Leaf Cuticle Can Contribute to Non-Host Resistance to Poplar Leaf Rust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resistance to M. larici-populina of 36 Clones of Poplar Species and Hybrids
2.2. Poplar Materials for Experiments
2.3. Cuticle Extraction
2.4. Urediniospore Germination in Leaf Cuticle Extracts
2.5. Identification of Cuticle Compounds
2.6. Experiments with Cuticular Components
3. Results
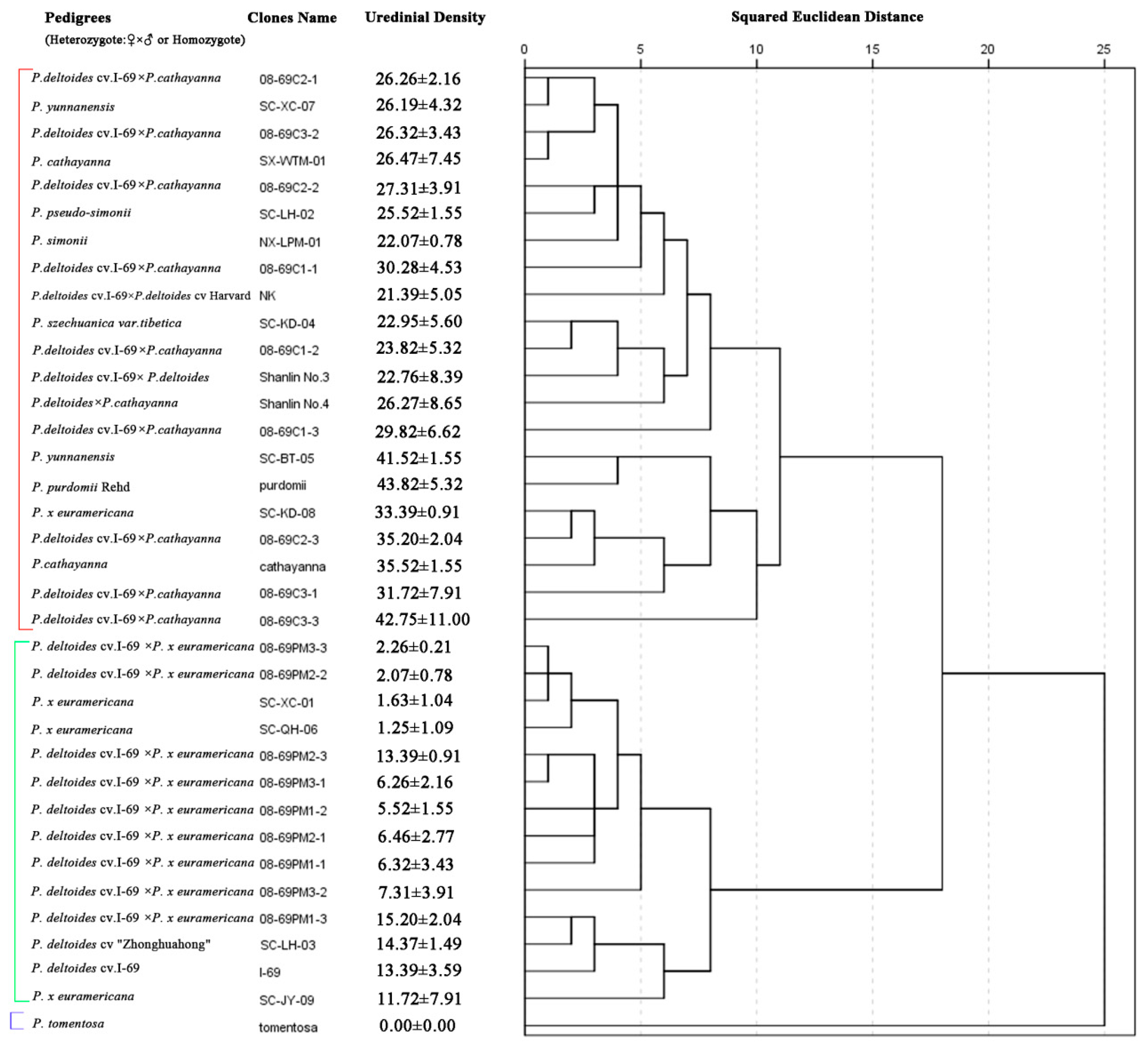
3.1. Rust Incidence and Severity in the Field
3.2. Urediniospore Germination in Leaf Cuticle Extracts
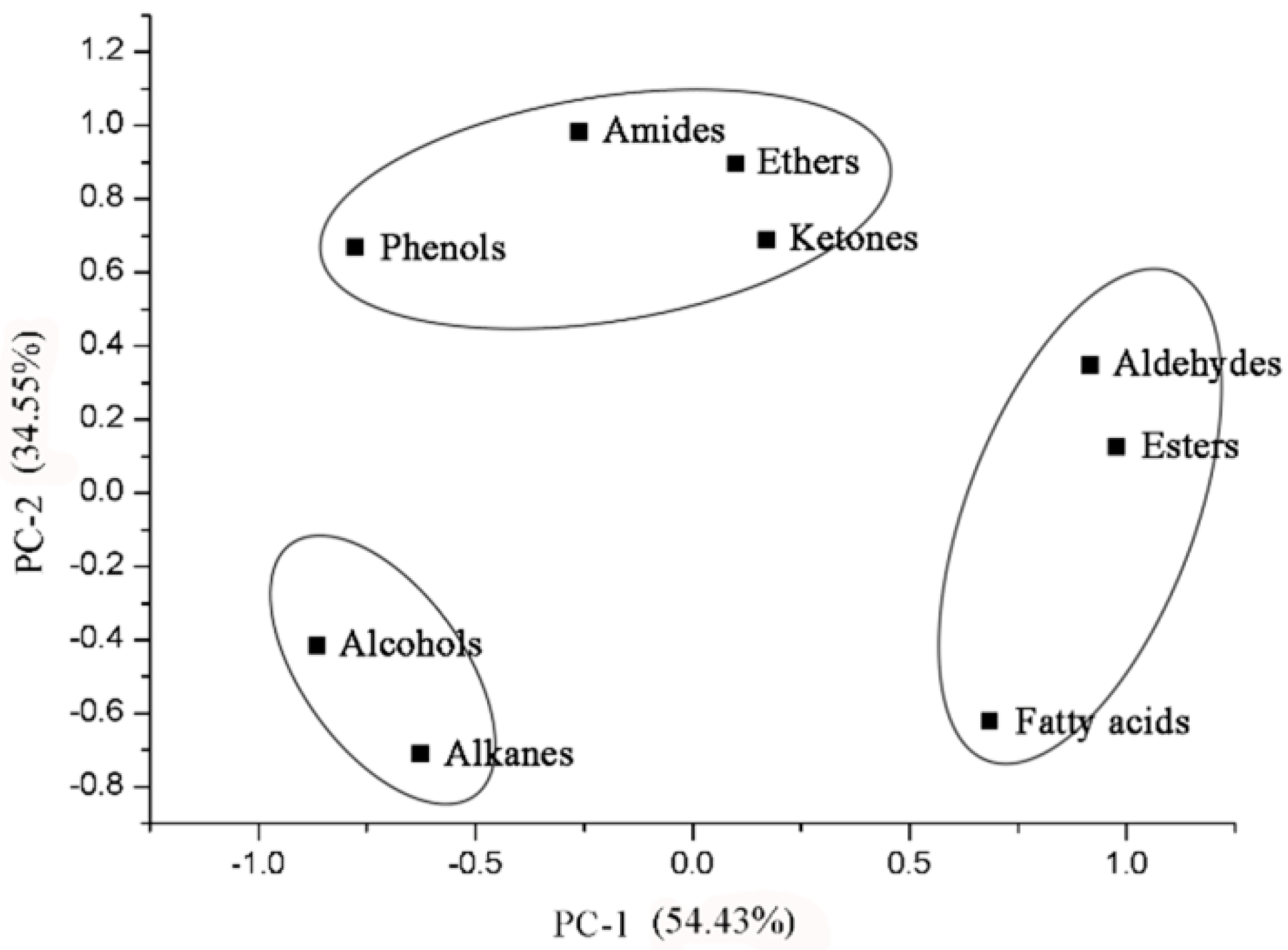
3.3. Chemical Composition of the Leaf Cuticle
3.4. Identification of Cuticle Components to Be Used in Further Assays
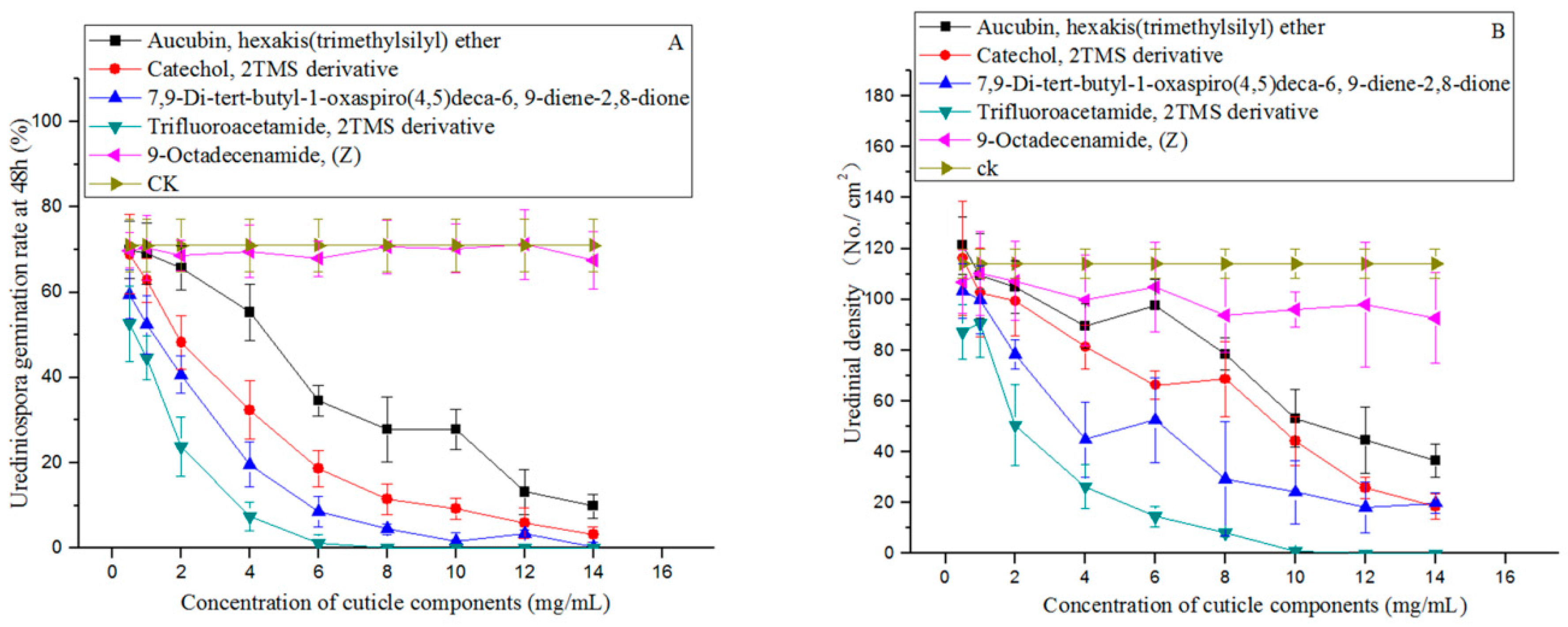
3.5. Assays of Candidate Cuticle Components
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Peng, S.; Ren, Z.; Wang, D.; Cao, Z. Infection behavior of Melampsora larici-populina on the leaf surface of P. purdomii. Agric. Sci. China 2011, 10, 1562–1569. [Google Scholar] [CrossRef]
- Hoch, H.C.; Staples, R.C.; Whitehead, B.; Comeau, J.; Wolf, E.D. Signaling for growth orientation and cell differentiation by surface topography in Uromyces. Science 1987, 235, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.A.; Hazen, B.E.; Hoch, H.C.; Kwon, Y.; Leinhos, G.M.E.; Staples, R.C.; Stumpf, M.A.; Terhune, B.T. Appressorium formation in response to topographical signals by 27 rust species. Phytopathology 1991, 81, 323–331. [Google Scholar] [CrossRef]
- Spiers, A.G.; Hopcroft, D.H. Penetration and infection of poplar leaves by urediniospores of Melampsora larici-populina and Melampsora medusae. N. Z. J. Bot. 1988, 26, 101–111. [Google Scholar] [CrossRef]
