Canopy Temperature Differences between Liana-Infested and Non-Liana Infested Areas in a Neotropical Dry Forest
Abstract
:1. Introduction
2. Materials and Methods
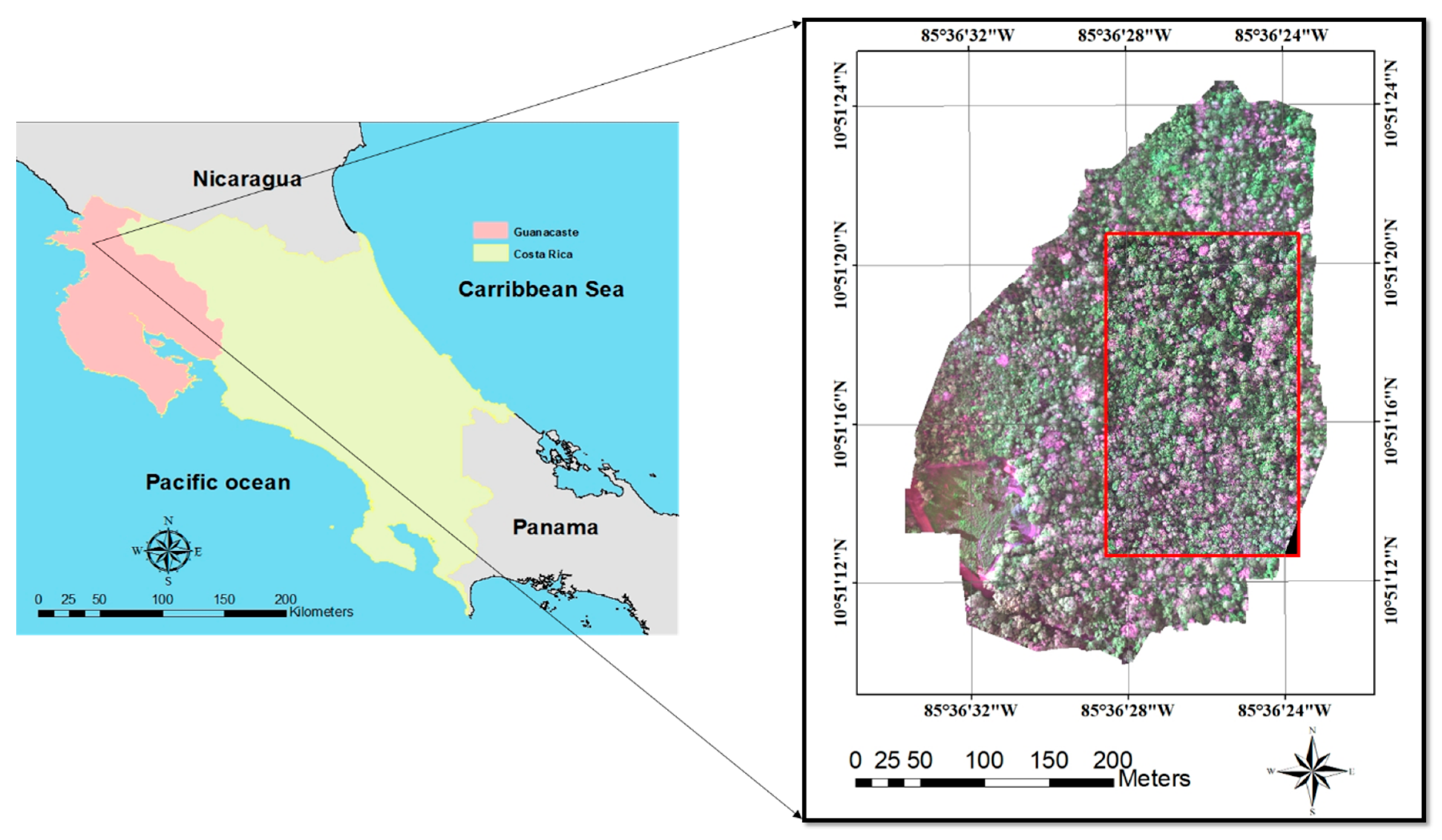
2.1. Study Area
2.2. Data Acquisition
2.3. Methods
3. Results
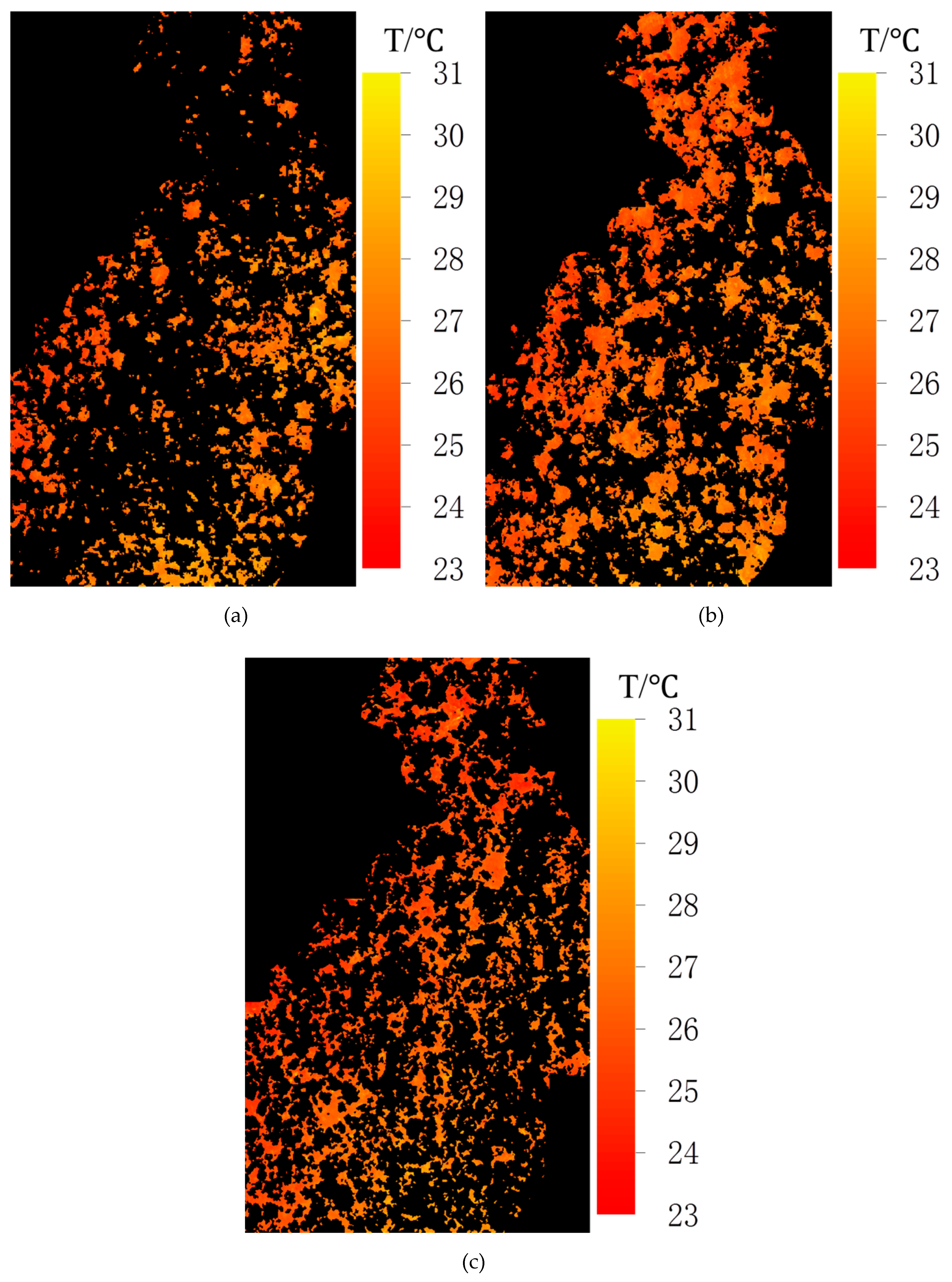
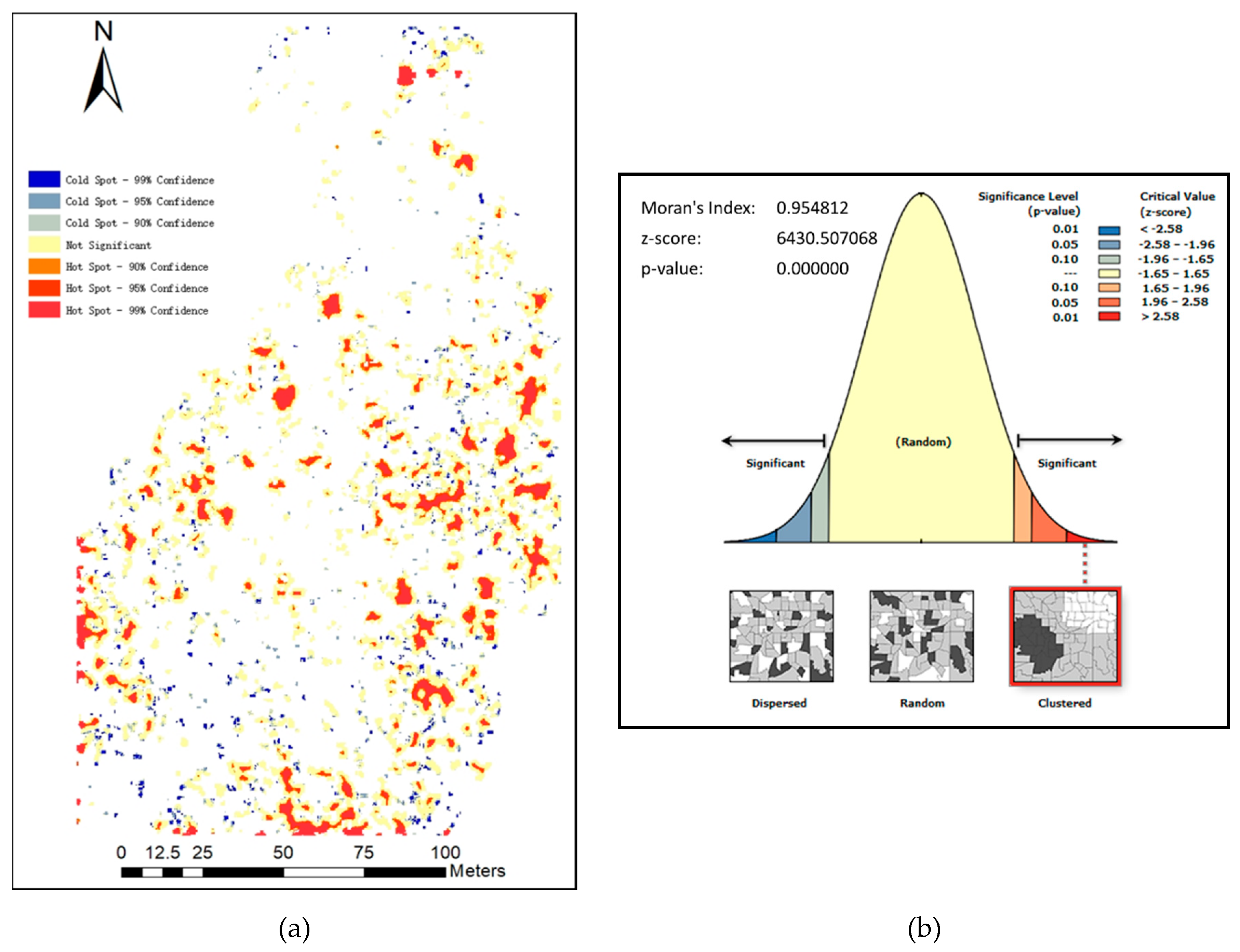
3.1. Spatial Distribution of the Image Components
3.2. Temperature Distributions of the Image Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schnitzer, S.A.; Bongers, F.; Wright, S.J. Community and ecosystem ramifications of increasing lianas in neotropical forests. Plant Signal. Behav. 2011, 6, 598–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janzen, D.H. Tropical dry forests. In Biodiversity, 1st ed.; Wilson, E.O., Ed.; National Academy Press: Washington, DC, USA, 1988; pp. 130–137. [Google Scholar]
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. Lond. Ser. B 2010, 365, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Balfour, D.A.; Bond, W.J. Factors limiting climber distribution and abundance in a southern African forest. J. Ecol. 1993, 81, 93–100. [Google Scholar] [CrossRef]
- Schnitzer, S.A. A mechanistic explanation for global patterns of liana abundance and distribution. Am. Nat. 2005, 166, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Ingwell, L.L.; Wright, S.J.; Becklund, K.K.; Hubbell, S.P.; Schnitzer, S.A. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. J. Ecol. 2010, 98, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Schnitzer, S.A.; Bongers, F. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, G.M.F.; Phillips, O.L. Liana infestation impacts tree growth in a lowland tropical moist forest. Biogeosciences 2009, 6, 2217–2226. [Google Scholar] [CrossRef] [Green Version]
