Different Molecular Characterization of Soil Particulate Fractions under N Deposition in a Subtropical Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Soil Sampling
2.3. Physical Fractionation and C Content Determination
2.4. Pyrolysis-GC/MS
2.5. Statistical Analysis
3. Results and Discussions
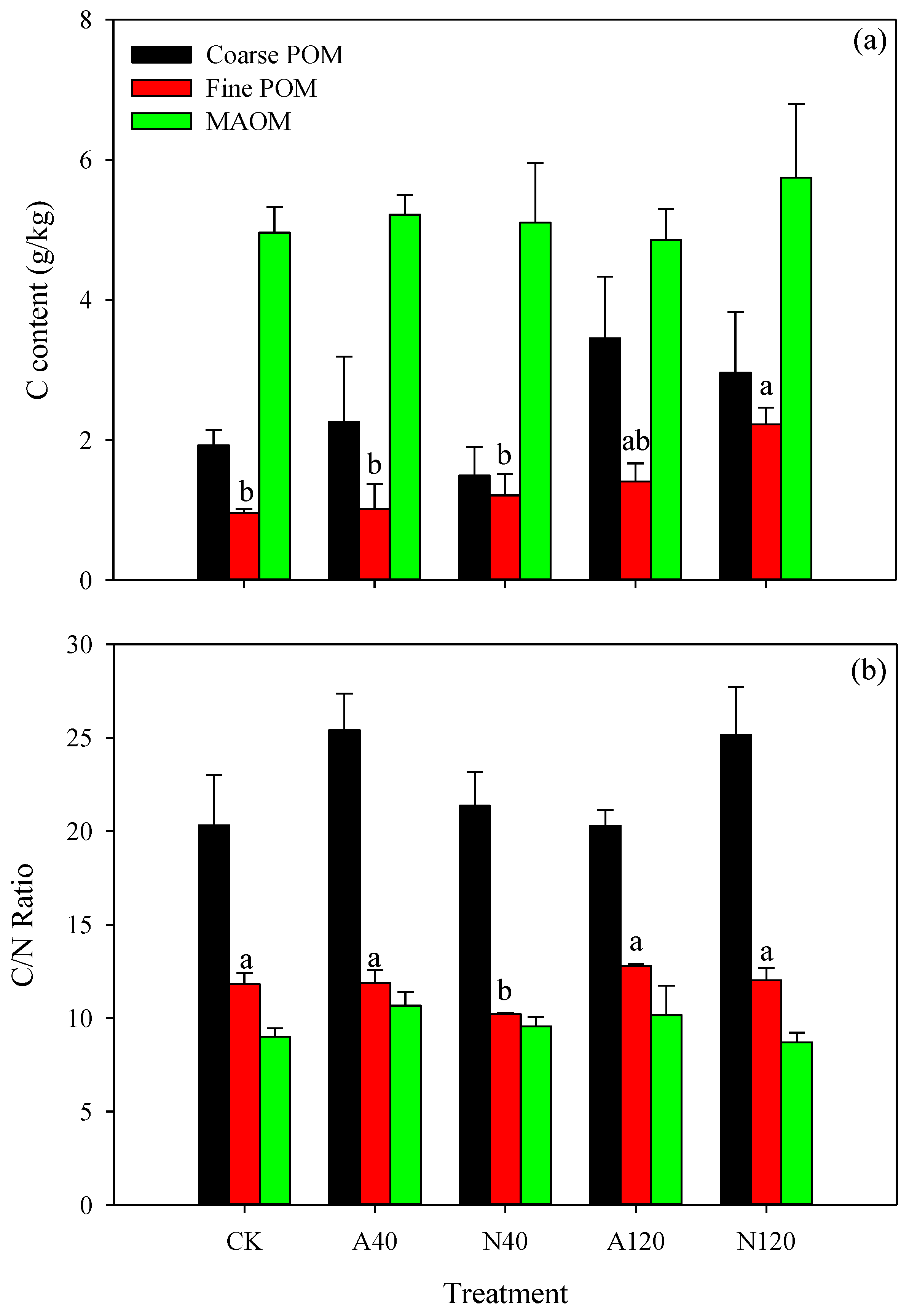
3.1. Percentages and C Contents of Particulate Fractions
3.2. General Distribution and Origin of Pyrolysis Products in the Soil Fractions
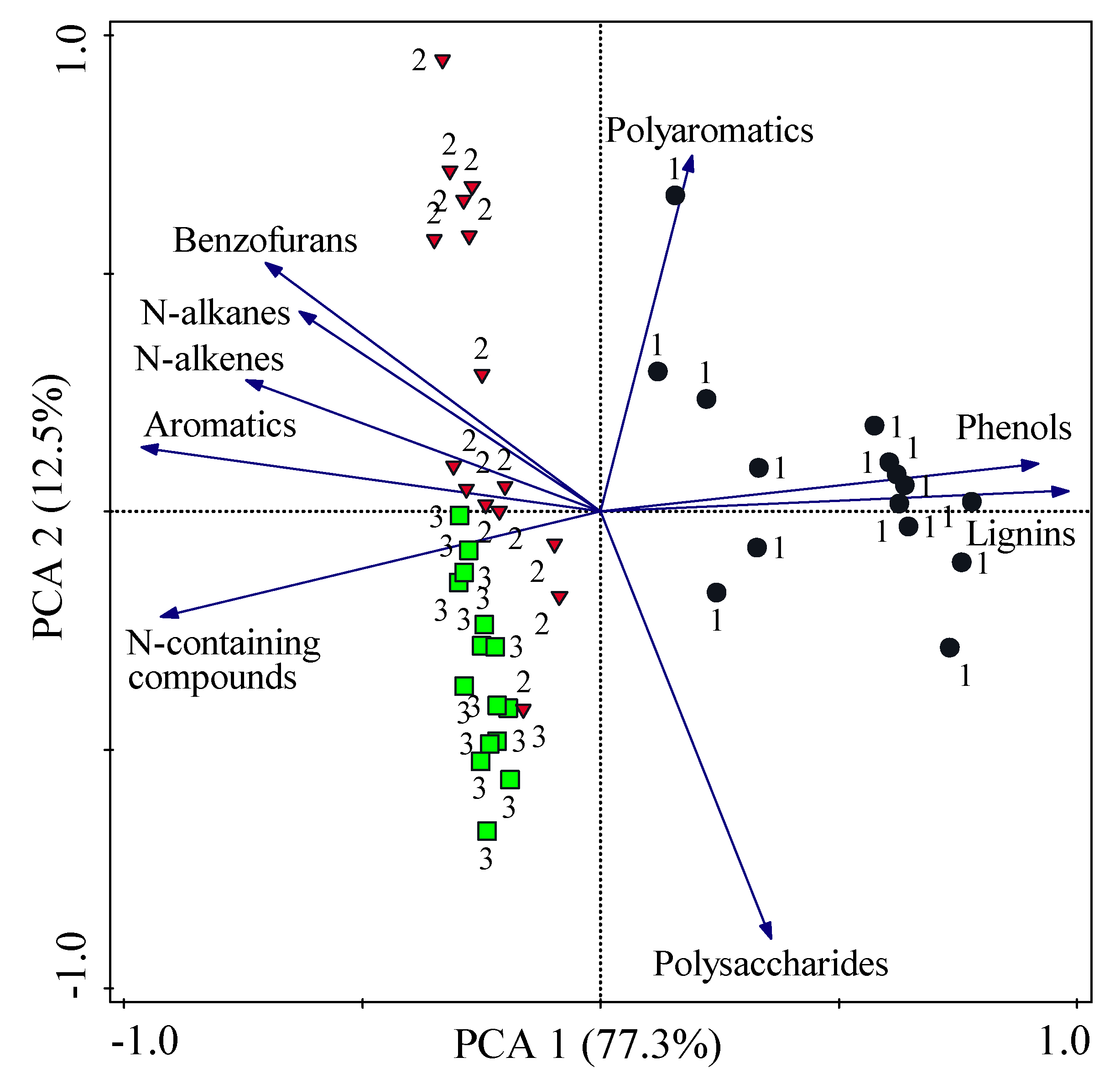
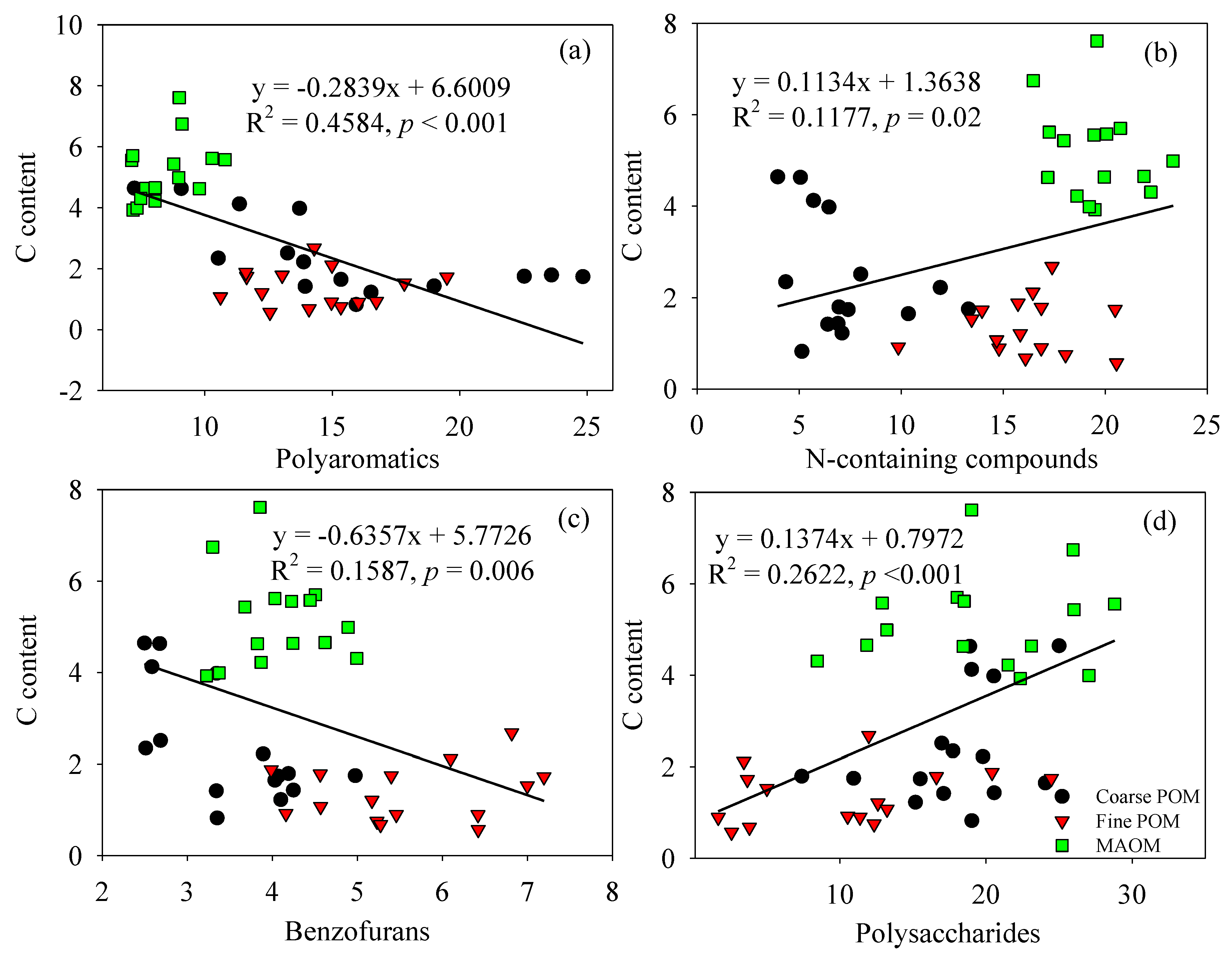
3.3. Factor Analysis Applied on Molecular Composition of Soil Particulate Fractions
3.4. Effects of N Addition on Chemical Compositions in Three Particulate Fractions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hedges, J.I.; Eglinton, G.; Hatcher, P.G.; Kirchman, D.L.; Arnosti, C.; Derenne, S.; Evershed, R.P.; Kögel-Knabner, I.; de Leeuw, J.W.; Littke, R. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem. 2000, 31, 945–958. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49. [Google Scholar] [CrossRef] [PubMed]
- Gleixner, G.; Poirier, N.; Bol, R.; Balesdent, J. Molecular dynamics of organic matter in a cultivated soil. Org. Geochem. 2002, 33, 357–366. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Christensen, B.T. Physical fractionation of soil and organic matter in primary particle size and density separates. In Advances in Soil Science; Springer: Berlin, Germany, 1992; pp. 1–90. [Google Scholar]
- Six, J.; Guggenberger, G.; Paustian, K.; Haumaier, L.; Elliott, E.; Zech, W. Sources and composition of soil organic matter fractions between and within soil aggregates. Eur. J. Soil Sci. 2001, 52, 607–618. [Google Scholar] [CrossRef]
- Sollins, P.; Swanston, C.; Kleber, M.; Filley, T.; Kramer, M.; Crow, S.; Caldwell, B.A.; Lajtha, K.; Bowden, R. Organic C and N stabilization in a forest soil: Evidence from sequential density fractionation. Soil Biol. Biochem. 2006, 38, 3313–3324. [Google Scholar] [CrossRef] [Green Version]
- Marx, M.-C.; Kandeler, E.; Wood, M.; Wermbter, N.; Jarvis, S. Exploring the enzymatic landscape: Distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol. Biochem. 2005, 37, 35–48. [Google Scholar] [CrossRef]
- Grandy, A.S.; Neff, J.C.; Weintraub, M.N. Carbon structure and enzyme activities in alpine and forest ecosystems. Soil Biol. Biochem. 2007, 39, 2701–2711. [Google Scholar] [CrossRef]
- Derrien, D.; Marol, C.; Balabane, M.; Balesdent, J. The turnover of carbohydrate carbon in a cultivated soil estimated by 13C natural abundances. Eur. J. Soil Sci. 2006, 57, 547–557. [Google Scholar] [CrossRef]