- Fink, W.; Haug, M.; Deising, H.; Mendgen, K. Early defence responses of cowpea (Vigna sinensis L.) induced by non-pathogenic rust fungi. Planta 1991, 185, 246–254. [Google Scholar] [CrossRef]
- Mamrutha, H.M.; Mogili, T.; Jhansi Lakshmi, K.; Rama, N.; Kosma, D.; Udaya Kumar, M.; Jenks, M.A.; Nataraja, K.N. Leaf cuticular wax amount and crystal morphology regulate post-harvest water loss in mulberry (Morus species). Plant Physiol. Biochem. 2010, 48, 690–696. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K.C. The Formation and Function of Plant Cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Broeckling, C.D.; Blancaflor, E.B.; Sledge, M.K.; Sumner, L.W.; Wang, Z.Y. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant J. 2005, 42, 689–707. [Google Scholar] [CrossRef]
- Tian, C.-M.; Shang, Y.-Z.; Zhuang, J.-Y.; Wang, Q.; Kakishima, M. Morphological and molecular phylogenetic analysis of Melampsora species on poplars in China. Mycoscience 2004, 45, 56–66. [Google Scholar] [CrossRef]
- Frey, P.; Pinon, J. Variability in pathogenicity of Melampsora allii-populina expressed on poplar cultivars. Eur. J. For. Pathol. 1997, 27, 397–407. [Google Scholar] [CrossRef]
- Bettgenhaeuser, J.; Gilbert, B.; Ayliffe, M.; Moscou, M.J. Nonhost resistance to rust pathogens - a continuation of continua. Front. Plant Sci. 2014, 5, 664. [Google Scholar] [CrossRef] [PubMed]
- Assante, G.; Maffi, D.; Saracchi, M.; Farina, G.; Moricca, S.; Ragazzi, A. Histological studies on the mycoparasitism of Cladosporium tenuissimum on urediniospores of Uromyces appendiculatus. Mycol. Res. 2004, 108, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Gao, H.; Chen, H.; Wu, W.; Fang, X. Changes in Cuticular Wax Composition of Two Blueberry Cultivars during Fruit Ripening and Postharvest Cold Storage. J. Agric. Food Chem. 2018, 66, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.J.; Gao, H.Y.; Cao, S.F.; Fang, X.J.; Chen, H.J.; Xiao, S.Y. