The Effect of Insect Defoliations and Seed Production on the Dynamics of Radial Growth Synchrony among Scots Pine Pinus sylvestris L. Provenances
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sites and Sample Collection
2.2. Seed Production
2.3. Insect Outbreaks
2.4. Sample Preparation
2.5. Dendrochronological Statistics
2.6. Clustering and PCGA
2.7. Pointer Years, Moving Inter–Series Correlation and Climate Sensitivity
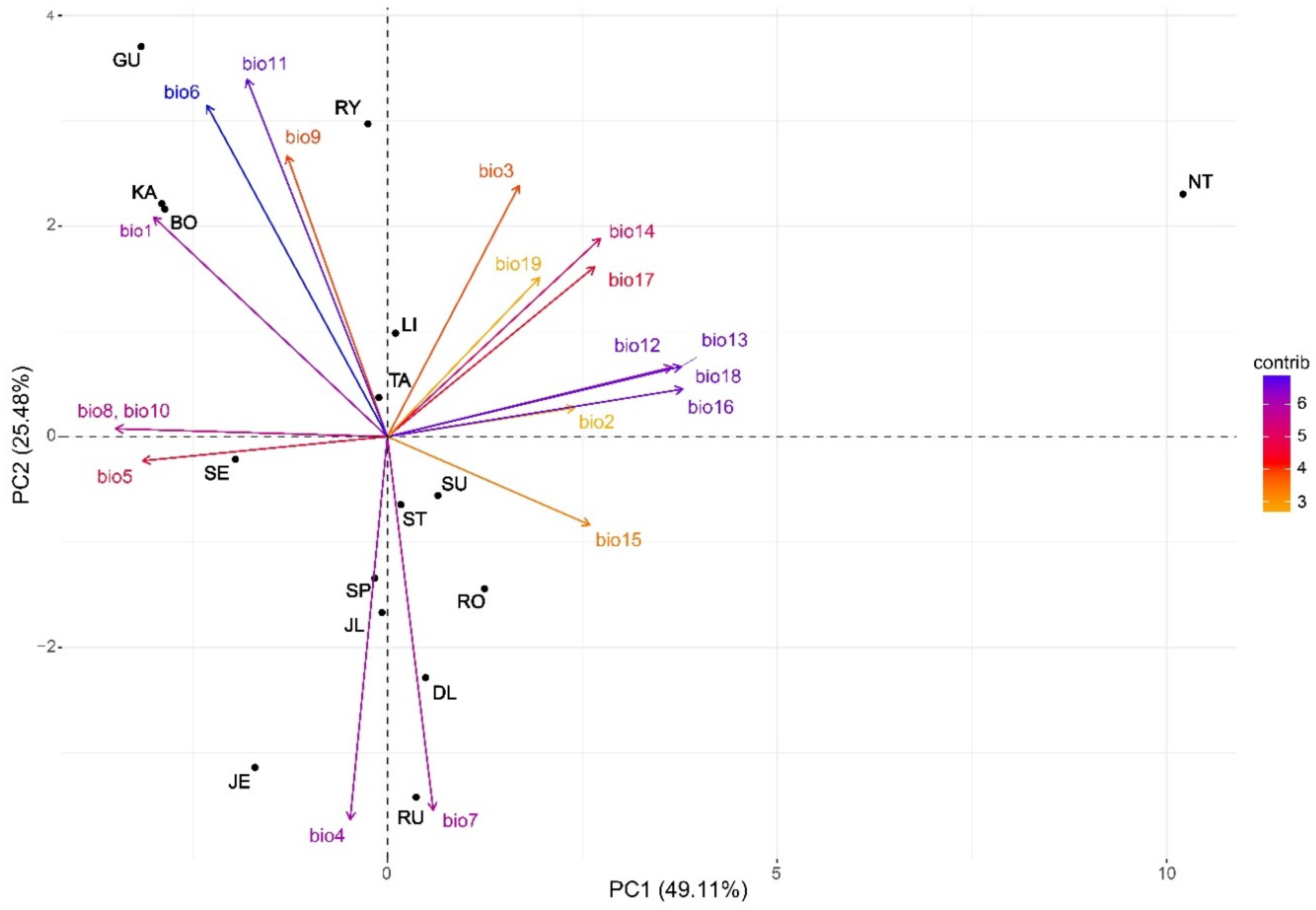
2.8. Bioclimatic Analyses
3. Results
3.1. Radial Growth Chronologies
3.2. Growth Responses to the Insect Outbreak
3.3. Clustering and PCGA
3.4. Pointer Years
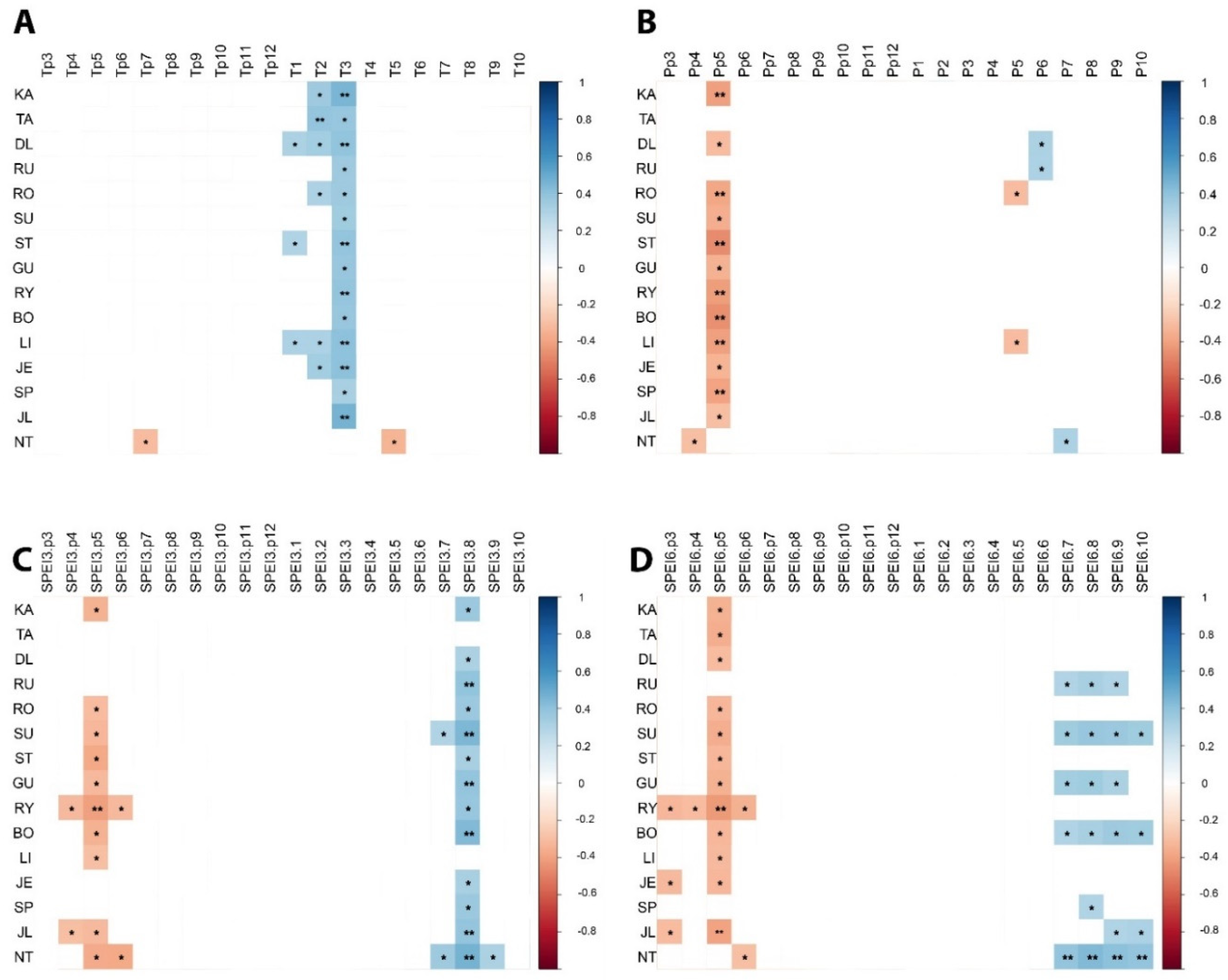
3.5. Climate–Growth Relationships
3.6. Climatic Transfer Effect
3.7. Temporal Variation in Climate Sensitivity
4. Discussion
4.1. Climate–Driven between–Provenance Variation
4.2. Biotic Determinants of Temporal Dynamics in Radial Growth
4.3. Individualistic Growth Response and Provenance Clustering
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, M.I.; Dumroese, R.K. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Jandl, R.; Bauhus, J.; Bolte, A.; Schindlbacher, A.; Schüler, S. Effect of climate–adapted forest management on carbon pools and greenhouse gas emissions. Curr. For. Rep. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Girona, M.M.; Navarro, L.; Morin, H. A secret hidden in the sediments: Lepidoptera scales. Front. Ecol. Evol. 2018, 6, 2. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Isaac–Renton, M.G.; Roberts, D.R.; Hamann, A.; Spiecker, H. Douglas–fir plantations in Europe: A retrospective test of assisted migration to address climate change. Glob. Chang. Biol. 2014, 20, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Jandl, R.; Kapeller, S.; Schueler, S. Disentangling the role of climate and soil on tree growth and its interaction with seed origin. Sci. Total Environ. 2019, 654, 393–401. [Google Scholar] [CrossRef]
- Klisz, M.; Buras, A.; Sass–Klaassen, U.; Puchałka, R.; Koprowski, M.; Ukalska, J. Limitations at the limit? Diminishing of genetic effects in Norway spruce provenance trials. Front. Plant Sci. 2019, 10, 306. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Vacchiano, G. Interactions between climate, growth and seed production in Spanish black pine (Pinus nigra Arn. ssp. salzmannii) forests in Cuenca Mountains (Spain). New For. 2018, 49, 399–414. [Google Scholar]
- Sangüesa–Barreda, G.; Linares, J.C.; Camarero, J.J. Reduced growth sensitivity to climate in bark–beetle infested Aleppo pines: Connecting climatic and biotic drivers of forest dieback. For. Ecol. Manag. 2015, 357, 126–137. [Google Scholar] [CrossRef]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in spatiotemporal patterns of 20th century spruce budworm outbreaks in eastern Canadian boreal forests. Front. Plant Sci. 2018, 9, 1905. [Google Scholar] [CrossRef]