- Quénéa, K.; Largeau, C.; Derenne, S.; Spaccini, R.; Bardoux, G.; Mariotti, A. Molecular and isotopic study of lipids in particle size fractions of a sandy cultivated soil (Cestas cultivation sequence, southwest France): Sources, degradation, and comparison with Cestas forest soil. Org. Geochem. 2006, 37, 20–44. [Google Scholar] [CrossRef]
- Christensen, B.T. Matching measurable soil organic matter fractions with conceptual pools in simulation models of carbon turnover: Revision of model structure. In Evaluation of Soil Organic Matter Models; Powlson, D.S., Smith, P., Smith, J.U., Eds.; Springer: Berlin, Germany, 1996; pp. 143–159. [Google Scholar]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Bol, R.; Poirier, N.; Balesdent, J.; Gleixner, G. Molecular turnover time of soil organic matter in particle-size fractions of an arable soil. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2009, 23, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Boom, A.; Chase, B.M.; Meadows, M.E.; Roberts, Z.E.; Britton, M.N.; Cumming, A.M. Biome-scale characterisation and differentiation of semi-arid and arid zone soil organic matter compositions using pyrolysis–GC/MS analysis. Geoderma 2013, 200, 189–201. [Google Scholar] [CrossRef]
- Buurman, P.; Roscoe, R. Different chemical composition of free light, occluded light and extractable SOM fractions in soils of Cerrado and tilled and untilled fields, Minas Gerais, Brazil: A pyrolysis-GC/MS study. Eur. J. Soil Sci. 2011, 62, 253–266. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob. Chang. Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Da Silva Oliveira, D.M.; Schellekens, J.; Cerri, C.E.P. Molecular characterization of soil organic matter from native vegetation–pasture–sugarcane transitions in Brazil. Sci. Total Environ. 2016, 548, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Derenne, S.; Quenea, K. Analytical pyrolysis as a tool to probe soil organic matter. J. Anal. Appl. Pyrolysis 2015, 111, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Nierop, K.G.; Pulleman, M.M.; Marinissen, J.C. Management induced organic matter differentiation in grassland and arable soil: A study using pyrolysis techniques. Soil Biol. Biochem. 2001, 33, 755–764. [Google Scholar] [CrossRef]
- Yassir, I.; Buurman, P. Soil organic matter chemistry changes upon secondary succession in Imperata Grasslands, Indonesia: A pyrolysis–GC/MS study. Geoderma 2012, 173, 94–103. [Google Scholar] [CrossRef]
- Parsi, Z.; Hartog, N.; Górecki, T.; Poerschmann, J. Analytical pyrolysis as a tool for the characterization of natural organic matter—A comparison of different approaches. J. Anal. Appl. Pyrolysis 2007, 79, 9–15. [Google Scholar] [CrossRef]
