Temporal Analysis of Bacterial Leaf Blight in Clonal Eucalyptus Plantations in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Experimental Area and Genetic Material
2.2. Bacterial Leaf Blight Progress
2.3. Area under the Disease-Progress Curve
2.4. Correlation Analysis
3. Results
3.1. Bacterial Leaf Blight Progress
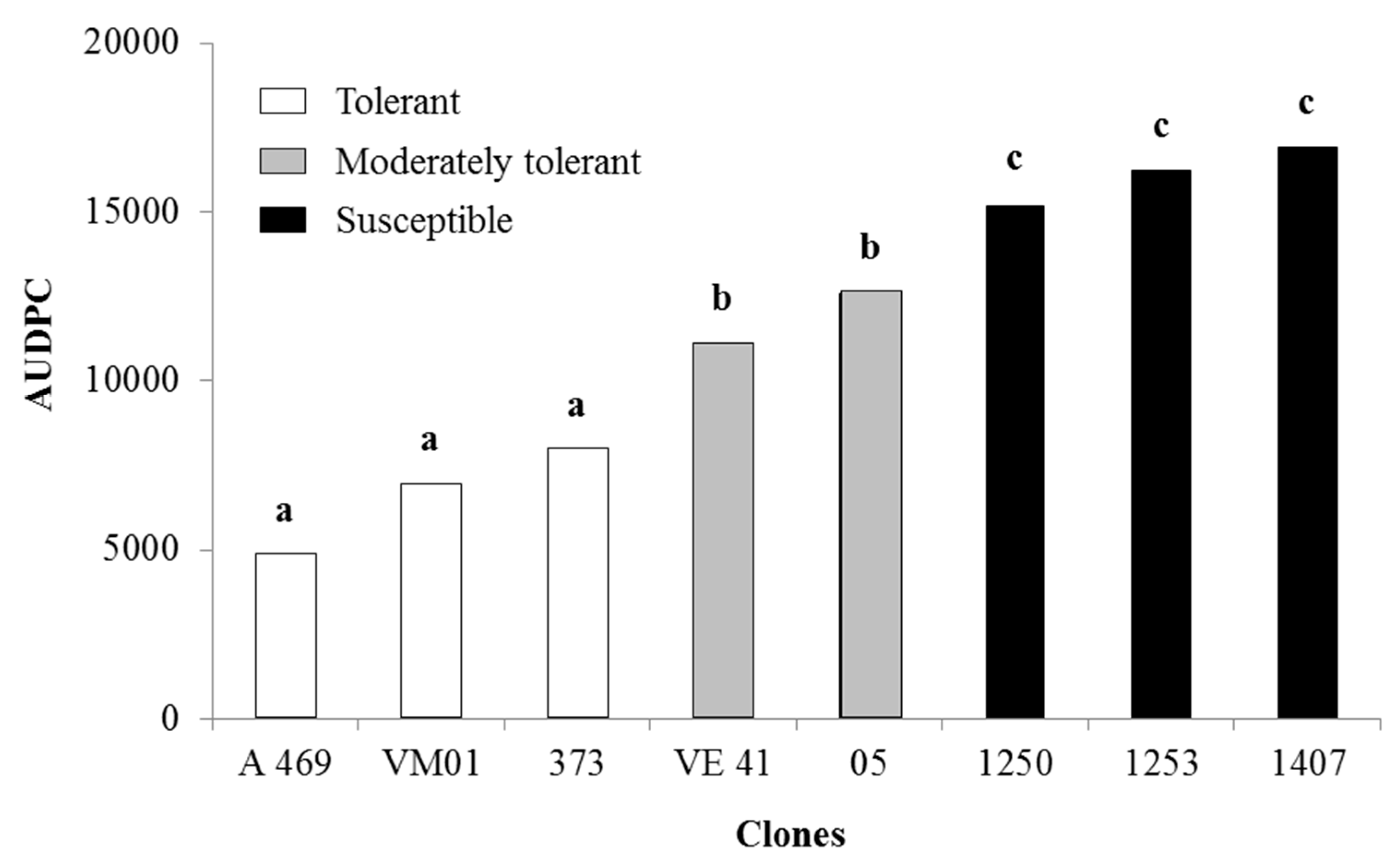
3.2. Area under the Disease-Progress Curve
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ibá. Indústria Brasileira de Árvores. Available online: http://iba.org/images/shared/Biblioteca/IBA_RelatorioAnual2017.pdf (accessed on 15 December 2018).