- Schnitzer, S.A.; Kuzee, M.E.; Bongers, F. Disentangling above-and below-ground competition between lianas and trees in a tropical forest. J. Ecol. 2005, 93, 1115–1125. [Google Scholar] [CrossRef]
- Alvira, D.; Putz, F.E.; Fredericksen, T.S. Liana loads and post-logging liana densities after liana cutting in a lowland forest in Bolivia. For. Ecol. Manag. 2004, 190, 73–86. [Google Scholar] [CrossRef]
- Phillips, O.L.; Vásquez Martínez, R.; Monteagudo Mendoza, A.; Baker, T.R.; Núñez Vargas, P. Large lianas as hyperdynamic elements of the tropical forest canopy. Ecology 2005, 86, 1250–1258. [Google Scholar] [CrossRef]
- Duran, S.M.; Gianoli, E. Carbon stocks in tropical forests decrease with liana density. Biol. Lett. 2013, 9, 20130301. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; Dalling, J.W.; Carson, W.P. The impact of lianas on tree regeneration in tropical forest canopy gaps: Evidence for an alternative pathway of gap-phase regeneration. J. Ecol. 2000, 88, 655–666. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Carson, W.P. Lianas suppress tree regeneration and diversity in treefall gaps. Ecol. Lett. 2010, 13, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kainer, K.A.; Wadt, L.H.O.; Gomes-Silva, D.A.P.; Capanu, M. Liana loads and their association with Bertholletia excelsa fruit and nut production, diameter growth and crown attributes. J. Trop. Ecol. 2006, 22, 147–154. [Google Scholar] [CrossRef]
- Grauel, W.T.; Putz, F.E. Effects of lianas on growth and regeneration of Prioria copaifera in Darien, Panama. For. Ecol. Manag. 2004, 190, 99–108. [Google Scholar] [CrossRef]
- Phillips, O.L.; Martínez, R.V.; Arroyo, L.; Baker, T.R.; Killeen, T.; Lewis, S.L.; Malhi, Y.; Mendoza, A.M.; Neill, D.; Vargas, P.N. Increasing dominance of large lianas in Amazonian forests. Nature 2002, 418, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Phillips, O.; Gentry, A.H. The useful plants of Tambopata, Peru: II. Additional hypothesis testing in quantitative ethnobotany. Econ. Bot. 1993, 47, 33–43. [Google Scholar] [CrossRef]
