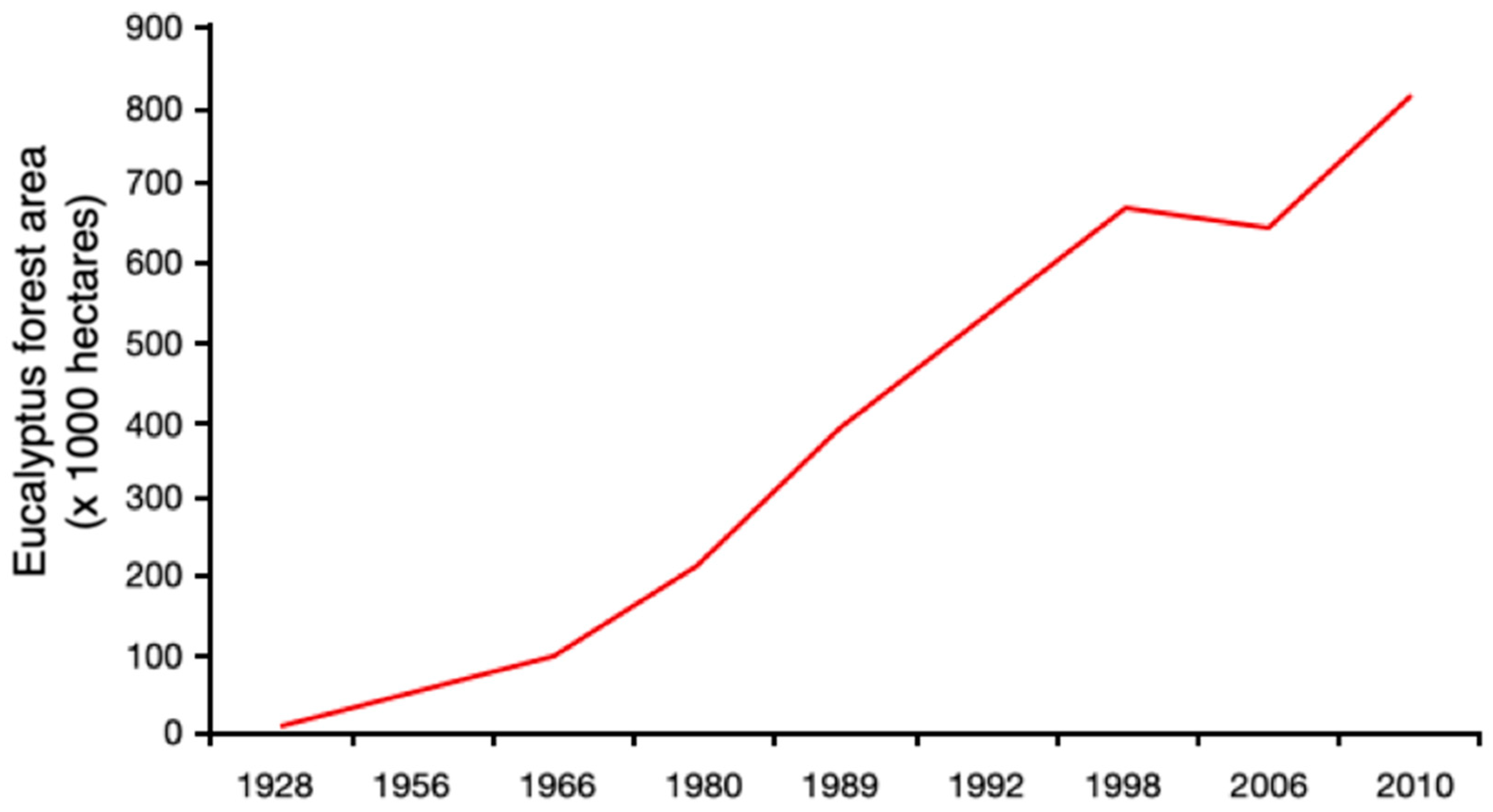
3.2. Eucalyptus
3.3. Acacias
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uva, J. IFN6–Áreas dos Usos dos Solos (Resultados Preliminares); ICNF: Lisboa, Portugal, 2013; Volume 1. [Google Scholar]
- Salvado, S. Eucalipto, a Árvore Que Reina Sobre a Floresta Nacional. Available online: https://www.rtp.pt/noticias/incendios-2015/eucalipto-a-arvore-que-reina-sobre-a-floresta-nacional_es869927 (accessed on 15 September 2018).