- Walter, J.A.; Sheppard, L.W.; Anderson, T.L.; Kastens, J.H.; Bjørnstad, O.N.; Liebhold, A.M.; Reuman, D.C. The geography of spatial synchrony. Ecol. Lett. 2017, 20, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Defriez, E.J.; Sheppard, L.W.; Reid, P.C.; Reuman, D.C. Climate change–related regime shifts have altered spatial synchrony of plankton dynamics in the North Sea. Glob. Chang. Biol. 2016, 22, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, T.A.; Gutiérrez, E.; Kirdyanov, A.V.; Camarero, J.J.; Génova, M.; Knorre, A.A.; Linares, J.C.; De Dios, V.R.; Sánchez–Salguero, R.; Voltas, J. Forests synchronize their growth in contrasting Eurasian regions in response to climate warming. Proc. Natl. Acad. Sci. USA 2016, 113, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camarero, J.J.; Gazol, A.; Sangüesa–Barreda, G.; Oliva, J.; Vicente–Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Housset, J.M.; Carcaillet, C.; Girardin, M.P.; Xu, H.; Tremblay, F.; Bergeron, Y. In situ comparison of tree–ring responses to climate and population genetics: The need to control for local climate and site variables. Front. Ecol. Evol. 2016, 4, 123. [Google Scholar] [CrossRef]
- George, J.P.; Schueler, S.; Karanitsch–Ackerl, S.; Mayer, K.; Klumpp, R.T.; Grabner, M. Inter–and intra–specific variation in drought sensitivity in Abies spec. and its relation to wood density and growth traits. Agric. For. Meteorol. 2015, 214, 430–443. [Google Scholar] [CrossRef]
- George, J.P.; Grabner, M.; Karanitsch–Ackerl, S.; Mayer, K.; Weißenbacher, L.; Schueler, S. Genetic variation, phenotypic stability, and repeatability of drought response in European larch throughout 50 years in a common garden experiment. Tree Physiol. 2017, 37, 33–46. [Google Scholar] [CrossRef]
- Merian, P.; Bontemps, J.; Berge, L.; Lebourgeois, F. Spatial variation and temporal instability in climate–growth relationships of sessile oak (Quercus petraea [Matt.] Liebl.) under temperate conditions. Plant Ecol. 2011, 212, 1855–1871. [Google Scholar] [CrossRef]
- Carrer, M. Individualistic and time–varying tree–ring growth to climate sensitivity. PLoS ONE 2011, 6, e22813. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Long–term change in the sensitivity of tree–ring growth to climate forcing in Larix decidua. New Phytol. 2006, 170, 861–872. [Google Scholar] [CrossRef]
- Chavardès, R.D.; Daniels, L.D.; Waeber, P.O.; Innes, J.L.; Nitschke, C.R. Unstable climate–growth relations for white spruce in southwest Yukon, Canada. Clim. Chang. 2013, 116, 593–611. [Google Scholar] [CrossRef]
- Biondi, F. Are climate–tree growth relationships changing in north–central Idaho, USA? Arct. Antarct. Alp. Res. 2000, 32, 111–116. [Google Scholar] [CrossRef]
- Dansgaard, W.; Johnsen, S.J.; Clausen, H.B.; Dahl–Jensen, D.; Gundestrup, N.S.; Hammer, C.U.; Hvidberg, C.S.; Steffensen, J.P.; Sveinbjörnsdottir, A.E.; Jouzel, J.; et al. Evidence for general instability of past climate from a 250–kyr ice–core record. Nature 1993, 364, 218–220. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Age–dependent tree–ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 2004, 85, 730–740. [Google Scholar] [CrossRef]
- Savva, Y.; Bergeron, Y.; Denneler, B.; Koubaa, A.; Tremblay, F. Effect of interannual climate variations on radial growth of jack pine provenances in Petawawa, Ontario. Can. J. For. Res. 2008, 38, 619–630. [Google Scholar] [CrossRef]
- Herrera, C.M.; Jordano, P.; Guitian, J.; Traveset, A. Annual variability in seed production by woody plants and the masting concept: Reassessment of principles and relationship to pollination and seed dispersal. Am. Nat. 1998, 152, 576–594. [Google Scholar] [CrossRef]