- Condon, M.A.; Sasek, T.W.; Strain, B.R. Allocation patterns in two tropical vines in response to increased atmospheric CO2. Funct. Ecol. 1992, 6, 680–685. [Google Scholar] [CrossRef]
- Van der Heijden, G.M.F.; Schnitzer, S.A.; Powers, J.S.; Phillips, O.L. Liana impacts on carbon cycling, storage and sequestration in tropical forests. Biotropica 2013, 45, 682–692. [Google Scholar] [CrossRef]
- Van der Heijden, G.M.F.; Healey, J.R.; Phillips, O.L. Infestation of trees by lianas in a tropical forest in Amazonian Peru. J. Veg. Sci. 2008, 19, 747–756. [Google Scholar] [CrossRef]
- Van der Heijden, G.M.F.; Powers, J.S.; Schnitzer, S.A. Lianas reduce carbon accumulation and storage in tropical forests. Proc. Natl. Acad. Sci. USA 2015, 112, 13267–13271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Azofeifa, A.; Rankine, C.; do Espirito Santo, M.M.; Fatland, R.; Garcia, M. Wireless sensing networks for environmental monitoring: Two case studies from tropical forests. In Proceedings of the IEEE 7th International Conference on eScience, Stockholm, Sweden, 5–8 December 2011. [Google Scholar]
- Guzmán, Q.J.A.; Sánchez-Azofeifa, G.A.; Rivard, B. Differences in leaf temperature between lianas and trees in the neotropical canopy. Forests 2018, 9, 307. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Schnitzer, S.A.; Bongers, F. Seasonal differences in leaf-level physiology give lianas a competitive advantage over trees in a tropical seasonal forest. Oecologia 2009, 161, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Rigling, A.; Dobbertin, M. Species-specific stomatal response of trees to drought—A link to vegetation dynamics? J. Veg. Sci. 2009, 20, 442–454. [Google Scholar] [CrossRef]