- Kaal, J.; Brodowski, S.; Baldock, J.A.; Nierop, K.G.; Cortizas, A.M. Characterisation of aged black carbon using pyrolysis-GC/MS, thermally assisted hydrolysis and methylation (THM), direct and cross-polarisation 13C nuclear magnetic resonance (DP/CP NMR) and the benzenepolycarboxylic acid (BPCA) method. Org. Geochem. 2008, 39, 1415–1426. [Google Scholar] [CrossRef]
- Abelenda, M.S.; Buurman, P.; Camps Arbestain, M.; Kaal, J.; Martinez-Cortizas, A.; Gartzia-Bengoetxea, N.; Macías, F. Comparing NaOH-extractable organic matter of acid forest soils that differ in their pedogenic trends: A pyrolysis-GC/MS study. Eur. J. Soil Sci. 2011, 62, 834–848. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.; Baek, G.; Yang, A.-R. Carbon and Nitrogen responses in litterfall and litter decomposition in red pine (Pinus densiflora S. et Z.) stands disturbed by pine wilt disease. Forests 2019, 10, 244. [Google Scholar] [CrossRef]
- De Assis, C.P.; González-Pérez, J.A.; de la Rosa, J.M.; Jucksch, I.; de Sá Mendonça, E.; González-Vila, F.J. Analytical pyrolysis of humic substances from a Latosol (Typic Hapludox) under different land uses in Minas Gerais, Brazil. J. Anal. Appl. Pyrolysis 2012, 93, 120–128. [Google Scholar] [CrossRef]
- Martin, F. Pyrolysis gas chromatography of humic substances from different origin. Zeitschrift Pflanzenernährung Bodenkunde 1975, 138, 407–416. [Google Scholar] [CrossRef]
- Martin, F.; Saiz-Jimenez, C.; Gonzalez-Vila, F. Pyrolysis-gas chromatography-mass spectrometry of lignins. Holzforschung 1979, 33, 210–212. [Google Scholar]
- De Vries, W.; Du, E.; Butterbach-Bahl, K. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest ecosystems. Curr. Opin. Environ. Sustain. 2014, 9, 90–104. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Neff, J.C.; Townsend, A.R.; Gleixner, G.; Lehman, S.J.; Turnbull, J.; Bowman, W.D. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 2002, 419, 915. [Google Scholar] [CrossRef]
- Maaroufi, N.I.; Nordin, A.; Hasselquist, N.J.; Bach, L.H.; Palmqvist, K.; Gundale, M.J. Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Glob. Chang. Biol. 2015, 21, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Currey, P.M.; Johnson, D.; Sheppard, L.J.; Leith, I.D.; Toberman, H.; Van Der WAL, R.; Dawson, L.A.; Artz, R.R. Turnover of labile and recalcitrant soil carbon differ in response to nitrate and ammonium deposition in an ombrotrophic peatland. Glob. Chang. Biol. 2010, 16, 2307–2321. [Google Scholar] [CrossRef]
- Nave, L.; Vance, E.; Swanston, C.; Curtis, P. Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 2009, 153, 231–240. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, X.; Luo, Y.; Yang, Y.; Fang, C.; Chen, J.; Li, B. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ. 2011, 140, 234–244. [Google Scholar] [CrossRef]