- Aguiar, C.; Pinto, B. Paleo-história e história antiga das florestas de Portugal continental: Até à Idade Média; LPN: Lisboa, Portugal, 2007. [Google Scholar]
- Hickey, L.J.; Doyle, J.A. Early Cretaceous fossil evidence for angiosperm evolution. Bot. Rev. 1977, 43, 3–104. [Google Scholar] [CrossRef]
- Reboredo, F.; Pais, J. Evolution of forest cover in Portugal: From the Miocene to the present. In Forest Context and Policies in Portugal; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–37. [Google Scholar]
- Thompson, J.D. Plant Evolution in the Mediterranean; Oxford University Press on Demand: Oxford, UK, 2005. [Google Scholar]
- Dinis, J.; Henriques, V.; Freitas, M.; Andrade, C.; Costa, P. Natural to anthropogenic forcing in the Holocene evolution of three coastal lagoons (Caldas da Rainha valley, western Portugal). Quat. Int. 2006, 150, 41–51. [Google Scholar] [CrossRef]
- Pinto, B.; Aguiar, C.; Partidário, M. Brief historical ecology of Northern Portugal during the Holocene. Environ. Hist. 2010, 16, 3–42. [Google Scholar] [CrossRef]
- Figueiral, I. Wood resources in north-west Portugal: Their availability and use from the late Bronze Age to the Roman period. Veg. Hist. Archaeobot. 1996, 5, 121–129. [Google Scholar] [CrossRef]
- Figueiral, I. Charcoal analysis and the history of Pinus pinaster (cluster pine) in Portugal. Rev. Palaeobot. Palynol. 1995, 89, 441–454. [Google Scholar] [CrossRef]
- Figueiral, I. Charcoal Analysis and the Vegetational Evolution of North-West Portugal. Oxf. J. Archaeol. 1993, 12, 209–222. [Google Scholar] [CrossRef]
- Castro, M. Silvopastoral systems in Portugal: Current status and future prospects. In Agroforestry in Europe; Springer: Berlin/Heidelberg, Germany, 2009; pp. 111–126. [Google Scholar]
- Boone, J.L.; Worman, F.S. Rural settlement and soil erosion from the late Roman period through the Medieval Islamic period in the lower Alentejo of Portugal. J. Field Archaeol. 2007, 32, 115–132. [Google Scholar] [CrossRef]
- Van den Brink, L.; Janssen, C. The effect of human activities during cultural phases on the development of montane vegetation in the Serra de Estrela, Portugal. Rev. Palaeobot. Palynol. 1985, 44, 193–215. [Google Scholar] [CrossRef]
- Parsons, J.J. The cork oak forests and the evolution of the cork industry in southern Spain and Portugal. Econ. Geogr. 1962, 38, 195–214. [Google Scholar] [CrossRef]
- Devy-Vareta, N. Para uma geografia histórica da floresta portuguesa: As matas medievais e a “coutada velha” do Rei. Revista da Faculdade de Letras Geografia I série 2013, 1, 47–73. [Google Scholar]
- Orellana, J.V.; de Mera, A.G. The vegetation in the Villuercas region (Extremadura, Spain) and in Serra de San Mamede (Alto Alenteio, Portugal). The effect of different land use on the vegetation pattern. Phytocoenologia 2003, 33, 727–748. [Google Scholar] [CrossRef]
- Reboredo, F.; Pais, J. Evolution of forest cover in Portugal: A review of the 12th–20th centuries. J. For. Res. 2014, 25, 249–256. [Google Scholar] [CrossRef]
- Devy-Vareta, N. Para uma geografia histórica da floresta portuguesa: Do declínio das matas medievais à política florestal do Renascimento (séc. XV e XVI). Revista da Faculdade de Letras Geografia I série 2013, 2, 5–40. [Google Scholar]
- Ribeiro, J.R.D.P. Modelação do Crescimento do Pinheiro-Manso e sua Aplicabilidade a Nível da Paisagem: Aplicação à Região da Margem Esquerda do Guadiana. Ph.D. Thesis, University of Évora, Évora, Portugal, 2014. [Google Scholar]