- Montoro Girona, M.; Lussier, J.M.; Morin, H.; Thiffault, N. Conifer regeneration after experimental shelterwood and seed–tree treatments in boreal forests: Finding silvicultural alternatives. Front. Plant Sci. 2018, 9, 1145. [Google Scholar] [CrossRef]
- Zamorano, J.G.; Hokkanen, T.; Lehikoinen, A. Climate–driven synchrony in seed production of masting deciduous and conifer tree species. J. Plant Ecol. 2018, 11, 180–188. [Google Scholar]
- Pearse, I.S.; Koenig, W.D.; Kelly, D. Mechanisms of mast seeding: Resources, weather, cues, and selection. New Phytol. 2016, 212, 546–562. [Google Scholar] [CrossRef]
- Suvanto, S.; Nöjd, P.; Henttonen, H.M.; Beuker, E.; Mäkinen, H. Geographical patterns in the radial growth response of Norway spruce provenances to climatic variation. Agric. For. Meteorol. 2016, 222, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Selås, V.; Piovesan, G.; Adams, J.M.; Bernabei, M. Climatic factors controlling reproduction and growth of Norway spruce in southern Norway. Can. J. For. Res. 2002, 32, 217–225. [Google Scholar] [CrossRef]
- Hacket–Pain, A.J.; Ascoli, D.; Berretti, R.; Mencuccini, M.; Motta, R.; Nola, P.; Piussi, P.; Ruffinatto, F.; Vacchiano, G. Temperature and masting control Norway spruce growth, but with high individual tree variability. For. Ecol. Manag. 2019, 438, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Caron, L.; Rossi, S. Carbon allocation during defoliation: Testing a defense–growth trade–off in balsam fir. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Hallsby, G.; Löfvenius, M.O.; Nilsson, M.C. Synergistic, additive and antagonistic impacts of drought and herbivory on Pinus sylvestris: Leaf, tissue and whole–plant responses and recovery. Tree Physiol. 2013, 33, 451–463. [Google Scholar] [CrossRef]
- Grüning, M.; Beule, L.; Meyer, S.; Karlovsky, P.; I–M–Arnold, A. The abundance of fungi, bacteria and denitrification genes during insect outbreaks in Scots pine forests. Forests 2018, 9, 497. [Google Scholar] [CrossRef]
- Grüning, M.M.; Simon, J.; Rennenberg, H.; L–M–Arnold, A. Defoliating insect mass outbreak affects soil n fluxes and tree n nutrition in Scots pine forests. Front. Plant Sci. 2017, 8, 954. [Google Scholar] [CrossRef]
- Bigler, C.; Bräker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an inciting mortality factor in scots pine stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef]
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Pennerstorfer, J.; Rosner, S.; Kikuta, S.; Schume, H.; et al. Do water–limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141. [Google Scholar] [CrossRef]
- Marini, L.; Ayres, M.P.; Battisti, A.; Faccoli, M. Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Clim. Chang. 2012, 115, 327–341. [Google Scholar] [CrossRef]
- Krams, I.; Daukšte, J.; Kivleniece, I.; Brumelis, G.; Cibuļskis, R.; Aboliņš–abols, M.; Rantala, M.J.; Mierauskas, P.; Krama, T. Drought–induced positive feedback in xylophagous insects: Easier invasion of Scots pine leading to greater investment in immunity of emerging individuals. For. Ecol. Manag. 2012, 270, 147–152. [Google Scholar] [CrossRef]
- Jacquet, J.S.; Bosc, A.; O′Grady, A.; Jactel, H. Combined effects of defoliation and water stress on pine growth and non–structural carbohydrates. Tree Physiol. 2014, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.L.; Lanta, V.; Kozlov, M.V. Effects of sap–feeding insect herbivores on growth and reproduction of woody plants: A meta–analysis of experimental studies. Oecologia 2010, 163, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Kaitaniemi, P.; Neuvonen, S.; Nyyssönen, T. Effects of cumulative defoliations on growth, reproduction, and insect resistance in mountain birch. Ecology 1999, 80, 524–532. [Google Scholar] [CrossRef]