- Yue, K.; Peng, Y.; Peng, C.; Yang, W.; Peng, X.; Wu, F. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: A meta-analysis. Sci. Rep.UK 2016, 6, 19895. [Google Scholar] [CrossRef]
- Liu, J.; Wu, N.; Wang, H.; Sun, J.; Peng, B.; Jiang, P.; Bai, E. Nitrogen addition affects chemical compositions of plant tissues, litter and soil organic matter. Ecology 2016, 97, 1796–1806. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Feng, W.; Niu, S.; Plante, A.; Luo, Y.; Wang, K. Different responses of soil organic carbon fractions to additions of nitrogen. Eur. J. Soil Sci. 2018, 69, 1098–1104. [Google Scholar] [CrossRef]
- Cheng, S.; He, S.; Fang, H.; Xia, J.; Tian, J.; Yu, G.; Geng, J.; Yu, G. Contrasting effects of NH4+ and NO3− amendments on amount and chemical characteristics of different density organic matter fractions in a boreal forest soil. Geoderma 2017, 293, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Fang, H.; Yu, G.; Xu, X.; Xu, M.; Wang, L.; Li, X.; Si, G.; Geng, J. Contrasting effects of ammonium and nitrate inputs on soil CO 2 emission in a subtropical coniferous plantation of southern China. Biol. Fert. Soils 2015, 51, 815–825. [Google Scholar] [CrossRef]
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Cambardella, C.; Elliott, E. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Elliott, E.; Cambardella, C. Physical separation of soil organic matter. Agric. Ecosyst. Environ. 1991, 34, 407–419. [Google Scholar] [CrossRef]
- Stemmer, M.; Gerzabek, M.H.; Kandeler, E. Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication. Soil Biol. Biochem. 1998, 30, 9–17. [Google Scholar] [CrossRef]
- Grandy, A.S.; Sinsabaugh, R.L.; Neff, J.C.; Stursova, M.; Zak, D.R. Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 2008, 91, 37–49. [Google Scholar] [CrossRef]
- Castellano, M.J.; Kaye, J.P.; Lin, H.; Schmidt, J.P. Linking carbon saturation concepts to nitrogen saturation and retention. Ecosystems 2012, 15, 175–187. [Google Scholar] [CrossRef]
- Grandy, A.S.; Neff, J.C. Molecular C dynamics downstream: The biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci. Total Environ. 2008, 404, 297–307. [Google Scholar] [CrossRef]
- Bingham, A.H.; Cotrufo, M.F. Organic nitrogen storage in mineral soil: Implications for policy and management. Sci. Total Environ. 2016, 551, 116–126. [Google Scholar] [CrossRef]
- Yu, H.; Ding, W.; Luo, J.; Geng, R.; Ghani, A.; Cai, Z. Effects of long-term compost and fertilizer application on stability of aggregate-associated organic carbon in an intensively cultivated sandy loam soil. Biol. Fert. Soils 2012, 48, 325–336. [Google Scholar] [CrossRef]