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef]
- Özer, N.; Şabudak, T.; Özer, C.; Gindro, K.; Schnee, S.; Solak, E. Investigations on the role of cuticular wax in resistance to powdery mildew in grapevine. J. Gener. Plant Pathol. 2017, 83, 316–328. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 4 April 2019).
- Newcombe, G. A review of exapted resistance to diseases of Populus. Eur. J. For. Pathol. 1998, 28, 209–216. [Google Scholar] [CrossRef]
- Newcombe, G. Inheritance of resistance to Glomerella cingulata in Populus. Can. J. For. Res. 2000, 30, 639–644. [Google Scholar] [CrossRef]
- Newcombe, G. Puccinia tanaceti: Specialist or generalist? Mycol. Res. 2003, 107, 797–802. [Google Scholar]
- Newcombe, G. Genes for parasite-specific, nonhost resistance in Populus. Phytopathology 2005, 95, 779–783. [Google Scholar] [CrossRef]
- Newcombe, G.; Bradshaw, H.D. Quantitative trait loci conferring resistance in hybrid poplar to Septoria populicola, the cause of leaf spot. Can. J. For. Res. 1996, 26, 1943–1950. [Google Scholar] [CrossRef]
- Newcombe, G.; Robb, J. The function and relative importance of the vascular coating response in highly resistant, moderately resistant and susceptible alfalfa infected by Verticillium albo-atrum. Physiol. Mol. Plant Pathol. 1988, 33, 47–58. [Google Scholar] [CrossRef]