- Matras, J. Provenances studies on Scots pine conducted by the Section of Seed Science and Selection of the Forest Research Institute. Sylwan 1989, 133, 53–56, (In Polish with English abstract). [Google Scholar]
- Boczoń, A.; Kowalska, A.; Gawryś, R. Soil−water determinants of forest management in the perspective of climate change. Sylwan 2017, 161, 763–771, (In Polish with English abstract). [Google Scholar]
- Beguería, S.; Vicente–Serrano, S.M.; Reig, F.; Latorre, B. Standardized Precipitation Evapotranspiration Index (SPEI) revisited: Parameter fitting, evapotranspiration models, tools, datasets and drought monitoring. Int. J. Climatol. 2014, 34, 3001–3023. [Google Scholar] [CrossRef]
- Hargreaves, G.H.; Samani, Z.A. Reference crop evapotranspiration from temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. FAO Irrigation and Drainage Paper No. 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; p. e156. [Google Scholar]
- IUSS Working Group WRB. World reference base for soil resources 2014, update 2015: International soil classification system for naming soils and creating legends for soil maps. World Soil Resour. Rep. 2015, 160, 192. [Google Scholar]
- Kantorowicz, W. Half a century of seed years in major tree species of Poland. Silvae Genet. 2000, 49, 245–249. [Google Scholar]
- Geri, C. The pine sawfly in central France. In Dynamics of Forest Insect Populations (Theory and Application); Berryman, A., Ed.; Springer: Boston, MA, USA, 1988; pp. 377–405. [Google Scholar]
- Schönherr, J. Nun moth outbreak in Poland 1978–1984. J. Appl. Entomol. 1985, 99, 73. [Google Scholar] [CrossRef]
- Barbour, D. The Pine Looper in Britain and Europe. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A., Ed.; Springer: New York, NY, USA, 1988; pp. 291–309. [Google Scholar]
- Straw, N.A.; Armour, H.L.; Day, K.R. The financial costs of defoliation of Scots pine (Pinus sylvestris) by pine looper moth (Bupalus piniaria). Forestry 2002, 75, 525–536. [Google Scholar] [CrossRef]
- Straw, N.A. The impact of pine looper moth, Bupalus piniaria L. (Lepidoptera; Geometridae) on the growth of scots pine in Tentsmuir Forest, Scotland. For. Ecol. Manag. 1996, 87, 209–232. [Google Scholar] [CrossRef]
- Cedervind, J. Impact of Pine looper Defoliation in Scots Pine: Secondary Attack by Pine Shoot Beetles, Tree Mortality, Top.–Kill, Growth Losses, and Foliage Recovery; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2003. [Google Scholar]
- Nakládal, O.; Brinkeová, H. Review of historical outbreaks of the nun moth (Lymantria monacha) with respect to host tree species. J. For. Sci. 2015, 61, 18–26. [Google Scholar]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Krause, C.; Morin, H. Changes in radial increment in stems and roots of balsam fir [Abies balsamea (L.) Mill.] after defoliation spruce budworm. For. Chron. 1995, 71, 747–754. [Google Scholar] [CrossRef]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree–ring width series for dendroclimatic studies. Tree Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Speer, J.H. Fundamentals of Tree–Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Cook, E.; Briffa, K.; Shiyatov, S.; Mazepa, A.; Jones, P. Data analysis. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E., Kairiukstis, L., Eds.; International Institute for Applied Systems Analysis, Kluwer: Dordrecht, Switzerland, 1990; pp. 97–162. [Google Scholar]