- Cheng, S.; Fang, H.; Yu, G. Threshold responses of soil organic carbon concentration and composition to multi-level nitrogen addition in a temperate needle-broadleaved forest. Biogeochemistry 2018, 137, 219–233. [Google Scholar] [CrossRef]
- Flessa, H.; Amelung, W.; Helfrich, M.; Wiesenberg, G.L.; Gleixner, G.; Brodowski, S.; Rethemeyer, J.; Kramer, C.; Grootes, P.M. Storage and stability of organic matter and fossil carbon in a Luvisol and Phaeozem with continuous maize cropping: A synthesis. J. Plant Nutr. Soil Sci. 2008, 171, 36–51. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Lou, Y.; Gao, Y.; Fang, H.; Liu, S.; Xu, M.; Blagodatskaya, E.; Kuzyakov, Y. Response of soil organic matter fractions and composition of microbial community to long-term organic and mineral fertilization. Biol. Fert. Soils 2017, 53, 523–532. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Zhong, X.-L.; Li, J.-T.; Li, X.-J.; Ye, Y.-C.; Liu, S.-S.; Hallett, P.D.; Ogden, M.R.; Naveed, M. Physical protection by soil aggregates stabilizes soil organic carbon under simulated N deposition in a subtropical forest of China. Geoderma 2017, 285, 323–332. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Zhang, X.; Liang, W. Contributions of soil biota to C sequestration varied with aggregate fractions under different tillage systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- DeForest, J.L.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci. Soc. Am. J. 2004, 68, 132–138. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D. Pyrolysis-GC-MS characterization of forage materials. J. Agr. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; Almendros, G.; De la Rosa, J.; González-Vila, F.J. Appraisal of polycyclic aromatic hydrocarbons (PAHs) in environmental matrices by analytical pyrolysis (Py–GC/MS). J. Anal. Appl. Pyrolysis 2014, 109, 1–8. [Google Scholar] [CrossRef]
- Buurman, P.; Peterse, F.; Almendros Martin, G. Soil organic matter chemistry in allophanic soils: A pyrolysis-GC/MS study of a Costa Rican Andosol catena. Eur. J. Soil Sci. 2007, 58, 1330–1347. [Google Scholar] [CrossRef]
- Buurman, P.; Nierop, K.; Pontevedra-Pombal, X.; Cortizas, A.M. Molecular chemistry by pyrolysis–GC/MS of selected samples of the Penido Vello peat deposit, Galicia, NW Spain. Dev. Earth Surf. Process. 2006, 9, 217–240. [Google Scholar]
- Geng, J.; Cheng, S.L.; Fang, H.J.; Pei, J.; Xu, M.; Lu, M.Z.; Yang, Y.; Cao, Z.C. Nitrogen fertilization changes the molecular composition of soil organic matter in a subtropical plantation forest. Soil Sci. Soc. Am. J. in press.