- Alfenas, A.C.; Zauza, E.A.V.; Mafia, R.G.; Assis, T.F. Clonagem e Doenças do Eucalipto, 2nd ed.; UFV: Viçosa, Brazil, 2009; p. 500. [Google Scholar]
- Ferraz, H.G.M.; Badel, J.L.; Da Silva Guimarães, L.M.; Reis, B.P.; Tótola, M.R.; Gonçalves, R.C.; Alfenas, A.C. Xanthomonas axonopodis pv. eucalyptorum pv. nov. Causing Bacterial Leaf Blight on Eucalypt in Brazil. Plant Pathol. J. 2018, 34, 269–285. [Google Scholar]
- Gonçalves, R.C.; Lau, D.; Oliveira, J.R.; Maffia, L.A.; Cascardo, J.C.M.; Alfenas, A.C. Etiology of bacterial leaf blight of eucalyptus in Brazil. Trop. Plant Pathol. 2008, 33, 180–188. [Google Scholar] [CrossRef]
- Mafia, R.G.; Teixeira, L.P.; Ferreira, M.A. Produção de mudas de eucalipto sob dois sistemas de irrigação e a ocorrência da bacteriose foliar Xanthomonas axonopodis Vauterin. Sci. For. 2017, 45, 697–704. [Google Scholar] [CrossRef]
- Assis, T.F.; Mafia, R.G. Hibridação e clonagem. In Biotecnologia Florestal; Borém, A., Ed.; Suprema: Viçosa, Brazil, 2007; pp. 93–121. [Google Scholar]
- Arriel, D.A.A.; Fonseca, N.R.; Guimarães, L.M.S.; Hermenegildo, P.S.; Mafia, R.G.; Borges, N.; Souza, H.P.; Alfenas, A.C. Wilt and die-back of Eucalyptus spp. caused by Erwinia psidii in Brazil. For. Pathol. 2014, 44, 255–265. [Google Scholar] [CrossRef]
- EMBRAPA. Sistema Brasileiro de Classificação de Solos, 2nd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2006; p. 306. [Google Scholar]
- Jesus Junior, W.C.; Pozza, E.A.; Vale, F.X.R.; Aguilera, G.M. Análise temporal de epidemias. In Epidemiologia Aplicada ao Manejo de Doenças de Plantas; Vale, F.X.R., Jesus Junior, W.C., Zambolim, L., Eds.; Perffil: Belo Horizonte, Brazil, 2004; pp. 127–188. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990; p. 532. [Google Scholar]
- Cornell, J.A.; Berger, R.D. Factors that influence the value of the coefficient of determination in simple linear and non linear regression models. Phytopathology 1987, 77, 63–70. [Google Scholar] [CrossRef]