- Haldimann, P.; Gallé, A.; Feller, U. Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiol. 2008, 28, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnitzer, S.A.; Bongers, F. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecol. Lett. 2011, 14, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, Y.; Zhang, H.; Fu, P.; Fan, Z. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.; Kustas, W. Thermal remote sensing of drought and evapotranspiration. Eos Trans. Am. Geophys. Union 2008, 89, 233–240. [Google Scholar] [CrossRef]
- Gates, D.M. Leaf temperature and transpiration. Agron. J. 1964, 56, 273–277. [Google Scholar] [CrossRef]
- Monteith, J.L.; Szeicz, G. Radiative temperature in the heat balance of natural surfaces. Q. J. R. Meteorol. Soc. 1962, 88, 496–507. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J., Jr. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Carlson, T.N.; Dodd, J.K.; Benjamin, S.G.; Cooper, J.N. Satellite estimation of the surface energy balance, moisture availability and thermal inertia. J. Appl. Meteorol. 1981, 20, 67–87. [Google Scholar] [CrossRef]
- Taconet, O.; Bernard, R.; Vidal-Madjar, D. Evapotranspiration over an agricultural region using a surface flux/temperature model based on NOAA-AVHRR data. J. Appl. Meteorol. Climatol. 1986, 25, 284–307. [Google Scholar] [CrossRef]
- Heilman, J.L.; Kanemasu, E.T.; Rosenberg, N.J.; Blad, B.L. Thermal scanner measurement of canopy temperatures to estimate evapotranspiration. Remote Sens. Environ. 1976, 5, 137–145. [Google Scholar] [CrossRef]
- Sullivan, D.G.; Fulton, J.P.; Shaw, J.N.; Bland, G. Evaluating the sensitivity of an unmanned thermal infrared aerial system to detect water stress in a cotton canopy. Trans. ASABE 2007, 50, 1963–1969. [Google Scholar] [CrossRef]