- Radich, M.C.; Baptista, F.O. Floresta e sociedade: Um percurso (1875–2005). Silva Lusit. 2005, 13, 143–157. [Google Scholar]
- Devy-Vareta, N. Os Serviços Florestais no século XIX; Finisterra: Lisboa, Portugal, 1989. [Google Scholar]
- Pinho, R.J. As ciências da vegetação e a intervenção dos serviços florestais. Gestão e conservação da flora e da vegetação de Portugal e da África Lusófona. In Honorium do Professor Catedrático Emérito Ilídio Rosário dos Santos Moreira; ISA Press: Lisboa, Portugal, 2012; pp. 217–235. [Google Scholar]
- Reboredo, F.; Pais, J. A construção naval e a destruição do coberto florestal em Portugal–do século XII ao século XX. Ecologia 2012, 4, 31–42. [Google Scholar]
- Radich, M.C. A floresta no Portugal Oitocentista; Análise Social: Lisboa, Portugal, 2000. [Google Scholar]
- Devy-Vareta, N. A floresta na construção das paisagens rurais. Geografia de Portugal 2005, 3, 115–135. [Google Scholar]
- Nobre, C. Alvorecer do turismo cultural na primeira metade do séc. XX: Afonso Lopes Vieira e a Valorização do Património da Região de Leiria. M.Sc. Thesis, Instituto Politécnico de Leiria, Leiria, Portugal, 2006. [Google Scholar]
- Caldas, E.d.C. A Agricultura Portuguesa no Limiar da Reforma Agrária; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2005. [Google Scholar]
- Baptista, F.O.; Santos, R.T. Os Proprietários Florestais: Resultados de um Inquérito; Celta Editora: Lisboa, Portugal, 2005. [Google Scholar]
- Mendes, A.; Fernandes, L. Políticas e instituições florestais em Portugal-desde o final do Antigo Regime até à actualidade; LPN: Lisboa, Portugal, 2007. [Google Scholar]
- ICNF. IFN6-Áreas dos usos do solo e das Espécies Florestais de Portugal Continental (Resultados preliminares); ICNF: Lisboa, Portugal, 2013. [Google Scholar]
- ICNF. Instituto da Conservação da Natureza e das Florestas. Available online: http://www.icnf.pt (accessed on 19 September 2018).
- DGRF. Estratégia nacional para as Florestas—Atualização; DGRF: Lisboa, Portugal, 2015. [Google Scholar]
- ICNF. Perfil Florestal; ICNF: Lisboa, Portugal, 2017. [Google Scholar]
- INE. Indicadores Agro-ambientais 1989–2007. Agricultura, Floresta e Pescas. Available online: http://doi.org/10.1017/CBO9781107415324.004 (accessed on 7 February 2019).
- Ferreira-Leite, F.; Bento-Gonçalves, A.; Lourenço, L. Grandes incêndios florestais em Portugal Continental. Da história recente à atualidade. Cadernos de Geografia 2012, 30–31, 81–86. [Google Scholar] [CrossRef]
- Sarmento, E.; Dores, V. A Fileira Florestal no Contexto da Economia Nacional: A Produtividade e a Especialização Regional. Silva Lusit. 2013, 21, 21–37. [Google Scholar]
- Martins, J.B.; Gomes, L.M.F.; Beato, C.S.M. A revitalização do espaço rural e propostas de intervenção no sentido do turismo local: O caso de estudo de Santo André (Interior-Norte de Portugal). In Proceedings of the ICEUBI2011 International Conference on Engineering UBI2011 Innovation & Development, Covilhã, Portugal, 28–30 November 2011. [Google Scholar]
- Perlin, A.P.; Guedes, G.; Nunes, M.; Ferreira, P. Indicadores de sustentabilidade da indústria de cortiça portuguesa. Revista de Gestão dos Países de Língua Portuguesa 2013, 12, 47–56. [Google Scholar]
- Pestana, M.; Tinoco, I. A indústria eo comércio da cortiça em Portugal durante o século XX. Silva Lusit. 2009, 17, 1–26. [Google Scholar]
- Amorim. Grupo Amorim. Available online: www.amorim.pt (accessed on 14 September 2018).