- Systat Software. SigmaPlot for Windows Version 12.0; Systat Software Inc.: San Jose, CA, USA, 2011. [Google Scholar]
- Ferreira, D.F. SISVAR Versão 5.3; Departamento de Ciências Exatas-UFLA: Lavras, Brazil, 2010. [Google Scholar]
- Bergamin Filho, A.; Amorim, L.; Hau, B. Análise temporal e espacial de epidemias. In O Essencial da Fitopatologia: Epidemiologia de Doenças de Planta; Zambolim, L., Jesus Júnior, W.C., Rodrigues, F.A., Eds.; UFV: Viçosa, Brazil, 2014; pp. 101–165. [Google Scholar]
- Marcuzzo, L.L.; Becker, W.F.; Fernandes, J.M.C. Alguns aspectos epidemiológicos da mancha bacteriana (Xanthomonas spp.) do tomateiro na região de Caçador/SC. Summa Phytopathol. 2009, 35, 132–135. [Google Scholar] [CrossRef]
- Romeiro, R.S. Bactérias Fitopatogênicas, 2nd ed.; UFV: Viçosa, Brazil, 2007. [Google Scholar]
- Neves, D.A.; Guimarães, L.M.S.; Ferraz, H.G.M.; Alfenas, A.C. Favorable conditions for X. axonopodis infection in Eucalyptus spp. Trop. Plant Pathol. 2014, 39, 428–433. [Google Scholar] [CrossRef]
- Behlau, F.; Belasque Junior, J.; Bergamin Filho, A.; Leite Junior, R.P. Incidência e severidade de cancro cítrico em laranja ‘Pêra Rio’ sob condições de controle químico e proteção com quebra-vento. Fitopatol. Bras. 2007, 32, 311–317. [Google Scholar] [CrossRef]
- Fininsa, C.; Yuen, J. Temporal progression of bean common bacterial blight (Xanthomonas campestris pv. Phaseoli) in sole and intercropping systems. Eur. J. Plant Pathol. 2002, 108, 485–495. [Google Scholar] [CrossRef]
- Vargas, R.G.; Gonçalves-Zuliani, A.M.O.; Croce Filho, J.; Carvalho, S.A.; Nocchi, P.T.R.; Nunes, W.M.C. Avaliação da resistência de variedades de Citrus spp. à Xanthomonas citri subsp. citri na região Noroeste Paranaense, em condições de campo. Summa Phytopathol. 2013, 39, 235–241. [Google Scholar] [CrossRef]
- Nociti, L.A.S.; Camargo, M.; Rodrigues Neto, J.; Francischini, F.J.B.; Belasque Júnior, J. Agressividade de linhagens de X. axonopodis pv. aurantifolii Tipo C em lima ácida ‘Galego’. Fitopatol. Bras. 2006, 31, 140–146. [Google Scholar] [CrossRef]