- Li, W.; Campos-Vargas, C.; Marzahn, P.; Sanchez-Azofeifa, A. On the estimation of tree mortality and liana infestation using a deep self-encoding network. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 1–13. [Google Scholar] [CrossRef]
- Runkle, J.R. Guidelines and sample protocol for sampling forest gaps. In Gen. Tech. Rep. PNW-GTR-283; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1992; 44p. [Google Scholar]
- Castro, S.M.; Sanchez-Azofeifa, G.A.; Sato, H. Effect of drought on productivity in a Costa Rican tropical dry forest. Environ. Res. Lett. 2018, 13, 045001. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Quesada, M.; Rodríguez, J.P.; Nassar, J.M.; Stoner, K.E.; Castillo, A.; Garvin, T.; Zent, E.L.; Calvo-Alvarado, J.C.; Kalacska, M.E.R.; et al. Research priorities for neotropical dry forests. Biotropica 2005, 37, 477–485. [Google Scholar]
- Kalacska, M.; Sanchez-Azofeifa, G.A.; Calvo-Alvarado, J.C.; Quesada, M.; Rivard, B.; Janzen, D.H. Species composition, similarity and diversity in three successional stages of a seasonally dry tropical forest. For. Ecol. Manag. 2004, 200, 227–247. [Google Scholar] [CrossRef]
- Iqbal, F.; Lucieer, A.; Barry, K. Simplified radiometric calibration for UAS-mounted multispectral sensor. Eur. J. Remote Sens. 2018, 51, 301–313. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics, 1st ed.; Olkin, I., Ed.; Stanford University Press: Palo Alto, CA, USA, 1961; pp. 278–292. [Google Scholar]
- Massey, F.J., Jr. The Kolmogorov-Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Welch, B.L. The significance of the difference between two means when the population variances are unequal. Biometrika 1938, 29, 350–362. [Google Scholar] [CrossRef]
- Welch, B.L. On the comparison of several mean values: An alternative approach. Biometrika 1951, 38, 330–336. [Google Scholar] [CrossRef]