- Newcombe, G.; Stirling, B.; Bradshaw, H.D. Abundant pathogenic variation in the new hybrid rust Melampsora x columbiana on hybrid poplar. Phytopathology 2001, 91, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Eckenwalder, J.E. Systematics and evolution of Populus. In Biology of Populus and its Implications for Management and Conservation; NCR Research Press: Ottawa, ON, Canada, 1996; Volume 7, p. 32. [Google Scholar]
- Zsuffa, L. A summary review of interspecific breeding in the genus L. In Proceedings of the 14th Meeting of the Canadian Tree Improvement Association, Petawawa, ON, Canada, 18–22 August 1975; pp. 107–123. [Google Scholar]
- Heath, M.C. Thoughts on the role and evolution of induced resistance in natural ecosystems, and its relationship to other types of plant defenses against disease. In Induced Resistance to Disease in Plants; Springer: Dordrecht, The Netherlands, 1995; pp. 141–151. [Google Scholar]
- Zheng, W.; Newcombe, G.; Hu, D.; Cao, Z.; Yu, Z.; Peng, Z. The First Record of a North American Poplar Leaf Rust Fungus, Melampsora medusae, in China. Forests 2019, 10, 182. [Google Scholar] [CrossRef]
- Pinon, J.; Frey, P.; Husson, C. Wettability of poplar leaves influences dew formation and infection by Melampsora larici-populina. Plant Dis. 2006, 90, 177–184. [Google Scholar] [CrossRef]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Schreiber, L. Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci. 2010, 15, 546–553. [Google Scholar] [CrossRef]


| P. tomentosa | P. deltoides cv. ‘I-69’ | P. purdomii | Control | |
|---|---|---|---|---|
| Urediniospore germination (%) | 7.8 ± 2.3 a | 27.8 ± 8.5 b | 58.2 ± 4.8 c | 62.0 ± 5.4 c |
| Tube length (µm) | 7.7 ± 5.6 a | 62.3 ± 29.9 b | 278.0 ± 126.7 c | 282.5 ± 113.3 c |
| Model | Coefficients | Collinearity Statistics | R | Residual (δ) | ||
|---|---|---|---|---|---|---|