- Eckstein, D.; Bauch, J. Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss. Cent. 1969, 88, 230–250. [Google Scholar] [CrossRef]
- Buras, A.; Wilmking, M. Correcting the calculation of Gleichläufigkeit. Dendrochronologia 2015, 34, 29–30. [Google Scholar] [CrossRef]
- Wigley, T.; Briffa, K.; Jones, P. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Douglass, A.E. Evidence of climatic effects in the annual rings of trees. Ecology 1920, 1, 24–32. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Sokal, R.; Michener, C. A statistical method for evaluating systematic relationships. Univ. Kans. Sci. Bull. 1958, 38, 1409–1438. [Google Scholar]
- Kaufman, L.; Rousseeuw, P. Clustering large applications (Program CLARA). In Finding Groups in Data: An Introduction to Cluster Analysis; Kaufman, L., Rousseeuw, P., Eds.; Willey: Hoboken, NJ, USA, 2008; pp. 126–163. [Google Scholar]
- Buras, A.; Van Der Maaten–theunissen, M.; Van Der Maaten, E. Tuning the Voices of a Choir: Detecting ecological gradients in time–series populations. PLoS ONE 2016, 11, e0158346. [Google Scholar] [CrossRef]
- Simes, J.R. Improved Bonferroni procedure for multiple tests of significance. Biometrika 1986, 73, 751–754. [Google Scholar] [CrossRef]
- Shestakova, T.; Gutiérrez, E.; Voltas, J. A roadmap to disentangling ecogeographical patterns of spatial synchrony in dendrosciences. Trees Struct. Funct. 2018, 32, 359–370. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Eckstein, D.; Serre–Bachet, F.; Bräker, O.U. Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 1990, 8, 9–38. [Google Scholar]
- Cropper, J. Tree–ring skeleton plotting by computer. Tree Ring Bull. 1979, 39, 47–60. [Google Scholar]
- Neuwirth, B.; Schweingruber, F.H.; Winiger, M. Spatial patterns of central European pointer years from 1901 to 1971. Dendrochronologia 2007, 24, 79–89. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 19 November 2017).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Species Distribution Modeling. Available online: http://rspatial.org/sdm/ (accessed on 28 October 2018).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. Factominer: Multivariate exploratory data analysis and data mining. R Package Version 2018, 1, 29. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Rousseeuw, P.; Struyf, A.; Hubert, M.; Studer, M.; Roudier, P.; Gonzalez, J.; Kozlowski, K. Finding Groups in Data: Cluster Analysis Extended Rousseeuw. Available online: https://svn.r-project.org/R-packages/trunk/cluster (accessed on 28 October 2018).
- van der Maaten–Theunissen, M.; van der Maaten, E.; Bouriaud, O. PointRes: An R package to analyze pointer years and components of resilience. Dendrochronologia 2015, 35, 34–38. [Google Scholar] [CrossRef]
- Begueria, S.; Vicente–Serrano, S.M.; Sawasawa, H. SPEI: Calculation of Standardised Precipitation–Evapotranspiration Index. R Package Version 2017, 1, 6. [Google Scholar]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy–climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Antonova, G.F.; Stasova, V.V. Effects of environmental factors on wood formation in Scots pine stems. Trees 1993, 7, 214–219. [Google Scholar] [CrossRef]