- Games, P.A.; Howell, J.F. Pairwise multiple comparison procedures with unequal n’s and/or variances: A Monte Carlo study. J. Educ. Behav. Stat. 1976, 1, 113–125. [Google Scholar]
- Cribbie, R.A.; Wilcox, R.R.; Bewell, C.; Keselman, H.J. Tests for treatment group equality when data are nonnormal and heteroscedastic. J. Mod. Appl. Stat. Methods 2007, 6, 117–132. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Chissom, B.S. Interpretation of the kurtosis statistic. Am. Stat. 1970, 24, 19–22. [Google Scholar]
- Waldock, C.; Dornelas, M.; Bates, A.E. Temperature-driven biodiversity change: Disentangling space and time. Bioscience 2018, 68, 873–884. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Castro-Esau, K. Canopy observations on the hyperspectral properties of a community of tropical dry forest lianas and their host trees. Int. J. Remote Sens. 2006, 27, 2101–2109. [Google Scholar] [CrossRef]
- Pau, S.; Detto, M.; Kim, Y.; Still, C.J. Tropical forest temperature thresholds for gross primary productivity. Ecosphere 2018, 9, e02311. [Google Scholar] [CrossRef] [Green Version]
- Mildrexler, D.J.; Zhao, M.; Running, S.W. A global comparison between station air temperatures and MODIS land surface temperatures reveals the cooling role of forests. J. Geophys. Res. Biogeosci. 2011, 116, G03025. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Schnitzer, S.A.; Zhang, Y.-J.; Fan, Z.-X.; Goldstein, G.; Tomlinson, K.W.; Lin, H.; Zhang, J.-L.; Cao, K.-F. Physiological regulation and efficient xylem water transport regulate diurnal water and carbon balances of tropical lianas. Funct. Ecol. 2017, 31, 306–317. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar]
- Grace, J. Plant response to wind. In Windbreak Technology, 1st ed.; Brandle, J.R., Hintz, D.L., Sturrock, J.W., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1988; pp. 71–88. [Google Scholar]