| B | VIF | Eigenvalue | Condition Index | |||
| Constant | 61.555 | 1.728 | 1.000 | 0.952 | ||
| Alkanes | 1.012 | 1.004 | 0.086 | 5.809 | 0.000 a | |
| Total load | −20.758 | 1.213 | 0.272 | 2.522 | ||
| Variety | Carbon Number | Compound Name | Molecular Formula | Chemical Structure | SI | RSI |
|---|---|---|---|---|---|---|
| Ether | C33 | Aucubin, hexakis(trimethylsilyl) ether | C33H70O9Si6 | 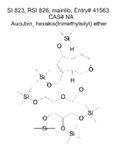 | 823 | 826 |
| Phenol | C12 | Catechol, 2TMS derivative | C12H22O2Si2 | 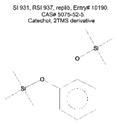 | 931 | 937 |
| Ketone | C17 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6, 9-diene-2,8-dione | C17H24O3 | 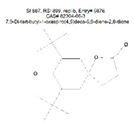 | 929 | 934 |
| Amide | C8 | Trifluoroacetamide, 2TMS derivative | C8H18F3NOSi2 | 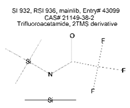 | 921 | 922 |
| C18 | 9-Octadecenamide, (Z) | C18H35NO |  | 885 | 911.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Shen, K.; Newcombe, G.; Fan, J.; Chen, Q. Leaf Cuticle Can Contribute to Non-Host Resistance to Poplar Leaf Rust. Forests 2019, 10, 870. https://doi.org/10.3390/f10100870
Yu Z, Shen K, Newcombe G, Fan J, Chen Q. Leaf Cuticle Can Contribute to Non-Host Resistance to Poplar Leaf Rust. Forests. 2019; 10(10):870. https://doi.org/10.3390/f10100870
Chicago/Turabian StyleYu, Zhongdong, Kuocheng Shen, George Newcombe, Junfeng Fan, and Qianwen Chen. 2019. "Leaf Cuticle Can Contribute to Non-Host Resistance to Poplar Leaf Rust" Forests 10, no. 10: 870. https://doi.org/10.3390/f10100870
APA StyleYu, Z., Shen, K., Newcombe, G., Fan, J., & Chen, Q. (2019). Leaf Cuticle Can Contribute to Non-Host Resistance to Poplar Leaf Rust. Forests, 10(10), 870. https://doi.org/10.3390/f10100870