- Gruber, A.; Strobl, S.; Veit, B.; Oberhuber, W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol. 2010, 30, 490–501. [Google Scholar] [CrossRef]
- Buras, A.; Schunk, C.; Zeiträg, C.; Herrmann, C.; Kaiser, L.; Lemme, H.; Straub, C.; Taeger, S.; Gößwein, S.; Klemmt, H.J.; et al. Are Scots pine forest edges particularly prone to drought–induced mortality? Environ. Res. Lett. 2018, 13. [Google Scholar] [CrossRef]
- Matisons, R.; Jansone, D.; Elferts, D.; Adamovičs, A.; Schneck, V.; Jansons, Ā. Plasticity of response of tree–ring width of Scots pine provenances to weather extremes in Latvia. Dendrochronologia 2019, 54, 1–10. [Google Scholar] [CrossRef]
- Gričar, J.; Zupančič, M.; Čufar, K.; Koch, G.; Schmitt, U.; Owen, P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Ann. Bot. 2006, 97, 943–951. [Google Scholar] [CrossRef]
- Oribe, Y.; Funada, R.; Kubo, T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees Struct. Funct. 2003, 17, 185–192. [Google Scholar]
- Hari, P.; Kerminen, V.M.; Kulmala, L.; Kulmala, M.; Noe, S.; Petäjä, T.; Vanhatalo, A.; Bäck, J. Annual cycle of Scots pine photosynthesis. Atmos. Chem. Phys. 2017, 17, 15045–15053. [Google Scholar] [CrossRef] [Green Version]
- Oleksyn, J.; Tjoelker, M.G.; Reich, P.B. Adaptation to changing environment in Scots pine populations across a latitudinal gradient. Silva Fenn. 1998, 32, 129–140. [Google Scholar] [CrossRef]
- Taeger, S.; Zang, C.; Liesebach, M.; Schneck, V.; Menzel, A. Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. For. Ecol. Manag. 2013, 307, 30–42. [Google Scholar] [CrossRef]
- Semerci, A.; Semerci, H.; Çalişkan, B.; Çiçek, N.; Ekmekçi, Y.; Mencuccini, M. Morphological and physiological responses to drought stress of European provenances of Scots pine. Eur. J. For. Res. 2017, 136, 91–104. [Google Scholar] [CrossRef]
- Savva, Y.V.; Schweingruber, F.H.; Kuzmina, N.A.; Vaganov, E.A. Sensitivity of diameter growth to annual weather conditions in Scots pine provenances at a Central Siberian location. Silvae Genet. 2002, 51, 49–55. [Google Scholar]
- Davi, H.; Pichot, C.; Fady, B.; Cailleret, M.; Restoux, G.; Amm, A. Disentangling the factors driving tree reproduction. Ecosphere 2016, 7, e01389. [Google Scholar] [CrossRef]
- Greene, D.F.; Messier, C.; Asselin, H.; Fortin, M.J. The effect of light availability and basal area on cone production in Abies balsamea and Picea glauca. Can. J. Bot. 2002, 80, 370–377. [Google Scholar] [CrossRef]
- Krouchi, F.; Derridj, A.; Lefèvre, F. Year and tree effect on reproductive organisation of Cedrus atlantica in a natural forest. For. Ecol. Manag. 2004, 197, 181–189. [Google Scholar] [CrossRef]
- Seifert, T.; Muller–Starck, G. Impacts of fructification on biomass production and correlated genetic effects in Norway spruce (Picea abies [L.] Karst.). Eur. J. For. Res. 2009, 128, 155–169. [Google Scholar] [CrossRef]
- Nussbaumer, A.; Waldner, P.; Etzold, S.; Gessler, A.; Benham, S.; Thomsen, I.M.; Jørgensen, B.B.; Timmermann, V.; Verstraeten, A.; Sioen, G.; et al. Patterns of mast fruiting of Common beech, Sessile and Common oak, Norway spruce and Scots pine in Central and Northern Europe. For. Ecol. Manag. 2016, 363, 237–251. [Google Scholar] [CrossRef]