- Halfeld-Vieira, B.A.; Sousa, R.M. Virulência de isolados de X. axonopodis pv. phaseoli e sua variante fuscans. Ciênc. Agrotéc. 2000, 24, 94–102. [Google Scholar]
- Rossi, A.P. Ferrugem do Eucalipto: Análise Temporal e Quantificação de Danos. Master’s Thesis, Universidade Federal do Espírito Santo; Centro de Ciências Agrárias, Jerônimo Monteiro, Brazil, 2013. [Google Scholar]
- Silva, D.E.M. Quantificação da Resistência Parcial em Espécies de Eucalipto à Ferrugem (Puccinia Psidii Winter). Master’s Thesis, Universidade Federal do Rio de Janeiro, Seropédica, Brazil, 2012. [Google Scholar]
- Gonçalves, J.L. Sazonalidade de Ocorrência de Mofo Cinzento, Oídio e Manchas Bacterianas Foliares em Dois Viveiros de Produção de Mudas Clonais de Eucalipto. Master’s Thesis, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Faculdade de Ciências Agronômicas, Botucatu, Brazil, 2014. [Google Scholar]
- Valeriano, R.; Pozza, E.A.; Santos, L.A.; Chaves, E.; Barbosa Junior, M.P.; Ferreira, M.A. Escala diagramática e reação diferencial de clones para Oídio do Eucalipto. Sci. For. 2015, 43, 51–61. [Google Scholar]
- Auer, C.G.; Santos, A.F.; Rodrigues, L.M.R.; Rodrigues Neto, J. Ocorrência de mancha foliar bacteriana em plantios de eucalipto no Estado do Paraná. Summa Phytopathol. 2010, 36, 82. [Google Scholar]
- Palladino, C.; Pérez, G.; Alonso, R.; Pérez, C.A. Identificación y control de enfermedades bacterianas em plantaciones jóvenes de Eucalyptus dunnii Y E. grandis. In Jornada Técnica de Protección Forestal: Avances de Investigación en Plagas y Enfermedades Forestales; Balmelli, G., Simeto, S., Martínez, G., Gómez, D.V., Eds.; INIA: Montevideo, Uruguay, 2013; pp. 29–38. [Google Scholar]
- Fukui, R.; Fukui, H.; Alvarez, A.M. Effect of temperature on the incubation period and leaf colonization in bacterial blight of Anthurim. Phytopathology 1999, 89, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Zerh, E.; Shepard, D.P.; Bridges, W.C. Bacterial spot of peach as influenced by water congestion, leaf, wetness duration, and temperature. Plant Dis. 1996, 80, 339–341. [Google Scholar]