- Chiavari, G.; Galletti, G.C. Pyrolysis—Gas chromatography/mass spectrometry of amino acids. J. Anal. Appl. Pyrolysis 1992, 24, 123–137. [Google Scholar] [CrossRef]
- Kaal, J.; Rumpel, C. Can pyrolysis-GC/MS be used to estimate the degree of thermal alteration of black carbon? Org. Geochem. 2009, 40, 1179–1187. [Google Scholar] [CrossRef]
- Simoneit, B.; Rogge, W.; Lang, Q.; Jaffé, R. Molecular characterization of smoke from campfire burning of pine wood (Pinus elliottii). Chemosphere Glob. Chang. Sci. 2000, 2, 107–122. [Google Scholar] [CrossRef]
- Mayle, F.E.; Burbridge, R.; Killeen, T.J. Millennial-scale dynamics of southern Amazonian rain forests. Science 2000, 290, 2291–2294. [Google Scholar] [CrossRef] [PubMed]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Van Heemst, J.D.; van Bergen, P.F.; Stankiewicz, B.A.; de Leeuw, J.W. Multiple sources of alkylphenols produced upon pyrolysis of DOM, POM and recent sediments. J. Anal. Appl. Pyrolysis 1999, 52, 239–256. [Google Scholar] [CrossRef]
- Galletti, G.C.; Reeves, J.B. Pyrolysis/gas chromatography/ion-trap detection of polyphenols (vegetable tannins): Preliminary results. Org. Mass Spectrom. 1992, 27, 226–230. [Google Scholar] [CrossRef]
- Vancampenhout, K.; Schellekens, J.; Slaets, J.; Hatté, C.; Buurman, P. Fossil redox-conditions influence organic matter composition in loess paleosols. Quat. Int. 2016, 418, 105–115. [Google Scholar] [CrossRef]
- Gleixner, G.; Bol, R.; Balesdent, J. Molecular insight into soil carbon turnover. Rapid Commun. Mass Spectrom. 1999, 13, 1278–1283. [Google Scholar] [CrossRef]
- Justi, M.; Schellekens, J.; de Camargo, P.B.; Vidal-Torrado, P. Long-term degradation effect on the molecular composition of black carbon in Brazilian Cerrado soils. Org. Geochem. 2017, 113, 196–209. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.A.; Buurman, P.; Schellekens, J.; Vidal-Torrado, P. Molecular chemistry in humic Ferralsols from Brazilian Cerrado and forest biomes indicates a major contribution from black carbon in the subsoil. J. Anal. Appl. Pyrolysis 2015, 113, 518–528. [Google Scholar] [CrossRef]
- Bento-Gonçalves, A.; Vieira, A.; Úbeda, X.; Martin, D. Fire and soils: Key concepts and recent advances. Geoderma 2012, 191, 3–13. [Google Scholar] [CrossRef]
- Kleber, M.; Mertz, C.; Zikeli, S.; Knicker, H.; Jahn, R. Changes in surface reactivity and organic matter composition of clay subfractions with duration of fertilizer deprivation. Eur. J. Soil Sci. 2004, 55, 381–391. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.-Y.; Zou, H.-T.; Kou, L.; Yang, Y.; Wen, X.-F.; Li, S.-G.; Wang, H.-M.; Sun, X.-M. Contrasting effects of ammonium and nitrate additions on the biomass of soil microbial communities and enzyme activities in subtropical China. Biogeosciences 2017, 14, 4815–4827. [Google Scholar] [CrossRef] [Green Version]
- Naafs, D.F.W. What Are Humic Substances?: A Molecular Approach to the Study of Organic Matter in Acid Soils. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2004. [Google Scholar]
- Spaccini, R.; Piccolo, A.; Haberhauer, G.; Gerzabek, M. Transformation of organic matter from maize residues into labile and humic fractions of three European soils as revealed by 13C distribution and CPMAS-NMR spectra. Eur. J. Soil Sci. 2000, 51, 583–594. [Google Scholar] [CrossRef]