- Rebetez, M.; Mayer, H.; Dupont, O. Heat and drought 2003 in Europe: A climate synthesis. Ann. For. Sci. 2006, 63, 569–577. [Google Scholar] [CrossRef]
- He, B.; Liu, J.; Guo, L.; Wu, X.; Xie, X.; Zhang, Y.; Chen, C.; Zhong, Z.; Chen, Z. Recovery of ecosystem carbon and energy fluxes from the 2003 drought in Europe and the 2012 drought in the United States. Geophys. Res. Lett. 2018, 45, 4879–4888. [Google Scholar] [CrossRef]
- Ericsson, A.; Larsson, S.; Tenow, O. Effects of early and late season defoliation on growth and carbohydrate dynamics in Scots pine. J. Appl. Ecol. 1980, 17, 747–769. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis–McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evolut. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Savva, Y.; Schweingruber, F.; Milyutin, L.; Vaganov, E. Genetic and environmental signals in tree rings from different provenances of Pinus sylvestris L. planted in the southern taiga, central Siberia. Trees 2002, 16, 313–324. [Google Scholar] [CrossRef]
- Ståhl, E.G. Transfer effects and variations in basic density and tracheid length of Pinus sylvestris L. populations. Stud. For. Suec. 1988, 180, 1–15. [Google Scholar]
- DeWitt, T.J.; Scheiner, S.M. Phenotypic variation from single genotypes. In Phenotypic Plasticity: Functional and Conceptual Approaches; DeWitt, T.J., Scheiner, S.M., Eds.; Oxford University Press: Oxford, UK, 2004; Volume 13, pp. 1–10. [Google Scholar]
- Wójkiewicz, B.; Cavers, S.; Wachowiak, W. Current approaches and perspectives in population genetics of Scots pine (Pinus sylvestris L.). For. Sci. 2016, 62, 343–354. [Google Scholar] [CrossRef]
- Hebda, A.; Wójkiewicz, B.; Wachowiak, W. Genetic characteristics of Scots pine in Poland and reference populations based on nuclear and chloroplast microsatellite markers. Silva Fenn. 2017, 51, 1721. [Google Scholar] [CrossRef]
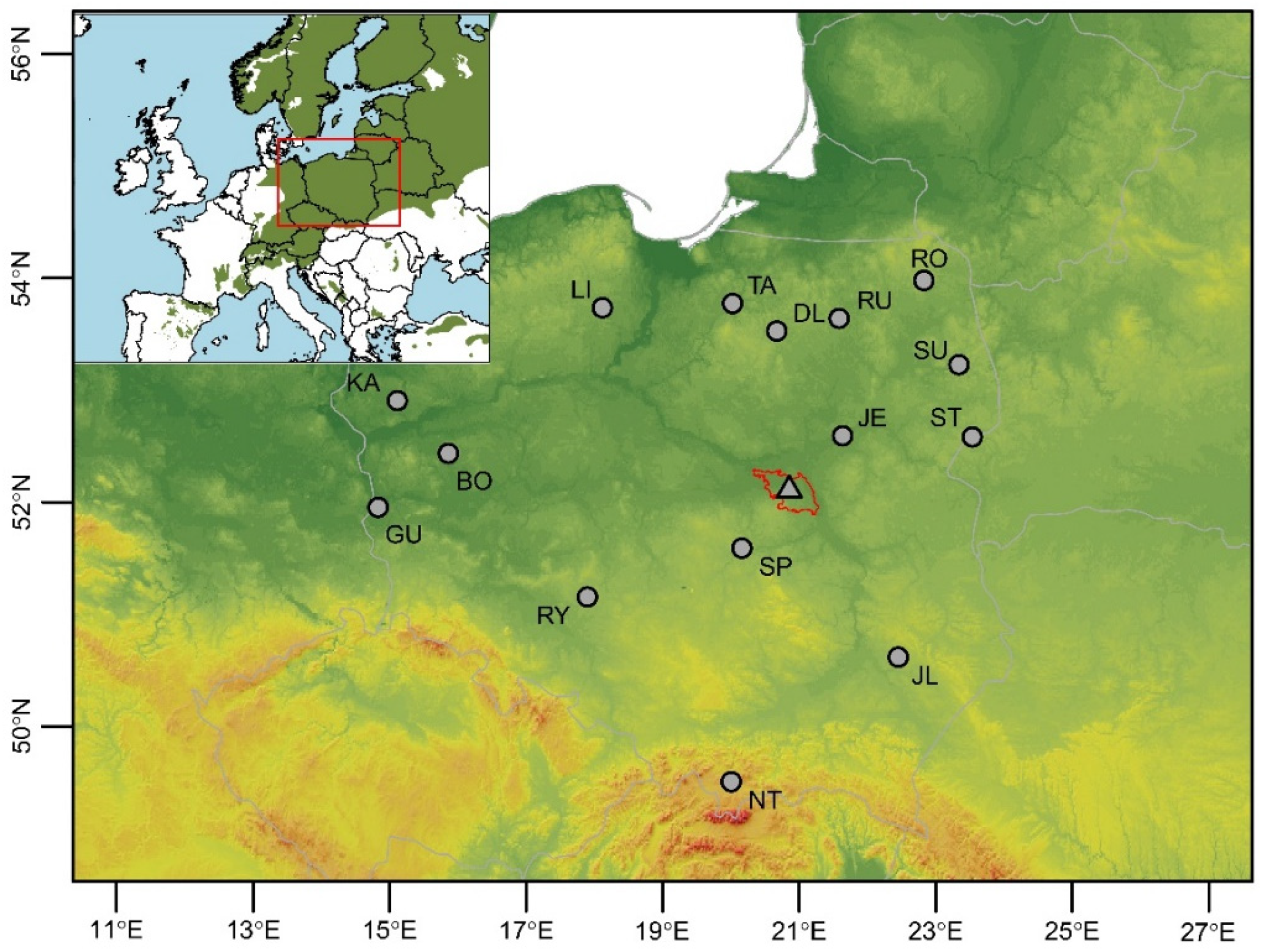
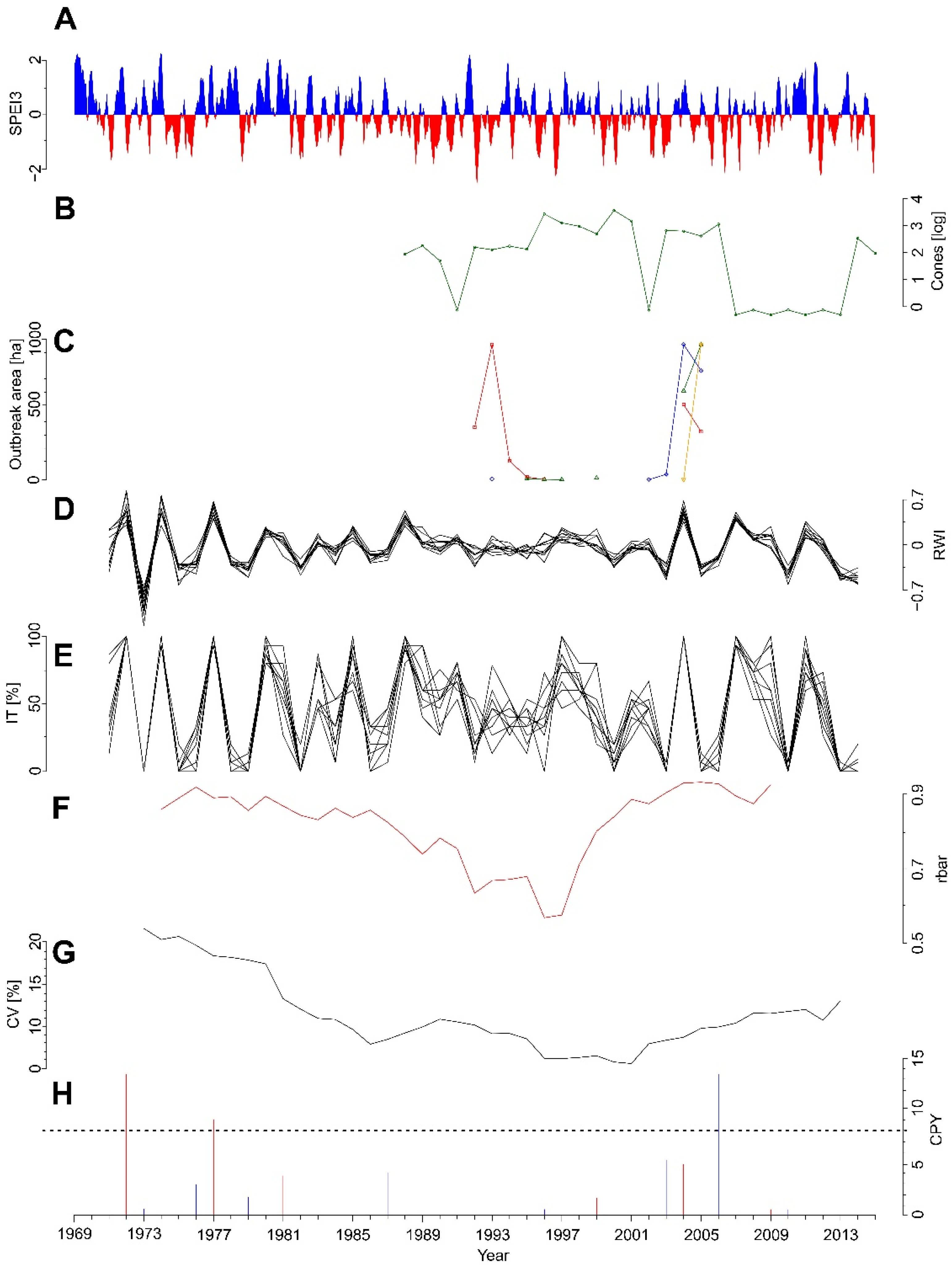
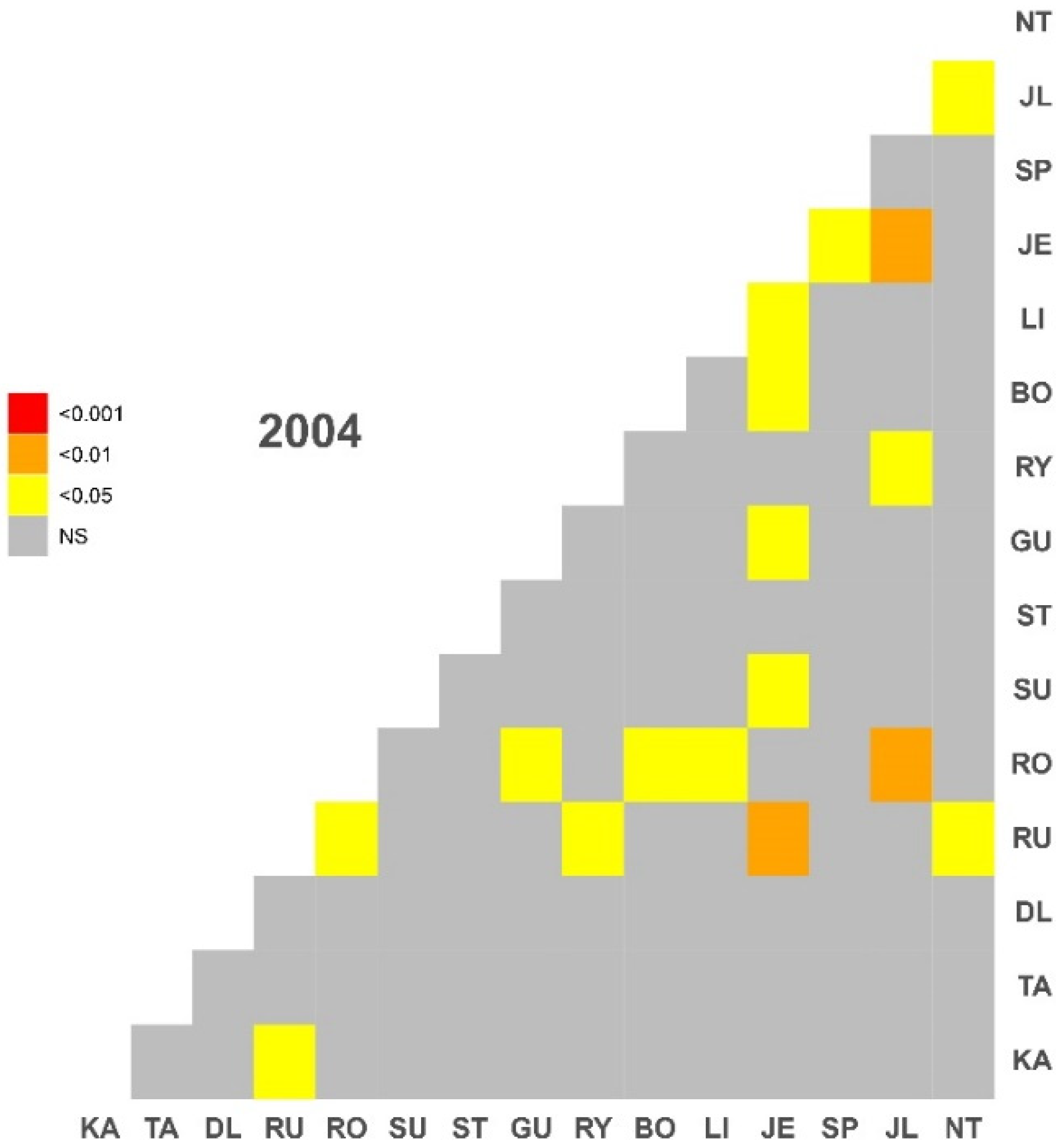

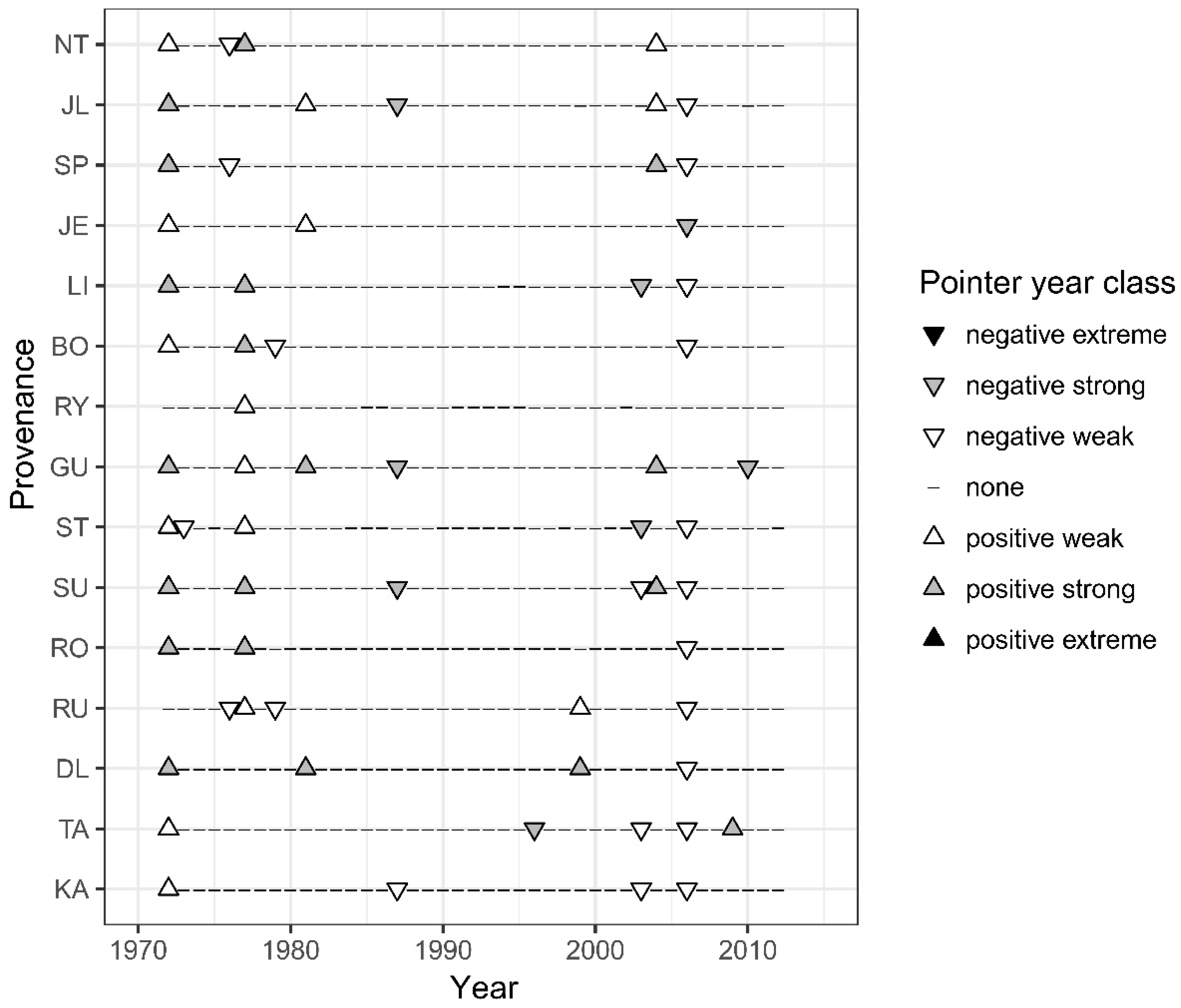
| Provenance ID | Latitude | Longitude | Altitude | MAP [mm] | Tmin [°C] | Tmax [°C] |
|---|---|---|---|---|---|---|
| KA | 52.9087 | 15.1120 | 76 | 558 | −5.2 | 24.0 |
| TA | 53.7759 | 20.0241 | 121 | 650 | −7.4 | 23.3 |
| DL | 53.5296 | 20.6719 | 171 | 634 | −8.6 | 23.3 |
| RU | 53.6424 | 21.5867 | 150 | 630 | −9.2 | 23.6 |
| RO | 53.9820 | 22.8266 | 168 | 598 | −8.1 | 22.0 |
| SU | 53.2298 | 23.3395 | 154 | 573 | −7.7 | 22.7 |
| ST | 52.5850 | 23.5362 | 178 | 572 | −7.5 | 23.4 |
| GU | 51.9563 | 14.8310 | 61 | 554 | −4.3 | 24.2 |
| RY | 51.1572 | 17.8977 | 202 | 575 | −5.4 | 23.2 |
| BO | 52.4379 | 15.8613 | 74 | 552 | −5.2 | 23.9 |
| LI | 53.7377 | 18.1175 | 132 | 585 | −6.7 | 21.8 |
| JE | 52.5956 | 21.6365 | 104 | 523 | −8.3 | 24.0 |
| SP | 51.5927 | 20.1617 | 195 | 565 | −7.8 | 23.4 |
| JL | 50.6207 | 22.4525 | 212 | 571 | −8.1 | 23.4 |
| NT | 49.5087 | 20.0055 | 700 | 927 | −8.8 | 21.3 |
| Provenance ID | TRW | glk | MS | rbt | EPS | AR1 |
|---|---|---|---|---|---|---|
| KA | 2.69 | 0.76 | 0.72 | 0.52 | 0.94 | 0.61 |
| TA | 2.50 | 0.76 | 0.78 | 0.52 | 0.94 | 0.67 |
| DL | 2.42 | 0.75 | 0.76 | 0.56 | 0.95 | 0.63 |
| RU | 2.55 | 0.81 | 0.74 | 0.50 | 0.94 | 0.53 |
| RO | 2.55 | 0.80 | 0.77 | 0.52 | 0.94 | 0.69 |