- Pria, M.D.; Christiano, R.C.S.; Furtado, E.L.; Amorim, L.; Bergamin Filho, A. Effects of temperature and leaf wetness duration on infection of sweet oranges by Asiatic citrus canker. Plant Pathol. 2006, 55, 657–663. [Google Scholar] [CrossRef]
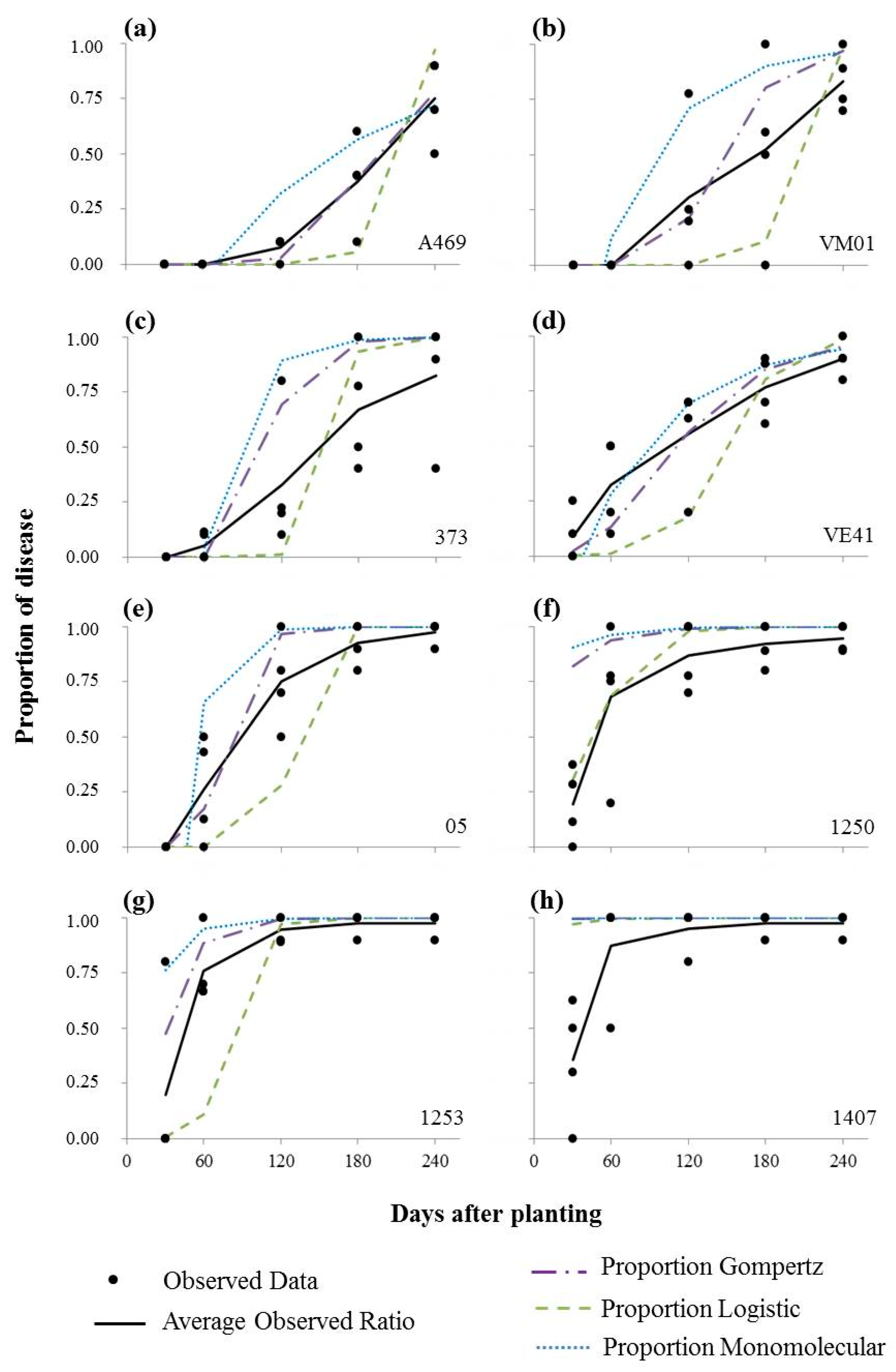
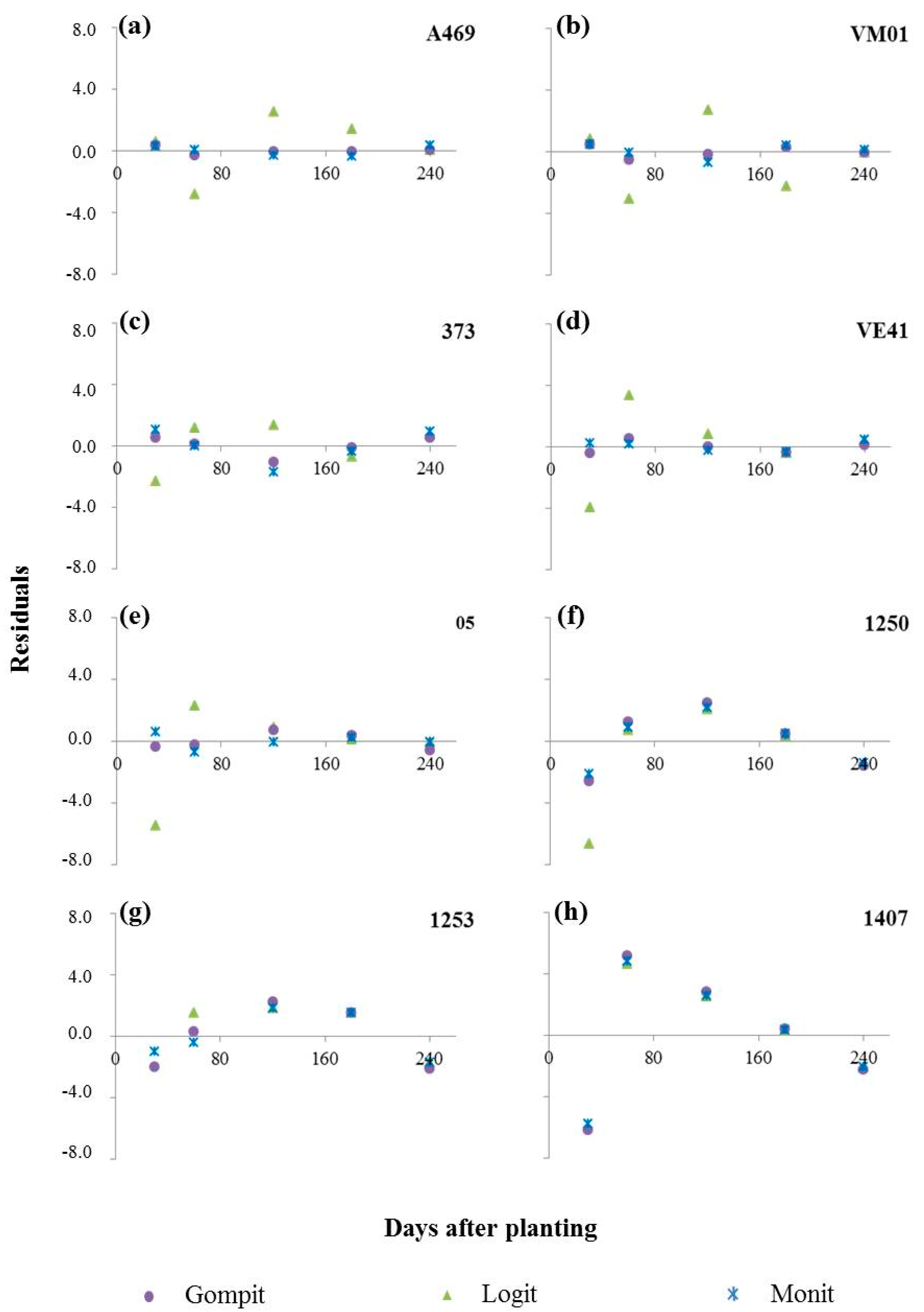


| Clones | Model | R2 | R2* | MSE(1) | Yo | Xo (Intercept) | Xo (SD) | r (rate) | r (SD) |
|---|---|---|---|---|---|---|---|---|---|
| A469 | Log | 0.798 ** | 0.817 | 18.289 ** | 0.000 | −21.918 ** | 1.965 | 0.106 * | 0.013 |
| Gom | 0.873 ** | 0.996 | 0.462 ** | 0.000 | −3.967 ** | 0.312 | 0.022 ** | 0.002 | |
| Mon | 0.610 ** | 0.738 | 0.225 ** | −0.660 | −0.507 * | 0.218 | 0.007 ** | 0.001 | |
| VM01 | Log | 0.644 ** | 0.697 | 44.533 ** | 0.000 | −22.095 ** | 2.866 | 0.111 ** | 0.019 |
| Gom | 0.477 ** | 0.936 | 7.658 ** | 0.000 | −4.344 * | 1.188 | 0.033 ** | 0.008 | |
| Mon | 0.273 * | 0.734 | 5.876 * | −1.643 | −0.972 | 1.041 | 0.018 * | 0.007 | |
| 373 | Log | 0.699 ** | 0.876 | 39.479 ** | 0.000 | −18.598 ** | 2.699 | 0.118 ** | 0.018 |
| Gom | 0.486 ** | 0.919 | 17.144 ** | 0.000 | −4.052 * | 1.778 | 0.050 ** | 0.012 | |
| Mon | 0.344 | 0.593 | 16.824 | −7.645 | −2.157 | 1.762 | 0.037 | 0.012 | |
| VE41 | Log | 0.392 ** | 0.847 | 24.773 ** | 0.0005 | −7.454 ** | 2.138 | 0.049 ** | 0.015 |
| Gom | 0.630 ** | 0.969 | 1.714 ** | 0.0007 | −1.973 ** | 0.562 | 0.021 ** | 0.004 | |
| Mon | 0.519 ** | 0.968 | 1.222 ** | −0.6820 | −0.520 | 0.475 | 0.014 ** | 0.003 | |
| 05 | Log | 0.667 ** | 0.795 | 52.971 ** | 0.000 | −16.179 ** | 3.126 | 0.127 ** | 0.021 |
| Gom | 0.582 ** | 0.962 | 21.055 ** | 0.000 | −4.579 * | 1.971 | 0.067 ** | 0.013 | |
| Mon | 0.495 ** | 0.745 | 20.258 ** | −8.309 | −2.231 | 1.933 | 0.055 ** | 0.013 | |
| 1250 | Log | 0.249 * | 0.979 | 55.791 * | 0.082 | −2.409 | 3.208 | 0.053 * | 0.022 |
| Gom | 0.209 * | 0.996 | 35.723 * | 0.542 | 0.489 | 2.567 | 0.038 * | 0.017 | |
| Mon | 0.181 | 0.997 | 33.542 | 0.741 | 1.349 | 2.487 | 0.034 | 0.017 | |