- Navigator. The Navigator Company. Available online: www.thenavigatorcompany.com (accessed on 15 September 2018).
- SONAE. SONAE. Available online: www.sonaeindustria.com (accessed on 15 September 2018).
- Vasques, A.; Keizer, J. Desenvolvimento de bases ecológicas para o uso de espécies vegetais em restauro ecológico após graves perturbações; ISA Press: Lisboa, Portugal, 2012. [Google Scholar]
- De Almeida, J.D.; Freitas, H. Exotic flora of continental Portugal—A new assessment. Bocconea 2012, 24, 231–237. [Google Scholar]
- Marchante, H.; Morais, M.; Freitas, H.; Marchante, E. Guia Prático Para a Identificação de Plantas Invasoras em Portugal; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2014. [Google Scholar]
- Marchante, H.; Marchante, E.; Freitas, H. Invasive plant species in Portugal: An overview. In International Workshop on Invasive Plants in Mediterranean Type Regions of the World; LIPOR: Porto, Portugal, 2005. [Google Scholar]
- Vieira, C.G. Espécies Exóticas Invasoras—Breves Apontamentos. ICNF—Instituto da Conservação da Natureza 2012. Available online: http://www2.icnf.pt/portal/agir/resource/doc/sab-ma/invasor2012-brev-apont (accessed on 7 February 2019).
- N.A., Um pequeno Insecto Vai Lutar Contra Uma Das Piores Plantas Invasoras em Portugal. Available online: https://www.ambientemagazine.com/um-pequeno-insecto-vai-lutar-contra-uma-das-piores-plantas-invasoras-em-portugal/ (accessed on 7 February 2019).
- Radich, M.C. Uma exótica em Portugal. Ler História 1994, 25, 11–26. [Google Scholar]
- Coutinho, A.X.P. Curso de Silvicultura; Tipografia da Academia Real das Ciências: Lisboa, Portugal, 1886. [Google Scholar]
- Pimentel, C.S. Eucalipto globulus. In Descrição, Cultura e Aproveitamento d’esta Árvore; Typografia Universal: Lisboa, Portugal, 1884. [Google Scholar]
- Alves, A.M.; Pereira, J.S.; Silva, J.M.N. A Introdução e a Expansão do Eucalipto em Portugal; ISA Press: Lisboa, Portugal, 2007. [Google Scholar]
- Radich, M. Introdução e Expansão do Eucalipto em Portugal; Pinhais e eucaliptais (Árvores e Florestas de Portugal, Vol. 4); Público/Fundação Luso-Americana para o Desenvolvimento/Liga para a Protecção da Natureza: Lisboa, Portugal, 2007; pp. 151–165. [Google Scholar]
- Pereda, I.G. Experts Florestais: Os Primeiros Silvicultores em Portugal. Ph.D. Thesis, University of Évora, Évora, Portugal, 2018. [Google Scholar]
- Alves, J.F. A estruturação de um sector industrial: A pasta de papel. Revista da Faculdade de Letras História III série 2014, 1, 153–182. [Google Scholar]
- Fernandes, M.M. Acácias e geografia histórica: Rotas de um percurso global (parte1). Cad. Do Curso De Doutor. Em Geogr. 2012, 4, 23–40. [Google Scholar]