| Compound | Code | m/z1 | RT 2 | Compound | Code | m/z | RT |
|---|---|---|---|---|---|---|---|
| n-alkanes | Aromatics | ||||||
| n-C6:0 | A1 | 57 + 71 | 2.47 | Benzene | Ar1 | 78 | 3.06 |
| n-C7:0 | A2 | 57 + 71 | 3.56 | Indane | Ar2 | 117 + 118 | 17.47 |
| n-C8:0 | A3 | 57 + 71 | 5.86 | Indene | Ar3 | 115 + 116 | 18.01 |
| n-C9:0 | A4 | 57 + 71 | 9.92 | Toluene | Ar4 | 91 + 92 | 4.92 |
| n-C10:0 | A5 | 57 + 71 | 15.63 | Methylindene | Ar5 | 115 + 130 | 24.98 |
| n-C11:0 | A6 | 57 + 71 | 22.24 | Lignins | |||
| n-C12:0 | A7 | 57 + 71 | 29.09 | 4-Acetylphenol | Lg1 | 121 + 136 | 25.96 |
| n-C13:0 | A8 | 57 + 71 | 35.81 | Guaiacol | Lg2 | 109 + 124 | 21.25 |
| n-C14:0 | A9 | 57 + 71 | 42.27 | 4-Methylguaiacol | Lg3 | 123 + 138 | 28.32 |
| n-C15:0 | A10 | 57 + 71 | 48.41 | 4-Ethylguaiacol | Lg4 | 137 + 152 | 34.10 |
| n-C16:0 | A11 | 57 + 71 | 54.24 | 4-Vinylguaiacol | Lg5 | 135 + 150 | 36.38 |
| n-C17:0 | A12 | 57 + 71 | 59.82 | 4-Formylguaiacol | Lg6 | 151 + 152 | 41.787 |
| n-C18:0 | A13 | 57 + 71 | 65.10 | 4-(Prop-2-enyl)guaiacol, trans | Lg7 | 164 | 45.00 |
| n-C19:0 | A14 | 57 + 71 | 70.15 | 4-Acetylguaiacol | Lg8 | 151 + 166 | 47.19 |
| n-C20:0 | A15 | 57 + 71 | 74.96 | Syringol | Lg9 | 139 + 154 | 38.884 |
| n-C21:0 | A16 | 57 + 71 | 79.56 | 4-Ethylsyringol | Lg10 | 167 + 182 | 49.13 |
| n-C22:0 | A17 | 57 + 71 | 84.01 | Polyaromatics | |||
| n-C23:0 | A18 | 57 + 71 | 87.63 | Naphthalene | PA1 | 128 | 27.18 |
| n-C24:0 | A19 | 57 + 71 | 90.58 | Methylnaphthalene | PA2 | 141 + 142 | 34.61 |
| n-C25:0 | A20 | 57 + 71 | 93.13 | Biphenyl | PA3 | 154 | 40.22 |
| n-C26:0 | A21 | 57 + 71 | 95.40 | C2 naphthalene | PA4 | 141 + 156 | 41.05 |
| n-C27:0 | A22 | 57 + 71 | 97.49 | Methylbiphenyl | PA5 | 168 + 167 | 41.64 |
| n-C28:0 | A23 | 57 + 71 | 99.44 | Fluorene | PA6 | 165 + 166 | 52.19 |
| n-alkenes | Phenanthrene | PA7 | 178 | 62.77 | |||
| n-C4:1 | E1 | 57 + 71 | 1.78 | Anthracene | PA8 | 178 | 63.28 |
| n-C5:1 | E2 | 57 + 71 | 1.92 | Methylphenanthrene | PA9 | 192 + 191 | 68.68 |
| n-C6:1 | E3 | 57 + 71 | 2.41 | 2-Phenylnaphthalene | PA10 | 204 + 202 | 72.53 |
| n-C7:1 | E4 | 57 + 71 | 3.43 | Fluoranthene | PA11 | 202 | 76.36 |
| n-C8:1 | E5 | 57 + 71 | 5.59 | Pyrene | PA12 | 202 | 78.63 |
| n-C9:1 | E6 | 57 + 71 | 9.53 | Retene | PA13 | 219 + 234 | 83.65 |
| n-C10:1 | E7 | 57 + 71 | 15.08 | Perylene | PA14 | 252 + 250 | 84.91 |
| n-C11:1 | E8 | 57 + 71 | 21.66 | N-containing compounds | |||
| n-C12:1 | E9 | 57 + 71 | 28.50 | Pyridine | N1 | 52 + 79 | 4.41 |
| n-C13:1 | E10 | 57 + 71 | 35.24 | Pyrrole | N2 | 67 | 4.71 |
| n-C14:1 | E11 | 57 + 71 | 41.74 | Methyl-1 H-pyrrole | N3 | 80 + 81 | 7.32 |
| n-C15:1 | E12 | 57 + 71 | 47.92 | Benzonitrile | N4 | 76 + 103 | 14.35 |
| n-C16:1 | E13 | 57 + 71 | 53.81 | Indole | N5 | 90 + 117 | 34.94 |
| n-C17:1 | E14 | 57 + 71 | 59.40 | Isoquinolin | N6 | 129 | 30.82 |
| n-C18:1 | E15 | 57 + 71 | 64.72 | Benzofurans | |||
| n-C19:1 | E16 | 57 + 71 | 69.79 | Benzofuran | Bf1 | 89 + 118 | 15.06 |
| n-C20:1 | E17 | 57 + 71 | 74.65 | Methylbenzofuran | Bf2 | 131 + 132 | 22.00 |
| n-C21:1 | E18 | 57 + 71 | 79.26 | Dibenzofuran | Bf3 | 139 + 168 | 48.34 |
| n-C22:1 | E19 | 57 + 71 | 83.74 | Phenols | |||
| n-C23:1 | E20 | 57 + 71 | 87.43 | Phenol | Ph1 | 66 + 94 | 14.88 |
| n-C24:1 | E21 | 57 + 71 | 90.43 | Methylphenol | Ph2 | 107 | 19.35 |