| SU | 2.65 | 0.74 | 0.73 | 0.54 | 0.95 | 0.49 |
| ST | 2.40 | 0.71 | 0.70 | 0.48 | 0.93 | 0.55 |
| GU | 2.53 | 0.75 | 0.68 | 0.49 | 0.94 | 0.51 |
| RY | 2.62 | 0.68 | 0.68 | 0.42 | 0.92 | 0.46 |
| BO | 2.96 | 0.71 | 0.72 | 0.47 | 0.93 | 0.57 |
| LI | 2.70 | 0.70 | 0.74 | 0.58 | 0.95 | 0.56 |
| JE | 2.71 | 0.71 | 0.70 | 0.44 | 0.92 | 0.55 |
| SP | 2.70 | 0.73 | 0.73 | 0.56 | 0.95 | 0.54 |
| JL | 2.53 | 0.73 | 0.68 | 0.45 | 0.93 | 0.60 |
| NT | 2.47 | 0.75 | 0.68 | 0.47 | 0.93 | 0.67 |
| Bioclimatic Variables | Abbreviation | PC 1 | PC 2 |
|---|---|---|---|
| Annual mean temperature | bio1 | −0.250 (<0.001) | 0.241 (0.075) |
| Mean monthly temperature range | bio2 | 0.201 (0.252) | 0.0316 (0.987) |
| Isothermality [(bio2/bio7) × 100)] | bio3 | 0.141 (0.837) | 0.276 (0.013) |
| Temperature seasonality (std × 100) | bio4 | −0.040 (0.385) | −0.420 (<0.001) |
| Max temperature of warmest month | bio5 | −0.262 (0.001) | −0.026 (0.785) |
| Min temperature of coldest month | bio6 | −0.193 (0.001) | 0.364 (0.001) |
| Temperature annual range (bio5–bio6) | bio7 | 0.049 (0.130) | −0.409 (<0.001) |
| Mean temperature of wettest quarter | bio8 | −0.290 (<0.001) | 0.009 (0.892) |
| Mean temperature of driest quarter | bio9 | −0.107 (0.010) | 0.308 (0.001) |
| Mean temperature of warmest quarter | bio10 | −0.290 (<0.001) | 0.009 (0.892) |
| Mean temperature of coldest quarter | bio11 | −0.150 (0.002) | 0.392 (<0.001) |
| Annual precipitation | bio12 | 0.305 (0.001) | 0.076 (0.780) |
| Precipitation of wettest month | bio13 | 0.314 (0.010) | 0.077 (0.433) |
| Precipitation of driest month | bio14 | 0.228 (0.847) | 0.218 (0.036) |
| Precipitation seasonality (CV) | bio15 | 0.217 (0.200) | −0.097 (0.429) |
| Precipitation of wettest quarter | bio16 | 0.316 (0.004) | 0.052 (0.247) |
| Precipitation of driest quarter | bio17 | 0.222 (0.440) | 0.186 (0.164) |
| Precipitation of warmest quarter | bio18 | 0.316 (0.004) | 0.052 (0.247) |
| Precipitation of coldest quarter | bio19 | 0.163 (0.389) | 0.175 (0.204) |
| Eigenvalue | 9.33 | 4.84 | |
| Variance explained | 49.11 | 25.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klisz, M.; Puchałka, R.; Wilczyński, S.; Kantorowicz, W.; Jabłoński, T.; Kowalczyk, J. The Effect of Insect Defoliations and Seed Production on the Dynamics of Radial Growth Synchrony among Scots Pine Pinus sylvestris L. Provenances. Forests 2019, 10, 934. https://doi.org/10.3390/f10100934
Klisz M, Puchałka R, Wilczyński S, Kantorowicz W, Jabłoński T, Kowalczyk J. The Effect of Insect Defoliations and Seed Production on the Dynamics of Radial Growth Synchrony among Scots Pine Pinus sylvestris L. Provenances. Forests. 2019; 10(10):934. https://doi.org/10.3390/f10100934
Chicago/Turabian StyleKlisz, Marcin, Radosław Puchałka, Sławomir Wilczyński, Władysław Kantorowicz, Tomasz Jabłoński, and Jan Kowalczyk. 2019. "The Effect of Insect Defoliations and Seed Production on the Dynamics of Radial Growth Synchrony among Scots Pine Pinus sylvestris L. Provenances" Forests 10, no. 10: 934. https://doi.org/10.3390/f10100934
APA StyleKlisz, M., Puchałka, R., Wilczyński, S., Kantorowicz, W., Jabłoński, T., & Kowalczyk, J. (2019). The Effect of Insect Defoliations and Seed Production on the Dynamics of Radial Growth Synchrony among Scots Pine Pinus sylvestris L. Provenances. Forests, 10(10), 934. https://doi.org/10.3390/f10100934