- Talley, S.M.; Lawton, R.O.; Setzer, W.N. Host preferences of Rhus radicans (Anacardiaceae) in a southern deciduous hardwood forest. Ecology 1996, 77, 1271–1276. [Google Scholar] [CrossRef]
- Rice, K.; Brokaw, N.; Thompson, J. Liana abundance in a Puerto Rican forest. For. Ecol. Manag. 2004, 190, 33–41. [Google Scholar] [CrossRef]
- Allen, B.P.; Sharitz, R.R.; Goebel, P.C. Twelve years post-hurricane liana dynamics in an old-growth southeastern floodplain forest. For. Ecol. Manag. 2005, 218, 259–269. [Google Scholar] [CrossRef]
- Carsten, L.D.; Juola, F.A.; Male, T.D.; Cherry, S. Host associations of lianas in a south-east Queensland rain forest. J. Trop. Ecol. 2002, 18, 107–120. [Google Scholar] [CrossRef]
- Slot, M.; Rey-Sánchez, C.; Gerber, S.; Lichstein, J.W.; Winter, K.; Kitajima, K. Thermal acclimation of leaf respiration of tropical trees and lianas: Response to experimental canopy warming, and consequences for tropical forest carbon balance. Glob. Chang. Biol. 2014, 20, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Putz, F.E.; Chai, P. Ecological studies of lianas in Lambir National Park, Sarawak, Malaysia. J. Ecol. 1987, 75, 523–531. [Google Scholar] [CrossRef]
- Waite, C.E.; van der Heijden, G.M.F.; Field, R.; Boyd, D.S. A view from above: Unmanned aerial vehicles (UAVs) provide a new tool for assessing liana infestation in tropical forest canopies. J. Appl. Ecol. 2019, 56, 902–912. [Google Scholar] [CrossRef]
- Kimball, B.A.; Bernacchi, C.J. Evapotranspiration, canopy temperature, and plant water relations. In Managed Ecosystems and CO2, 1st ed.; Nösberger, J., Long, S.P., Norby, R.J., Stitt, M., Hendrey, G.R., Blum, H., Eds.; Springer: Berlin, Germany, 2006; pp. 311–324. [Google Scholar]
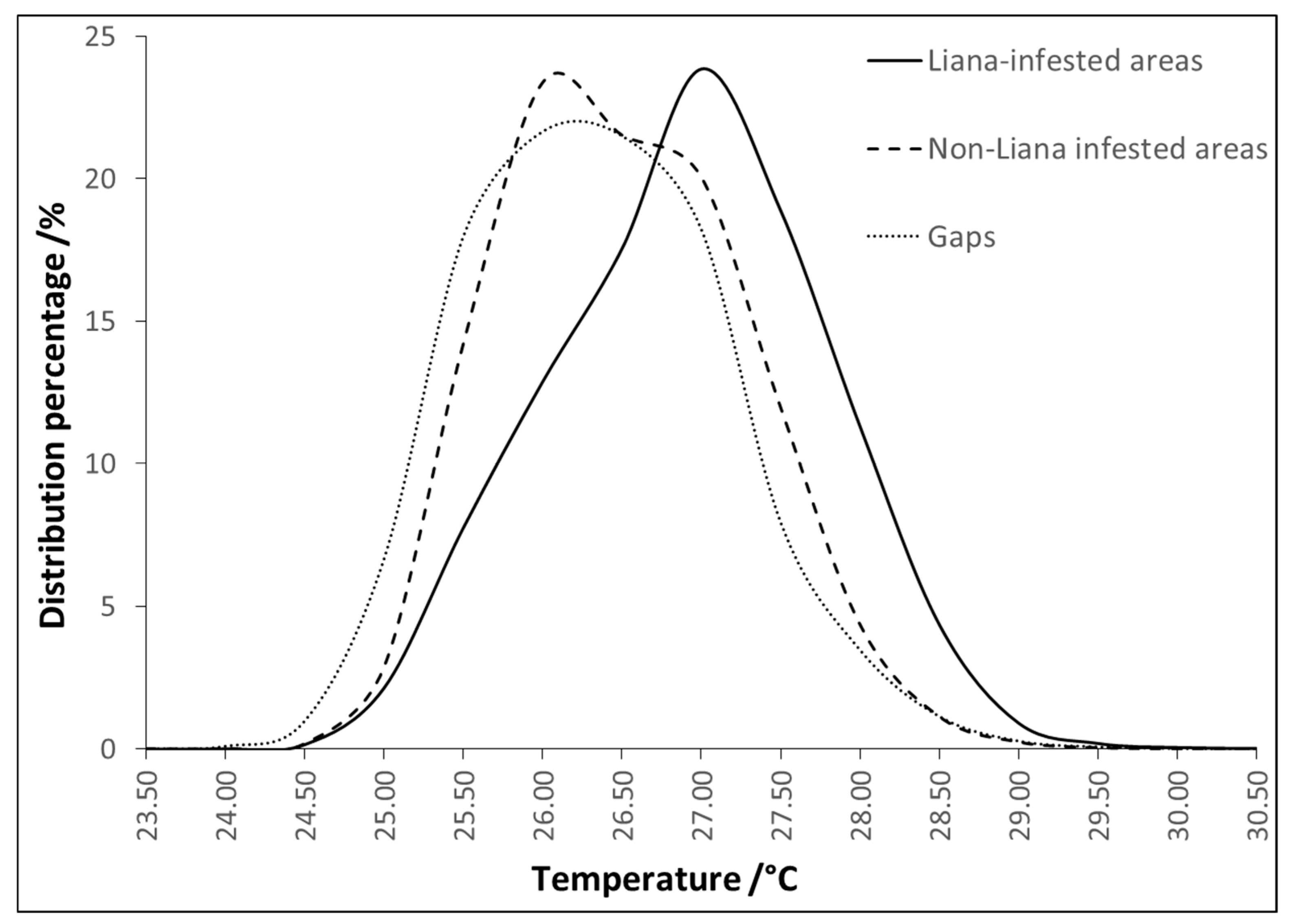