| n-C25:1 | E22 | 57 + 71 | 92.99 | C2 phenol | Ph3 | 107 + 122 | 25.54 |
| n-C26:1 | E23 | 57 + 71 | 95.30 | Polysaccharides | |||
| n-C27:1 | E24 | 57 + 71 | 97.39 | Acetic acid | Ps1 | 60 | 3.63 |
| n-C28:1 | E25 | 57 + 71 | 99.35 | 2-Furaldehyde | Ps2 | 95 + 96 | 6.25 |
| 5-Methyl-2-furaldehyde | Ps3 | 109 + 110 | 13.23 |
| Fractions | Treatments 1 | Relative Abundance (%) 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n-alkanes | n-alkenes | Aromatics | Lignins | Polyaromatics | N-Containing Compounds | Benzofurans | Phenols | Polysaccharides | ||
| Coarse POM | CK | 4.8 ± 1.9 | 2.0 ± 0.5 | 21.9 ± 5.0 | 11.2 ± 9.1 | 16.5 ± 3.8 | 7.2 ± 1.7 | 3.6 ± 0.5 | 16.5 ± 2.6 | 16.4 ± 4.9 |
| A40 | 3.3 ± 0.3 | 1.5 ± 0.2 | 19.0 ± 0.7 | 14.7 ± 2.1 | 13.9 ± 1.5 | 6.4 ± 0.4 | 3.3 ± 0.4 | 20.7 ± 2.0 | 17.1 ± 1.1 | |
| N40 | 3.6 ± 0.9 | 1.8 ± 0.4 | 22.9 ± 2.1 | 8.9 ± 4.3 | 16.3 ± 1.5 | 8.0 ± 2.0 | 3.8 ± 0.3 | 14.9 ± 1.1 | 19.8 ± 0.4 | |
| A120 | 3.0 ± 0.8 | 1.5 ± 0.4 | 19.1 ± 2.6 | 15.8 ± 7.9 | 15.3 ± 5.1 | 5.9 ± 1.0 | 3.3 ± 0.5 | 15.8 ± 2.4 | 20.3 ± 2.7 | |
| N120 | 3.1 ± 0.7 | 1.5 ± 0.4 | 19.2 ± 3.3 | 16.8 ± 8.4 | 15.0 ± 4.0 | 8.8 ± 2.4 | 3.4 ± 0.8 | 16.7 ± 0.3 | 15.6 ± 2.4 | |
| Fine POM | CK | 6.5 ± 0.5 | 3.3 ± 0.4 | 44.2 ± 2.6 | 0.1 ± 0.0 | 13.9 ± 1.7 | 15.4 ± 0.7 | 5.5 ± 0.5 | 2.3 ± 0.7 | 8.8 ± 3.6 |
| A40 | 4.9 ± 0.3 | 2.9 ± 0.4 | 43.5 ± 2.6 | 0.2 ± 0.1 | 15.8 ± 2.0 | 17.5 ± 1.9 | 6.3 ± 0.6 | 2.8 ± 0.4 | 6.2 ± 3.1 | |
| N40 | 4.6 ± 0.6 | 3.2 ± 0.7 | 40.8 ± 5.8 | 0.1 ± 0.0 | 12.7 ± 0.7 | 17.5 ± 1.5 | 5.3 ± 0.1 | 2.3 ± 0.5 | 13.6 ± 6.0 | |
| A120 | 6.9 ± 1.4 | 4.0 ± 1.1 | 39.3 ± 3.5 | 0.1 ± 0.0 | 15.9 ± 1.4 | 13.4 ± 2.0 | 5.2 ± 0.9 | 4.4 ± 2.2 | 10.7 ± 3.3 | |
| N120 | 5.6 ± 0.2 | 2.8 ± 0.4 | 39.5 ± 5.0 | 0.2 ± 0.0 | 13.6 ± 1.0 | 16.5 ± 0.5 | 5.6 ± 0.8 | 4.2 ± 2.3 | 11.9 ± 4.9 | |
| MAOM | CK | 4.6 ± 0.4 | 2.5 ± 0.4 | 43.6 ± 2.9 | 0.1 ± 0.0 | 9.1 ± 1.0 | 21.9 ± 1.0 | 4.8 ± 0.2 a | 1.9 ± 0.1 | 11.5 ± 1.5 b |
| A40 | 3.9 ± 0.7 | 2.3 ± 0.3 | 37.5 ± 3.6 | 0.1 ± 0.0 | 8.0 ± 0.5 | 19.8 ± 1.1 | 4.2 ± 0.3 ab | 2.0 ± 0.3 | 22.2 ± 5.3 a | |
| N40 | 4.3 ± 0.9 | 2.4 ± 0.2 | 36.4 ± 2.2 | 0.1 ± 0.0 | 8.0 ± 0.6 | 18.6 ± 1.1 | 3.6 ± 0.3 b | 2.8 ± 0.8 | 23.8 ± 1.1 a | |
| A120 | 4.3 ± 0.2 | 2.7 ± 0.1 | 40.2 ± 0.9 | 0.1 ± 0.0 | 8.3 ± 0.8 | 18.8 ± 1.0 | 4.1 ± 0.2 ab | 2.1 ± 0.4 | 19.3 ± 1.1 ab | |
| N120 | 5.4 ± 0.9 | 2.6 ± 0.2 | 36.2 ± 1.5 | 0.1 ± 0.0 | 8.9 ± 0.9 | 18.7 ± 0.7 | 3.8 ± 0.2 b | 2.8 ± 0.4 | 21.5 ± 2.8 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, J.; Cheng, S.; Fang, H.; Pei, J.; Xu, M.; Lu, M.; Yang, Y.; Cao, Z.; Li, Y. Different Molecular Characterization of Soil Particulate Fractions under N Deposition in a Subtropical Forest. Forests 2019, 10, 914. https://doi.org/10.3390/f10100914
Geng J, Cheng S, Fang H, Pei J, Xu M, Lu M, Yang Y, Cao Z, Li Y. Different Molecular Characterization of Soil Particulate Fractions under N Deposition in a Subtropical Forest. Forests. 2019; 10(10):914. https://doi.org/10.3390/f10100914
Chicago/Turabian StyleGeng, Jing, Shulan Cheng, Huajun Fang, Jie Pei, Meng Xu, Mingzhu Lu, Yan Yang, Zicheng Cao, and Yuna Li. 2019. "Different Molecular Characterization of Soil Particulate Fractions under N Deposition in a Subtropical Forest" Forests 10, no. 10: 914. https://doi.org/10.3390/f10100914