| 1253 | Log | 0.505 ** | 0.711 | 56.223 ** | 0.000 | −7.719 * | 3.220 | 0.094 ** | 0.022 |
| Gom | 0.497 ** | 0.996 | 24.445 ** | 0.010 | −1.527 | 2.123 | 0.061 ** | 0.014 | |
| Mon | 0.460 ** | 0.994 | 22.118 ** | −0.184 | −0.169 | 2.020 | 0.054 ** | 0.014 | |
| 1407 | Log | 0.213 * | 0.998 | 80.525 * | 0.853 | 1.762 | 3.854 | 0.058 * | 0.026 |
| Gom | 0.192 | 0.986 | 53.107 | 0.987 | 4.366 | 3.130 | 0.044 | 0.021 | |
| Mon | 0.178 | 0.979 | 49.555 | 0.993 | 5.019 | 3.023 | 0.041 | 0.021 |
| Variables | Spearman’s Correlation | Pearson’s Correlation |
|---|---|---|
| Incidence | AUDPC | |
| AUDPC | −0.037 * | |
| DBaH | −0.299 * | −0.425 * |
| DBH | −0.360 * | −0.379 * |
| Height | −0.523 ** | −0.564 ** |
| Rainfall | −0.587 ** | - |
| Average air temperature | 0.687 ** | - |
| Air relative humidity | −0.687 ** | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.E.S.; Silva Fernandes, F.; Glória Junior, M.A.; Soares de Oliveira, A.; Mafia, R.G.; Alves Ferreira, M. Temporal Analysis of Bacterial Leaf Blight in Clonal Eucalyptus Plantations in Brazil. Forests 2019, 10, 839. https://doi.org/10.3390/f10100839
de Oliveira MES, Silva Fernandes F, Glória Junior MA, Soares de Oliveira A, Mafia RG, Alves Ferreira M. Temporal Analysis of Bacterial Leaf Blight in Clonal Eucalyptus Plantations in Brazil. Forests. 2019; 10(10):839. https://doi.org/10.3390/f10100839
Chicago/Turabian Stylede Oliveira, Mara Elisa Soares, Fabiano Silva Fernandes, Murilo A. Glória Junior, Alvaro Soares de Oliveira, Reginaldo Gonçalves Mafia, and Maria Alves Ferreira. 2019. "Temporal Analysis of Bacterial Leaf Blight in Clonal Eucalyptus Plantations in Brazil" Forests 10, no. 10: 839. https://doi.org/10.3390/f10100839
APA Stylede Oliveira, M. E. S., Silva Fernandes, F., Glória Junior, M. A., Soares de Oliveira, A., Mafia, R. G., & Alves Ferreira, M. (2019). Temporal Analysis of Bacterial Leaf Blight in Clonal Eucalyptus Plantations in Brazil. Forests, 10(10), 839. https://doi.org/10.3390/f10100839