- Correia, A.C.; Pereira, J.S.; Mateus, J.; Pita, G.; Rodrigues, A.; Miranda, P.; Correia, A.V. Influência das alterações climáticas na cultura do eucalipto: Cenários possíveis; ISA Press: Lisboa, Portugal, 2007. [Google Scholar]
- Baptista, F.O. O. O rural depois da agricultura. In Desenvolvimento e Território: Espaços Rurais Pós-Agrícolas e Novos Lugares de Turismo e Lazer; M2-Artes Gráficas, Ltd.: Lisboa, Portugal, 2006; pp. 85–105. [Google Scholar]
- Lima, J.d.M. Eucalyptos e Acacias—Vinte Annos de Experiencias; Livraria do Lavrador: Porto, Portugal, 1920. [Google Scholar]
- Andrade, P. Das mimosas e outras acácias. In Histórias da Vida e da Terra—Um blog de História Natural; Wordpress.com: Lisboa, Portugal, 2012. [Google Scholar]
- Gibson, M.R.; Richardson, D.M.; Marchante, E.; Marchante, H.; Rodger, J.G.; Stone, G.N.; Byrne, M.; Fuentes-Ramírez, A.; George, N.; Harris, C.; et al. Reproductive biology of Australian acacias: Important mediator of invasiveness? Divers. Distrib. 2011, 17, 911–933. [Google Scholar] [CrossRef]
- Orchard, A.E.; Maslin, B.R. (1584) Proposal to conserve the name Acacia (Leguminosae: Mimosoideae) with a conserved type. Taxon 2003, 52, 362–363. [Google Scholar] [CrossRef]
- Bouchenak-Khelladi, Y.; Maurin, O.; Hurter, J.; Van der Bank, M. The evolutionary history and biogeography of Mimosoideae (Leguminosae): An emphasis on African acacias. Mol. Phylogenetics Evol. 2010, 57, 495–508. [Google Scholar] [CrossRef]
- Lorenzo, P.; González, L.; Reigosa, J.M. The genus Acacia as invader: The characteristic case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef]
- Aguiar, F.; Ferreira, M. Plant invasions in the rivers of the Iberian Peninsula, south-western Europe: A review. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2013, 147, 1107–1119. [Google Scholar] [CrossRef]
- Fernandes, P.M. Combining forest structure data and fuel modelling to classify fire hazard in Portugal. Ann. For. Sci. 2009, 66, 1–9. [Google Scholar] [CrossRef]
- Pedley, L. Derivation and dispersal of Acacia (Leguminosae), with particular reference to Australia, and the recognition of Senegalia and Racosperma. Bot. J. Linn. Soc. 1986, 92, 219–254. [Google Scholar] [CrossRef]
- Kriticos, D.; Sutherst, R.; Brown, J.; Adkins, S.; Maywald, G. Climate change and the potential distribution of an invasive alien plant: Acacia nilotica ssp. indica in Australia. J. Appl. Ecol. 2003, 40, 111–124. [Google Scholar] [CrossRef]
- Whibley, D.J. Acacias of South Australia; Government of South Australia Printer: Australia, 1980.