| Mean (°C) | Median (°C) | Std. Dev. | Min (°C) | Max (°C) | |
|---|---|---|---|---|---|
| Liana-infested areas | 26.67 | 26.71 | 0.85 | 23.96 | 30.86 |
| Non-liana infested areas | 26.26 | 26.22 | 0.77 | 23.73 | 30.88 |
| Gaps | 26.10 | 26.07 | 0.81 | 23.52 | 30.89 |
| Statistic a | Df1 | Df2 | Sig. | |
|---|---|---|---|---|
| Welch | 43.15510 | 2 | 764.85833 | 0.000 |
| Main Category | Comparative Categories | Mean Difference (I–J) | Std. Error | Sig. | 99% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 1 | 2 | 0.41238 | 0.06165 | 0.000 | 0.26760 | 0.55716 |
| 3 | 0.52990 | 0.05908 | 0.000 | 0.39117 | 0.66863 | |
| 2 | 1 | −0.41238 | 0.06165 | 0.000 | −0.55716 | −0.26760 |
| 3 | 0.11752 | 0.05965 | 0.120 | −0.02255 | 0.25759 | |
| 3 | 1 | −0.52990 | 0.05908 | 0.000 | −0.66863 | −0.39117 |
| 2 | −0.11752 | 0.05965 | 0.120 | −0.25759 | 0.02255 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Laakso, K.; Marzahn, P.; Sanchez-Azofeifa, G.A. Canopy Temperature Differences between Liana-Infested and Non-Liana Infested Areas in a Neotropical Dry Forest. Forests 2019, 10, 890. https://doi.org/10.3390/f10100890
Yuan X, Laakso K, Marzahn P, Sanchez-Azofeifa GA. Canopy Temperature Differences between Liana-Infested and Non-Liana Infested Areas in a Neotropical Dry Forest. Forests. 2019; 10(10):890. https://doi.org/10.3390/f10100890
Chicago/Turabian StyleYuan, Xu, Kati Laakso, Philip Marzahn, and G. Arturo Sanchez-Azofeifa. 2019. "Canopy Temperature Differences between Liana-Infested and Non-Liana Infested Areas in a Neotropical Dry Forest" Forests 10, no. 10: 890. https://doi.org/10.3390/f10100890
APA StyleYuan, X., Laakso, K., Marzahn, P., & Sanchez-Azofeifa, G. A. (2019). Canopy Temperature Differences between Liana-Infested and Non-Liana Infested Areas in a Neotropical Dry Forest. Forests, 10(10), 890. https://doi.org/10.3390/f10100890