- Bem, N.V.d. Gestão de Plantas Exóticas e Invasoras no Parque Nacional de Escotismo da Caparica. M.Sc. Thesis, University of Lisbon, Lisboa, Portugal, 2015. [Google Scholar]
- Fernandes, M. Recuperação Ecológica de Áreas Invadidas por Acacia Dealbata Link no vale do rio Gerês: Um Trabalho de Sísifo; Universidade de Trás-os-Montes e Alto-Douro: Vila Real, Portugal, 2008. [Google Scholar]
- Simões, C.A.d.C.P. A Degradação da Paisagem e a sua Perceção após Invasão Pela Espécie Acacia Dealbata Link.: O caso da Região do Alto Ceira. Ph.D. Thesis, University NOVA of Lisbon, Almada, Portugal, 2016. [Google Scholar]
- González, L.; Souto, X.C.; Reigosa, M. Allelopathic effects of Acacia melanoxylon R. Br. phyllodes during their decomposition. For. Ecol. Manag. 1995, 77, 53–63. [Google Scholar] [CrossRef]
- Ramoliya, P.; Patel, H.; Pandey, A. Effect of salinisation of soil on growth and macro-and micro-nutrient accumulation in seedlings of Acacia catechu (Mimosaceae). Ann. Appl. Biol. 2004, 144, 321–332. [Google Scholar] [CrossRef]
- Martins-Corder, M.P.; Borges, R.Z.; Junior, N.B. Fotoperiodismo e quebra de dormência em sementes de acácia-negra (Acacia mearnsii De Wild.). Ciência Florest. 1999, 9, 71–77. [Google Scholar] [CrossRef]
- Aguilera, N.; Guedes, L.M.; Becerra, J.; Baeza, C.; Hernández, V. Morphological effects at radicle level by direct contact of invasive Acacia dealbata Link. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 215, 54–59. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Antunes, P.M.; Martins-Loução, M.A.; Klironomos, J.N. Disturbance influences the outcome of plant–soil biota interactions in the invasive Acacia longifolia and in native species. Oikos 2010, 119, 1172–1180. [Google Scholar] [CrossRef]
- Coelho, S.I.D.B.F. Factores Facilitadores da Invasibilidade de Acacia Dealbata em Função do uso do solo. Ph.D. Thesis, University of Lisbon, Lisboa, Portugal, 2014. [Google Scholar]
- González-Muñoz, N.; Costa-Tenorio, M.; Espigares, T. Invasion of alien Acacia dealbata on Spanish Quercus robur forests: Impact on soils and vegetation. For. Ecol. Manag. 2012, 269, 214–221. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pereira, C.S.; Rodríguez-Echeverría, S. Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biol. Biochem. 2013, 57, 156–163. [Google Scholar] [CrossRef]
- Wilson, J.R.; Gairifo, C.; Gibson, M.R.; Arianoutsou, M.; Bakar, B.B.; Baret, S.; Celesti-Grapow, L.; DiTomaso, J.M.; Dufour-Dror, J.M.; Kueffer, C.; et al. Risk assessment, eradication, and biological control: Global efforts to limit Australian acacia invasions. Divers. Distrib. 2011, 17, 1030–1046. [Google Scholar] [CrossRef]
- Campbell, P.; Bell, R.; Kluge, R. Identifying the research requirements for the control of silver wattle (Acacia dealbata) in Natal. S. Afr. For. J. 1990, 155, 37–41. [Google Scholar] [CrossRef]
- Dennill, G.; Donnelly, D. Biological control of Acacia longifolia and related weed species (Fabaceae) in South Africa. Agric. Ecosyst. Environ. 1991, 37, 115–135. [Google Scholar] [CrossRef]
- Marchante, H.; Freitas, H.; Hoffmann, J. Assessing the suitability and safety of a well-known bud-galling wasp, Trichilogaster acaciaelongifoliae, for biological control of Acacia longifolia in Portugal. Biol. Control 2011, 56, 193–201. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M.C. Historical Development of the Portuguese Forest: The Introduction of Invasive Species. Forests 2019, 10, 974. https://doi.org/10.3390/f10110974
Nunes LJR, Meireles CIR, Pinto Gomes CJ, Almeida Ribeiro NMC. Historical Development of the Portuguese Forest: The Introduction of Invasive Species. Forests. 2019; 10(11):974. https://doi.org/10.3390/f10110974
Chicago/Turabian StyleNunes, Leonel J. R., Catarina I. R. Meireles, Carlos J. Pinto Gomes, and Nuno M. C. Almeida Ribeiro. 2019. "Historical Development of the Portuguese Forest: The Introduction of Invasive Species" Forests 10, no. 11: 974. https://doi.org/10.3390/f10110974
APA StyleNunes, L. J. R., Meireles, C. I. R., Pinto Gomes, C. J., & Almeida Ribeiro, N. M. C. (2019). Historical Development of the Portuguese Forest: The Introduction of Invasive Species. Forests, 10(11), 974. https://doi.org/10.3390/f10110974







